Abstract
The title compound, {(C4H12N2)[Bi2(C7H3NO4)4(H2O)]·H2O}n or {(pipzH2)[Bi2(pydc)4(H2O)]·H2O}n, where pydcH2 is pyridine-2,6-dicarboxylic acid and pipz is piperazine, was obtained by reaction of Bi(NO3)3·5H2O with (pipzH2)(pydcH)2·3H2O in a 1:2 molar ratio in aqueous solution. There are two independent BiIII atoms in the structure, one of which is eight-coordinate with a distorted bicapped trigonal-prismatic geometry, and another which is nine-coordinate with a distorted tricapped trigonal-prismatic geometry. The carboxylate groups of the (pydc)2− ligands link dinuclear [Bi2(C7H3NO4)4(H2O)]2− units into one-dimensional coordination polymers. The pipzH2
2+ cations (site symmetry  ) and non-coordinated water molecules lie between these polymers, forming N—H⋯O and O—H⋯O hydrogen bonds to the O atoms of the carboxylate groups.
) and non-coordinated water molecules lie between these polymers, forming N—H⋯O and O—H⋯O hydrogen bonds to the O atoms of the carboxylate groups.
Related literature
For related literature, see: Aghabozorg, Attar Gharamaleki, Ghadermazi et al. (2007 ▶); Aghabozorg, Attar Gharamaleki, Ghasemikhah et al. (2007 ▶); Aghabozorg, Motyeian et al. (2007 ▶); Aghabozorg, Daneshvar et al. (2007 ▶); Sharif et al. (2007 ▶); Sheshmani et al. (2006 ▶).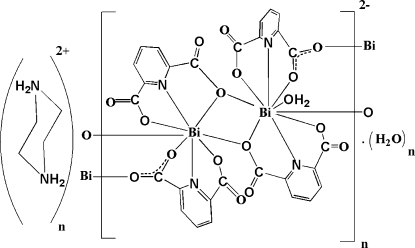
Experimental
Crystal data
(C4H12N2)[Bi2(C7H3NO4)4(H2O)]·H2O
M r = 1202.56
Triclinic,
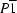
a = 10.8111 (4) Å
b = 12.1660 (5) Å
c = 14.0402 (5) Å
α = 96.094 (1)°
β = 93.169 (1)°
γ = 113.848 (1)°
V = 1669.65 (11) Å3
Z = 2
Mo Kα radiation
μ = 10.62 mm−1
T = 100 (2) K
0.15 × 0.15 × 0.10 mm
Data collection
Bruker SMART APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2005 ▶) T min = 0.204, T max = 0.346
22723 measured reflections
8005 independent reflections
6952 reflections with I > 2σ(I)
R int = 0.031
Refinement
R[F 2 > 2σ(F 2)] = 0.022
wR(F 2) = 0.048
S = 1.03
8005 reflections
523 parameters
H-atom parameters constrained
Δρmax = 1.32 e Å−3
Δρmin = −0.82 e Å−3
Data collection: APEX2 (Bruker, 2005 ▶); cell refinement: APEX2; data reduction: APEX2; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S160053680800161X/bi2264sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053680800161X/bi2264Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N5—H5N1⋯O2Wi | 0.90 | 2.00 | 2.761 (4) | 141 |
| N5—H5N2⋯O6 | 0.90 | 1.86 | 2.722 (4) | 160 |
| N6—H6N1⋯O16ii | 0.90 | 1.78 | 2.676 (4) | 176 |
| N6—H6N2⋯O12 | 0.90 | 1.95 | 2.762 (4) | 149 |
| O1W—H1W1⋯O7 | 0.85 | 2.08 | 2.891 (3) | 160 |
| O1W—H1W2⋯O9iii | 0.85 | 2.53 | 3.337 (4) | 158 |
| O2W—H2W1⋯O14 | 0.85 | 2.04 | 2.887 (4) | 176 |
| O2W—H2W2⋯O5 | 0.85 | 2.35 | 3.162 (4) | 159 |
Symmetry codes: (i) 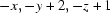 ; (ii)
; (ii) 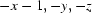 ; (iii)
; (iii) 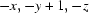 .
