Abstract
We have examined secretory antibody and cell-mediated immune responses to oral cholera vaccine in the human gastrointestinal mucosa. Freshly isolated peripheral blood lymphocytes and intestinal lymphocytes obtained by enzymatic dispersion of duodenal biopsies were assayed for numbers of total and vaccine specific immunoglobulin-secreting cells by enzyme-linked immunospot assay (ELISPOT) techniques; the frequency of cells secreting interferon-gamma (IFN-gamma) was also examined by a new modification of the ELISPOT technique. After booster immunizations with oral cholera vaccine, large numbers of cholera toxin-specific antibody-secreting cells (ASC) appeared in the small intestine. The responses were dominated by IgA ASC. A single immunization, performed 5 mo after the initial vaccinations, gave rise to an ASC response similar to that seen after the first booster immunization, with respect to both magnitude and isotype distribution. Each of the immunizations also evoked an ASC response in blood which was of lower magnitude than that seen in the small intestine, and comprised similar proportions of IgA and IgG ASC. A booster immunization also resulted in increased frequencies of IFN-gamma-secreting cells, but this increase was confined to the duodenal mucosa. This study establishes the feasibility of studying, at the single-cell level, intestinal immune reactivity in humans. Furthermore, it indicates that the small intestinal mucosa is an enriched source of IFN-gamma. It also demonstrates marked differences between intestinal and peripheral blood immune responses after enteric immunization, and confirms the notion that the mucosal immune system in humans displays immunological memory.
Full text
PDF

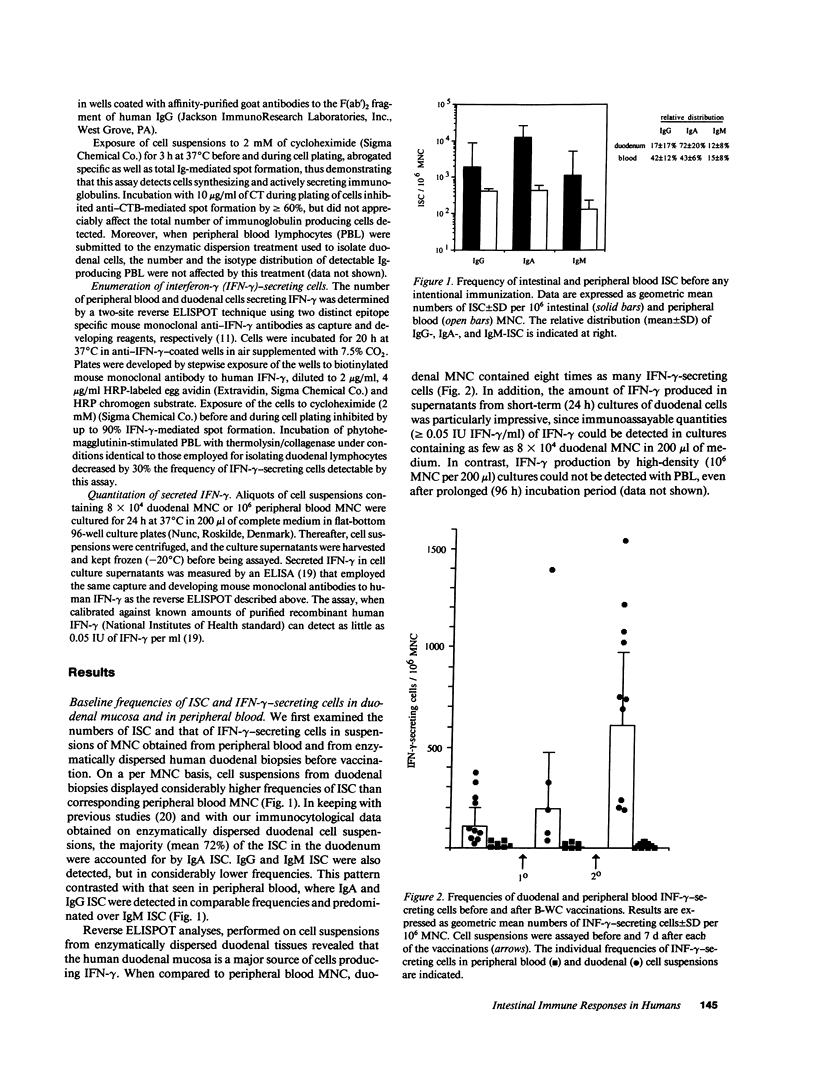
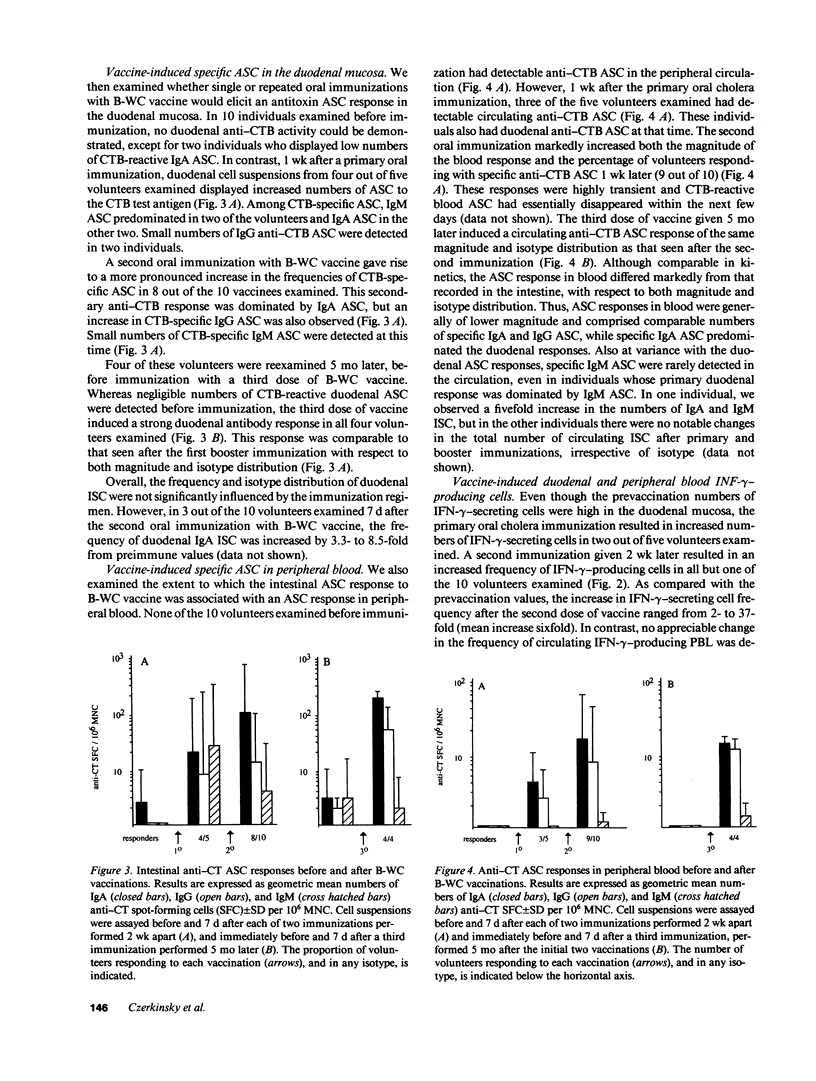


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson G., Ekre H. P., Alm G., Perlmann P. Monoclonal antibody two-site ELISA for human IFN-gamma. Adaptation for determinations in human serum or plasma. J Immunol Methods. 1989 Dec 20;125(1-2):89–96. doi: 10.1016/0022-1759(89)90081-1. [DOI] [PubMed] [Google Scholar]
- Bienenstock J., Befus D., McDermott M., Mirski S., Rosenthal K. Regulation of lymphoblast traffic and localization in mucosal tissues, with emphasis on IgA. Fed Proc. 1983 Dec;42(15):3213–3217. [PubMed] [Google Scholar]