.
supplementary crystallographic information
Comment
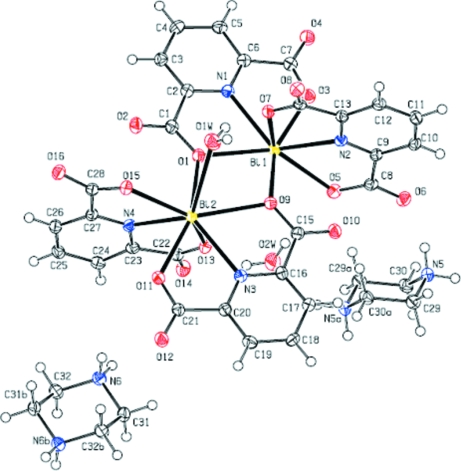
The preparation and characterization of self-assembling systems have been considered by chemists in recent years. A literature review shows that the (pydc)2- ligand can form complexes with transition metals (Aghabozorg, Attar Gharamaleki, Ghadermazi et al., 2007; Aghabozorg, Attar Gharamaleki, Ghasemikhah et al., 2007; Aghabozorg, Motyeian et al., 2007; Aghabozorg, Daneshvar et al., 2007; Sharif et al., 2007). In this work, (pydc)2- acts as tridentate ligand with one N atom of pyridine and two O atoms of carboxylates acting as donors and also has a bridging role between the binuclear units containing Bi1 and Bi2. The binuclear units consist of two BiIII atoms, four (pydc)2- ligands, and one coordinated water molecule, while one (pipzH2)2+ cation and one uncoordinated water molecule are also present in the asymmetric unit (Fig. 1).
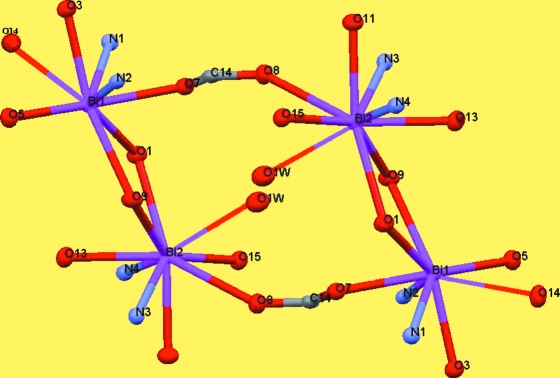
Two Bi1—O7—C14—O8—Bi2 bridging bonds between neighboring binuclear units link them together and form several rings with four BiIII atoms, six O atoms and two C atoms (Fig. 2). Atom Bi1 is eight coordinated by two tridentate (pydc)2- ligands, one O atom from the Bi1—O9—Bi2 bridge and one O atom from the neighbouring carboxylate group (Bi1—O14i = 2.971 (3) Å, symmetry code: (i) -x, 1 - y, 1 - z). Atom Bi2 is nine coordinated by two tridentate (pydc)2- ligands, one O atom from the Bi1—O1—Bi2 bridge, one O atom from a coordinated water molecule (Bi2—O1W = 2.960 (3) Å) and one O atom from a neighbouring carboxylate group (Bi2—O8ii = 2.883 (2) Å, symmetry code: (ii) -x, 1 - y, -z). The sum of the van der Waals radii for Bi and O is 3.86 Å, which is significantly longer than the bond distances for Bi1—O14i and Bi2—O8ii. The coordination polyhedron around Bi1 is a distorted bicapped trigonal prism which is nearly eclipsed, in which O1, O7, O9 and O3, O5, O14 form two triangles and N1 and N2 form two caps of the prism. The sum of the bond angles N3—Bi2—O1W (113.37 (8)°), O1W—Bi2—N4 (125.24 (8)°) and N4—Bi2—N3 (121.00 (9)°) is equal to 359.61°, indicating that Bi2 is located in the center of the O1W/N3/N4 plane. Atoms O8, O11, O15 and O1, O9, O13 build two triangles. So, a prism consisting of six O atoms and three caps (N3, N4 and O1W) on its faces is formed around Bi2, that is the coordination polyhedron may be described as a distorted tricapped trigonal prism.
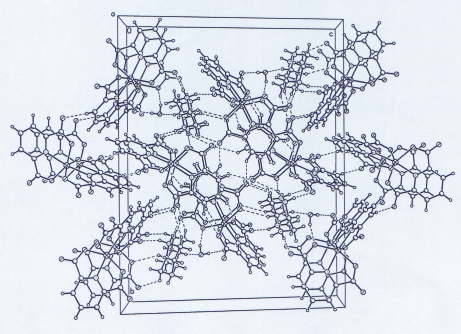
N—H···O hydrogen bonds are formed between (pipzH2)2+ and the carboxylate groups (Table1) and also O—H···O hydrogen bonds are formed between uncoordinated water molecules and the carboxylate groups. In addition, C—H···O contacts with C···O distances ranging from 2.887 (4)Å to 3.656 (4) Å are observed between (pipzH2)2+ cations and carboxylate groups. There are C29—H29B···Cg1 interactions (3.638 (5) Å; x, y + 1, z), where Cg1 is the centroid of the N4/C23—C27 ring and also C32—H32B···Cg2 interactions (3.656 (5) Å; x - 1,y, z), where Cg2 is the centroid of the N2/C9—C13 ring (Fig. 3).
Experimental
The proton transfer compound (pipzH2)(pydcH)2.3H2O was prepared by reaction of pyridine-2,6-dicarboxylic acid (pydcH2) with piperazine (pipz) (Sheshmani et al., 2006). A solution of Bi(NO3)3.5H2O (242 mg, 0.5 mmol) in water (25 ml) was added to (pipzH2)(pydcH)2.3H2O (253 mg, 1.0 mmol) in 25 ml water and colorless crystals were obtained by slow evaporation of the solvent at room temperature.
Refinement
H atoms attached to O and N atoms were found in difference Fourier maps, then their distances were normalized to O—H = 0.85, N—H = 0.90 Å along the observed O/N—H vector. H atoms bound to C atoms were placed in calculated positions. All H atoms were then refined as riding with Uiso(H) = 1.2Ueq(C/N) or 1.5Ueq(O).
Figures
Fig. 1.
The asymmetric unit of the title compound with displacement ellipsoids drawn at 50% probability for non-H atoms.
Fig. 2.
Rings of four BiIII atoms within the 1-D coordination polymers.
Fig. 3.
Crystal packing with hydrogen bonds shown as dashed lines.
Crystal data
| (C4H12N2)[Bi2(C7H3NO4)4(H2O)]·H2O | Z = 2 |
| Mr = 1202.56 | F000 = 1144 |
| Triclinic, P1 | Dx = 2.392 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation λ = 0.71073 Å |
| a = 10.8111 (4) Å | Cell parameters from 5207 reflections |
| b = 12.1660 (5) Å | θ = 2.6–29.4º |
| c = 14.0402 (5) Å | µ = 10.62 mm−1 |
| α = 96.094 (1)º | T = 100 (2) K |
| β = 93.169 (1)º | Prism, colourless |
| γ = 113.848 (1)º | 0.15 × 0.15 × 0.10 mm |
| V = 1669.65 (11) Å3 |
Data collection
| Bruker SMART APEXII CCD diffractometer | 8005 independent reflections |
| Radiation source: fine-focus sealed tube | 6952 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.031 |
| T = 100(2) K | θmax = 28.0º |
| φ and ω scans | θmin = 1.5º |
| Absorption correction: multi-scan(SADABS; Bruker, 2005) | h = −14→14 |