- Budd R. C., Cerottini J. C., MacDonald H. R. Selectively increased production of interferon-gamma by subsets of Lyt-2+ and L3T4+ T cells identified by expression of Pgp-1. J Immunol. 1987 Jun 1;138(11):3583–3586. [PubMed] [Google Scholar]
- Clemens J. D., Sack D. A., Harris J. R., Van Loon F., Chakraborty J., Ahmed F., Rao M. R., Khan M. R., Yunus M., Huda N. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet. 1990 Feb 3;335(8684):270–273. doi: 10.1016/0140-6736(90)90080-o. [DOI] [PubMed] [Google Scholar]
- Czerkinsky C. C., Nilsson L. A., Nygren H., Ouchterlony O., Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983 Dec 16;65(1-2):109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- Czerkinsky C., Andersson G., Ekre H. P., Nilsson L. A., Klareskog L., Ouchterlony O. Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J Immunol Methods. 1988 May 25;110(1):29–36. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- Czerkinsky C., Moldoveanu Z., Mestecky J., Nilsson L. A., Ouchterlony O. A novel two colour ELISPOT assay. I. Simultaneous detection of distinct types of antibody-secreting cells. J Immunol Methods. 1988 Nov 25;115(1):31–37. doi: 10.1016/0022-1759(88)90306-7. [DOI] [PubMed] [Google Scholar]
- Czerkinsky C., Prince S. J., Michalek S. M., Jackson S., Russell M. W., Moldoveanu Z., McGhee J. R., Mestecky J. IgA antibody-producing cells in peripheral blood after antigen ingestion: evidence for a common mucosal immune system in humans. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2449–2453. doi: 10.1073/pnas.84.8.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerkinsky C., Svennerholm A. M., Quiding M., Jonsson R., Holmgren J. Antibody-producing cells in peripheral blood and salivary glands after oral cholera vaccination of humans. Infect Immun. 1991 Mar;59(3):996–1001. doi: 10.1128/iai.59.3.996-1001.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangakis M. V., Koopman W. J., Kiyono H., Michalek S. M., McGhee J. R. An enzymatic method for preparation of dissociated murine Peyer's patch cells enriched for macrophages. J Immunol Methods. 1982;48(1):33–44. doi: 10.1016/0022-1759(82)90207-1. [DOI] [PubMed] [Google Scholar]
- Glass R. I., Becker S., Huq M. I., Stoll B. J., Khan M. U., Merson M. H., Lee J. V., Black R. E. Endemic cholera in rural Bangladesh, 1966-1980. Am J Epidemiol. 1982 Dec;116(6):959–970. doi: 10.1093/oxfordjournals.aje.a113498. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Fryklund J., Larsson H. Gamma-interferon-mediated down-regulation of electrolyte secretion by intestinal epithelial cells: a local immune mechanism? Scand J Immunol. 