| Tmin = 0.204, Tmax = 0.346 | k = −16→16 |
| 22723 measured reflections | l = −18→18 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.022 | H-atom parameters constrained |
| wR(F2) = 0.048 | w = 1/[σ2(Fo2) + (0.019P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 1.03 | (Δ/σ)max = 0.001 |
| 8005 reflections | Δρmax = 1.32 e Å−3 |
| 523 parameters | Δρmin = −0.82 e Å−3 |
| Primary atom site location: structure-invariant direct methods | Extinction correction: none |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Bi1 | 0.187854 (12) | 0.622892 (10) | 0.307230 (9) | 0.01470 (4) | |
| Bi2 | −0.184337 (12) | 0.356548 (10) | 0.178059 (9) | 0.01477 (4) | |
| O1 | 0.0427 (2) | 0.3934 (2) | 0.26028 (18) | 0.0193 (5) | |
| O2 | 0.0375 (3) | 0.2059 (2) | 0.23943 (18) | 0.0225 (5) | |
| O3 | 0.4159 (2) | 0.7303 (2) | 0.35552 (17) | 0.0195 (5) | |
| O4 | 0.6152 (3) | 0.7375 (2) | 0.41582 (19) | 0.0246 (6) | |
| O5 | 0.1821 (3) | 0.8179 (2) | 0.39201 (17) | 0.0202 (5) | |
| O6 | 0.2563 (3) | 1.0190 (2) | 0.39225 (18) | 0.0228 (5) | |
| O7 | 0.2296 (2) | 0.5669 (2) | 0.15750 (17) | 0.0175 (5) | |
| O8 | 0.2740 (3) | 0.6124 (2) | 0.01038 (17) | 0.0207 (5) | |
| O9 | −0.0239 (2) | 0.5885 (2) | 0.20041 (18) | 0.0196 (5) | |
| O10 | −0.0098 (2) | 0.7798 (2) | 0.21948 (19) | 0.0225 (5) | |
| O11 | −0.4218 (2) | 0.2606 (2) | 0.15982 (18) | 0.0203 (5) | |
| O12 | −0.6215 (2) | 0.2613 (2) | 0.11226 (19) | 0.0234 (5) | |
| O13 | −0.1855 (3) | 0.4370 (2) | 0.33567 (17) | 0.0193 (5) | |
| O14 | −0.2198 (3) | 0.4114 (2) | 0.48793 (18) | 0.0228 (5) | |
| O15 | −0.2214 (2) | 0.1481 (2) | 0.10123 (17) | 0.0183 (5) | |
| O16 | −0.2602 (3) | −0.0419 (2) | 0.12412 (18) | 0.0226 (5) | |
| N1 | 0.3057 (3) | 0.4903 (3) | 0.3281 (2) | 0.0165 (6) | |
| N2 | 0.2716 (3) | 0.7927 (2) | 0.2211 (2) | 0.0152 (6) | |
| N3 | −0.2965 (3) | 0.4993 (2) | 0.1700 (2) | 0.0161 (6) | |
| N4 | −0.2525 (3) | 0.2036 (2) | 0.2842 (2) | 0.0164 (6) | |
| C1 | 0.0944 (4) | 0.3157 (3) | 0.2662 (2) | 0.0180 (7) | |
| C2 | 0.2423 (4) | 0.3687 (3) | 0.3098 (2) | 0.0171 (7) | |
| C3 | 0.3090 (4) | 0.2964 (3) | 0.3301 (2) | 0.0191 (7) | |
| H3A | 0.2627 | 0.2105 | 0.3175 | 0.023* | |
| C4 | 0.4432 (4) | 0.3505 (3) | 0.3688 (3) | 0.0221 (7) | |
| H4A | 0.4899 | 0.3021 | 0.3841 | 0.026* | |
| C5 | 0.5101 (4) | 0.4771 (3) | 0.3854 (3) | 0.0204 (7) | |
| H5A | 0.6033 | 0.5165 | 0.4104 | 0.025* | |
| C6 | 0.4356 (3) | 0.5435 (3) | 0.3640 (2) | 0.0173 (7) | |
| C7 | 0.4970 (3) | 0.6814 (3) | 0.3806 (2) | 0.0174 (7) | |
| C8 | 0.2402 (3) | 0.9155 (3) | 0.3569 (3) | 0.0183 (7) | |
| C9 | 0.2945 (3) | 0.9050 (3) | 0.2603 (2) | 0.0160 (7) | |
| C10 | 0.3618 (3) | 1.0040 (3) | 0.2131 (3) | 0.0186 (7) | |
| H10A | 0.3786 | 1.0838 | 0.2414 | 0.022* | |
| C11 | 0.4038 (4) | 0.9842 (3) | 0.1244 (3) | 0.0213 (7) | |
| H11A | 0.4521 | 1.0509 | 0.0918 | 0.026* | |
| C12 | 0.3756 (3) | 0.8668 (3) | 0.0827 (3) | 0.0184 (7) | |
| H12A | 0.4016 | 0.8513 | 0.0209 | 0.022* | |
| C13 | 0.3087 (3) | 0.7735 (3) | 0.1339 (2) | 0.0169 (7) | |
| C14 | 0.2686 (3) | 0.6421 (3) | 0.0956 (2) | 0.0164 (7) | |
| C15 | −0.0753 (4) | 0.6690 (3) | 0.2024 (2) | 0.0181 (7) | |
| C16 | −0.2269 (3) | 0.6196 (3) | 0.1788 (2) | 0.0167 (7) | |
| C17 | −0.2899 (4) | 0.6960 (3) | 0.1640 (3) | 0.0200 (7) | |
| H17A | −0.2392 | 0.7816 | 0.1732 | 0.024* | |
| C18 | −0.4288 (4) | 0.6453 (3) | 0.1355 (3) | 0.0213 (7) | |
| H18A | −0.4743 | 0.6955 | 0.1229 | 0.026* | |
| C19 | −0.5008 (4) | 0.5192 (3) | 0.1255 (3) | 0.0216 (7) | |
| H19A | −0.5959 | 0.4819 | 0.1062 | 0.026* | |
| C20 | −0.4299 (3) | 0.4504 (3) | 0.1445 (2) | 0.0173 (7) | |
| C21 | −0.4988 (3) | 0.3139 (3) | 0.1373 (2) | 0.0169 (7) | |
| C22 | −0.2236 (3) | 0.3715 (3) | 0.4019 (3) | 0.0181 (7) | |
| C23 | −0.2778 (3) | 0.2360 (3) | 0.3722 (2) | 0.0169 (7) | |
| C24 | −0.3469 (3) | 0.1508 (3) | 0.4298 (2) | 0.0182 (7) | |
| H24A | −0.3693 | 0.1749 | 0.4907 | 0.022* | |
| C25 | −0.3832 (3) | 0.0283 (3) | 0.3965 (3) | 0.0191 (7) | |
| H25A | −0.4301 | −0.0325 | 0.4348 | 0.023* | |
| C26 | −0.3498 (3) | −0.0034 (3) | 0.3066 (3) | 0.0183 (7) | |
| H26A | −0.3702 | −0.0858 | 0.2834 | 0.022* | |
| C27 | −0.2863 (3) | 0.0871 (3) | 0.2518 (2) | 0.0167 (7) | |
| C28 | −0.2530 (3) | 0.0622 (3) | 0.1510 (3) | 0.0184 (7) | |
| N5 | 0.1037 (3) | 1.1154 (3) | 0.4887 (2) | 0.0199 (6) | |
| H5N1 | 0.1350 | 1.1969 | 0.4971 | 0.024* | |
| H5N2 | 0.1704 | 1.0994 | 0.4636 | 0.024* | |
| C29 | −0.0256 (4) | 1.0593 (3) | 0.4221 (3) | 0.0216 (7) | |
| H29A | −0.0078 | 1.0848 | 0.3577 | 0.026* | |
| H29B | −0.0931 | 1.0876 | 0.4471 | 0.026* | |
| C30 | 0.0819 (4) | 1.0776 (3) | 0.5865 (3) | 0.0210 (7) | |
| H30A | 0.0173 | 1.1065 | 0.6159 | 0.025* | |
| H30B | 0.1692 | 1.1143 | 0.6286 | 0.025* | |
| N6 | −0.8584 (3) | 0.0656 (3) | 0.0345 (2) | 0.0192 (6) | |
| H6N1 | −0.8147 | 0.0590 | −0.0171 | 0.023* | |
| H6N2 | −0.7844 | 0.1111 | 0.0761 | 0.023* | |