1989 Oct;30(4):499–503. doi: 10.1111/j.1365-3083.1989.tb02456.x. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M., Lönnroth I., Fall-Persson M., Markman B., Lundbeck H. Development of improved cholera vaccine based on subunit toxoid. Nature. 1977 Oct 13;269(5629):602–604. doi: 10.1038/269602a0. [DOI] [PubMed] [Google Scholar]
- Ijzermans J. N., Marquet R. L. Interferon-gamma: a review. Immunobiology. 1989 Oct;179(4-5):456–473. doi: 10.1016/S0171-2985(89)80049-X. [DOI] [PubMed] [Google Scholar]
- Jertborn M., Svennerholm A. M., Holmgren J. Five-year immunologic memory in Swedish volunteers after oral cholera vaccination. J Infect Dis. 1988 Feb;157(2):374–377. doi: 10.1093/infdis/157.2.374. [DOI] [PubMed] [Google Scholar]
- Kantele A., Arvilommi H., Jokinen I. Specific immunoglobulin-secreting human blood cells after peroral vaccination against Salmonella typhi. J Infect Dis. 1986 Jun;153(6):1126–1131. doi: 10.1093/infdis/153.6.1126. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Black R. E., Clements M. L., Cisneros L., Nalin D. R., Young C. R. Duration of infection-derived immunity to cholera. J Infect Dis. 1981 Jun;143(6):818–820. doi: 10.1093/infdis/143.6.818. [DOI] [PubMed] [Google Scholar]
- Lycke N., Holmgren J. Intestinal mucosal memory and presence of memory cells in lamina propria and Peyer's patches in mice 2 years after oral immunization with cholera toxin. Scand J Immunol. 1986 May;23(5):611–616. doi: 10.1111/j.1365-3083.1986.tb01995.x. [DOI] [PubMed] [Google Scholar]
- Madara J. L., Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989 Feb;83(2):724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott M. R., Bienenstock J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979 May;122(5):1892–1898. [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R., Arnold R. R., Michalek S. M., Prince S. J., Babb J. L. Selective induction of an immune response in human external secretions by ingestion of bacterial antigen. J Clin Invest. 1978 Mar;61(3):731–737. doi: 10.1172/JCI108986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987 Jul;7(4):265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- Ogra P. L., Chiba Y., Beutner K. R., Morag A. Vaccination by non-parenteral routes: characteristics of immune response. Dev Biol Stand. 1976;33:19–26. [PubMed] [Google Scholar]