| C31 | −0.9440 (4) | 0.1308 (3) | 0.0061 (3) | 0.0216 (7) | |
| H31A | −0.8868 | 0.2061 | −0.0189 | 0.026* | |
| H31B | −0.9830 | 0.1534 | 0.0632 | 0.026* | |
| C32 | −0.9431 (4) | −0.0495 (3) | 0.0703 (3) | 0.0231 (8) | |
| H32A | −0.9823 | −0.0312 | 0.1287 | 0.028* | |
| H32B | −0.8854 | −0.0917 | 0.0878 | 0.028* | |
| O1W | 0.0082 (3) | 0.3774 (2) | 0.0319 (2) | 0.0287 (6) | |
| H1W1 | 0.0826 | 0.4211 | 0.0666 | 0.043* | |
| H1W2 | 0.0135 | 0.4062 | −0.0211 | 0.043* | |
| O2W | −0.0696 (3) | 0.6708 (2) | 0.5000 (2) | 0.0348 (7) | |
| H2W1 | −0.1177 | 0.5951 | 0.4969 | 0.052* | |
| H2W2 | −0.0142 | 0.6958 | 0.4583 | 0.052* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Bi1 | 0.01464 (7) | 0.01425 (6) | 0.01526 (7) | 0.00582 (5) | 0.00170 (5) | 0.00288 (5) |
| Bi2 | 0.01454 (7) | 0.01311 (6) | 0.01629 (7) | 0.00520 (5) | 0.00116 (5) | 0.00279 (5) |
| O1 | 0.0178 (12) | 0.0164 (11) | 0.0229 (13) | 0.0061 (10) | 0.0002 (10) | 0.0041 (10) |
| O2 | 0.0236 (14) | 0.0161 (11) | 0.0245 (13) | 0.0058 (10) | −0.0020 (11) | 0.0011 (10) |
| O3 | 0.0184 (12) | 0.0182 (11) | 0.0207 (13) | 0.0064 (10) | 0.0001 (10) | 0.0037 (10) |
| O4 | 0.0189 (13) | 0.0227 (13) | 0.0304 (15) | 0.0072 (11) | −0.0034 (11) | 0.0043 (11) |
| O5 | 0.0240 (13) | 0.0219 (12) | 0.0168 (12) | 0.0112 (11) | 0.0038 (10) | 0.0043 (10) |
| O6 | 0.0265 (14) | 0.0198 (12) | 0.0238 (13) | 0.0114 (11) | 0.0052 (11) | 0.0010 (10) |
| O7 | 0.0166 (12) | 0.0164 (11) | 0.0193 (12) | 0.0065 (10) | 0.0015 (10) | 0.0035 (9) |
| O8 | 0.0232 (13) | 0.0215 (12) | 0.0180 (12) | 0.0102 (11) | 0.0017 (10) | 0.0016 (10) |
| O9 | 0.0154 (12) | 0.0173 (11) | 0.0265 (13) | 0.0069 (10) | 0.0014 (10) | 0.0049 (10) |
| O10 | 0.0180 (13) | 0.0172 (12) | 0.0287 (14) | 0.0043 (10) | −0.0010 (11) | 0.0033 (10) |
| O11 | 0.0161 (12) | 0.0177 (11) | 0.0258 (13) | 0.0053 (10) | 0.0019 (10) | 0.0048 (10) |
| O12 | 0.0181 (13) | 0.0197 (12) | 0.0299 (14) | 0.0052 (10) | −0.0018 (11) | 0.0051 (10) |
| O13 | 0.0245 (13) | 0.0161 (11) | 0.0178 (12) | 0.0091 (10) | −0.0005 (10) | 0.0025 (9) |
| O14 | 0.0295 (14) | 0.0201 (12) | 0.0177 (13) | 0.0100 (11) | 0.0021 (11) | 0.0000 (10) |
| O15 | 0.0207 (13) | 0.0157 (11) | 0.0182 (12) | 0.0074 (10) | 0.0007 (10) | 0.0027 (9) |
| O16 | 0.0294 (14) | 0.0185 (12) | 0.0233 (13) | 0.0122 (11) | 0.0086 (11) | 0.0048 (10) |
| N1 | 0.0167 (14) | 0.0187 (13) | 0.0154 (14) | 0.0078 (12) | 0.0025 (11) | 0.0057 (11) |
| N2 | 0.0144 (14) | 0.0173 (13) | 0.0147 (14) | 0.0077 (11) | −0.0016 (11) | 0.0020 (11) |