- Peters M. G., Secrist H., Anders K. R., Nash G. S., Rich S. R., MacDermott R. P. Normal human intestinal B lymphocytes. Increased activation compared with peripheral blood. J Clin Invest. 1989 Jun;83(6):1827–1833. doi: 10.1172/JCI114088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr Determinants of the localization, magnitude, and duration of a specific mucosal IgA plasma cell response in enterically immunized rats. J Immunol. 1982 Mar;128(3):1311–1315. [PubMed] [Google Scholar]
- Pierce N. F. The role of antigen form and function in the primary and secondary intestinal immune responses to cholera toxin and toxoid in rats. J Exp Med. 1978 Jul 1;148(1):195–206. doi: 10.1084/jem.148.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sharrow S. O., Stephany D., Springer T. A., Young H. A., Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988 Mar 1;140(5):1401–1407. [PubMed] [Google Scholar]
- Sedgwick J. D., Holt P. G. A solid-phase immunoenzymatic technique for the enumeration of specific antibody-secreting cells. J Immunol Methods. 1983 Feb 25;57(1-3):301–309. doi: 10.1016/0022-1759(83)90091-1. [DOI] [PubMed] [Google Scholar]
- Sollid L. M., Kvale D., Brandtzaeg P., Markussen G., Thorsby E. Interferon-gamma enhances expression of secretory component, the epithelial receptor for polymeric immunoglobulins. J Immunol. 1987 Jun 15;138(12):4303–4306. [PubMed] [Google Scholar]
- Svennerholm A. M., Gothefors L., Sack D. A., Bardhan P. K., Holmgren J. Local and systemic antibody responses and immunological memory in humans after immunization with cholera B subunit by different routes. Bull World Health Organ. 1984;62(6):909–918. [PMC free article] [PubMed] [Google Scholar]
- Svennerholm A. M., Jertborn M., Gothefors L., Karim A. M., Sack D. A., Holmgren J. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit-whole cell vaccine. J Infect Dis. 1984 Jun;149(6):884–893. doi: 10.1093/infdis/149.6.884. [DOI] [PubMed] [Google Scholar]
- Svennerholm A. M., Sack D. A., Holmgren J., Bardhan P. K. Intestinal antibody responses after immunisation with cholera B subunit. Lancet. 1982 Feb 6;1(8267):305–308. doi: 10.1016/s0140-6736(82)91568-9. [DOI] [PubMed] [Google Scholar]
- Weisz-Carrington P., Roux M. E., McWilliams M., PHILLIPS-Quagliata J. M., Lamm M. E. Organ and isotype distribution of plasma cells producing specific antibody after oral immunization: evidence for a generalized secretory immune system. J Immunol. 1979 Oct;123(4):1705–1708. [PubMed] [Google Scholar]