| N3 | 0.0174 (14) | 0.0177 (13) | 0.0143 (14) | 0.0079 (11) | 0.0020 (11) | 0.0032 (11) |
| N4 | 0.0157 (14) | 0.0158 (13) | 0.0181 (14) | 0.0074 (11) | 0.0000 (11) | 0.0014 (11) |
| C1 | 0.0195 (18) | 0.0192 (16) | 0.0161 (17) | 0.0080 (14) | 0.0023 (14) | 0.0050 (13) |
| C2 | 0.0215 (18) | 0.0185 (16) | 0.0123 (15) | 0.0089 (14) | 0.0039 (13) | 0.0030 (12) |
| C3 | 0.0212 (18) | 0.0184 (16) | 0.0193 (17) | 0.0092 (14) | 0.0047 (14) | 0.0034 (13) |
| C4 | 0.0236 (19) | 0.0238 (17) | 0.0235 (19) | 0.0145 (15) | 0.0005 (15) | 0.0049 (14) |
| C5 | 0.0173 (17) | 0.0251 (17) | 0.0204 (18) | 0.0100 (14) | 0.0018 (14) | 0.0042 (14) |
| C6 | 0.0185 (17) | 0.0208 (16) | 0.0136 (16) | 0.0090 (14) | 0.0022 (13) | 0.0040 (13) |
| C7 | 0.0188 (17) | 0.0200 (16) | 0.0135 (16) | 0.0078 (14) | 0.0013 (13) | 0.0034 (13) |
| C8 | 0.0159 (17) | 0.0205 (16) | 0.0200 (17) | 0.0092 (14) | −0.0008 (14) | 0.0031 (14) |
| C9 | 0.0149 (16) | 0.0167 (15) | 0.0175 (17) | 0.0076 (13) | −0.0004 (13) | 0.0029 (13) |
| C10 | 0.0162 (17) | 0.0150 (15) | 0.0240 (18) | 0.0069 (13) | −0.0034 (14) | 0.0014 (13) |
| C11 | 0.0173 (17) | 0.0181 (16) | 0.0267 (19) | 0.0050 (14) | 0.0015 (15) | 0.0054 (14) |
| C12 | 0.0182 (17) | 0.0208 (16) | 0.0182 (17) | 0.0095 (14) | 0.0028 (14) | 0.0050 (13) |
| C13 | 0.0143 (16) | 0.0173 (15) | 0.0187 (17) | 0.0061 (13) | 0.0007 (13) | 0.0029 (13) |
| C14 | 0.0107 (15) | 0.0185 (15) | 0.0203 (17) | 0.0065 (13) | 0.0005 (13) | 0.0020 (13) |
| C15 | 0.0188 (18) | 0.0201 (16) | 0.0178 (17) | 0.0095 (14) | 0.0026 (14) | 0.0058 (13) |
| C16 | 0.0182 (17) | 0.0163 (15) | 0.0140 (16) | 0.0054 (13) | 0.0025 (13) | 0.0028 (12) |
| C17 | 0.0189 (17) | 0.0171 (16) | 0.0244 (18) | 0.0077 (14) | 0.0031 (14) | 0.0031 (14) |
| C18 | 0.0211 (18) | 0.0194 (16) | 0.0275 (19) | 0.0126 (14) | 0.0022 (15) | 0.0027 (14) |
| C19 | 0.0163 (17) | 0.0231 (17) | 0.0237 (18) | 0.0067 (14) | 0.0018 (14) | 0.0027 (14) |
| C20 | 0.0186 (17) | 0.0165 (15) | 0.0162 (16) | 0.0071 (14) | 0.0023 (13) | 0.0006 (13) |
| C21 | 0.0179 (17) | 0.0171 (15) | 0.0154 (16) | 0.0068 (13) | 0.0027 (13) | 0.0028 (13) |
| C22 | 0.0164 (17) | 0.0182 (16) | 0.0201 (17) | 0.0076 (13) | 0.0009 (13) | 0.0023 (13) |
| C23 | 0.0146 (16) | 0.0191 (16) | 0.0171 (17) | 0.0076 (13) | −0.0007 (13) | 0.0015 (13) |
| C24 | 0.0152 (16) | 0.0231 (17) | 0.0150 (16) | 0.0071 (14) | 0.0000 (13) | 0.0009 (13) |
| C25 | 0.0171 (17) | 0.0194 (16) | 0.0203 (18) | 0.0059 (14) | 0.0021 (14) | 0.0074 (13) |
| C26 | 0.0175 (17) | 0.0161 (15) | 0.0203 (17) | 0.0064 (13) | −0.0003 (14) | 0.0029 (13) |
| C27 | 0.0153 (16) | 0.0145 (15) | 0.0199 (17) | 0.0063 (13) | −0.0021 (13) | 0.0015 (13) |
| C28 | 0.0140 (16) | 0.0170 (15) | 0.0237 (18) | 0.0065 (13) | −0.0017 (14) | 0.0024 (13) |
| N5 | 0.0190 (15) | 0.0161 (13) | 0.0242 (16) | 0.0061 (12) | 0.0037 (12) | 0.0046 (12) |
| C29 | 0.0200 (18) | 0.0232 (17) | 0.0218 (18) | 0.0087 (15) | 0.0016 (14) | 0.0054 (14) |
| C30 | 0.0167 (17) | 0.0198 (16) | 0.0216 (18) | 0.0039 (14) | −0.0002 (14) | −0.0009 (14) |
| N6 | 0.0178 (15) | 0.0197 (14) | 0.0181 (15) | 0.0060 (12) | 0.0002 (12) | 0.0025 (11) |
| C31 | 0.0225 (18) | 0.0157 (16) | 0.0247 (19) | 0.0063 (14) | 0.0034 (15) | 0.0015 (14) |
| C32 | 0.0187 (18) | 0.0221 (17) | 0.0271 (19) | 0.0059 (14) | 0.0016 (15) | 0.0079 (15) |
| O1W | 0.0237 (14) | 0.0264 (14) | 0.0319 (15) | 0.0067 (11) | 0.0002 (12) | 0.0035 (12) |
| O2W | 0.0361 (17) | 0.0240 (14) | 0.0426 (18) | 0.0106 (13) | 0.0117 (14) | 0.0016 (12) |
Geometric parameters (Å, °)
| Bi1—O7 | 2.266 (2) | C8—C9 | 1.521 (5) |
| Bi1—O3 | 2.295 (2) | C9—C10 | 1.390 (5) |
| Bi1—N2 | 2.385 (3) | C10—C11 | 1.379 (5) |
| Bi1—N1 | 2.456 (3) | C10—H10A | 0.950 |
| Bi1—O9 | 2.526 (2) | C11—C12 | 1.388 (5) |
| Bi1—O5 | 2.565 (2) | C11—H11A | 0.950 |
| Bi1—O1 | 2.578 (2) | C12—C13 | 1.376 (5) |
| Bi1—O14i | 2.971 (3) | C12—H12A | 0.950 |
| Bi2—O13 | 2.326 (2) | C13—C14 | 1.505 (4) |
| Bi2—O11 | 2.337 (2) | C15—C16 | 1.503 (5) |
| Bi2—N4 | 2.414 (3) | C16—C17 | 1.382 (5) |
| Bi2—N3 | 2.496 (3) | C17—C18 | 1.388 (5) |
| Bi2—O1 | 2.499 (2) | C17—H17A | 0.950 |
| Bi2—O15 | 2.512 (2) | C18—C19 | 1.398 (5) |
| Bi2—O9 | 2.620 (2) | C18—H18A | 0.950 |
| Bi2—O8ii | 2.883 (2) | C19—C20 | 1.379 (5) |
| Bi2—O1W | 2.960 (3) | C19—H19A | 0.950 |
| O1—C1 | 1.285 (4) | C20—C21 | 1.510 (5) |
| O2—C1 | 1.227 (4) | C22—C23 | 1.511 (5) |
| O3—C7 | 1.295 (4) | C23—C24 | 1.380 (5) |
| O4—C7 | 1.223 (4) | C24—C25 | 1.397 (5) |
| O5—C8 | 1.266 (4) | C24—H24A | 0.950 |
| O6—C8 | 1.242 (4) | C25—C26 | 1.391 (5) |
| O7—C14 | 1.292 (4) | C25—H25A | 0.950 |
| O8—C14 | 1.224 (4) | C26—C27 | 1.380 (5) |
| O8—Bi2ii | 2.883 (2) | C26—H26A | 0.950 |
| O9—C15 | 1.306 (4) | C27—C28 | 1.509 (5) |
| O10—C15 | 1.231 (4) | N5—C30 | 1.496 (5) |
| O11—C21 | 1.290 (4) | N5—C29 | 1.497 (4) |
| O12—C21 | 1.228 (4) | N5—H5N1 | 0.900 |
| O13—C22 | 1.267 (4) | N5—H5N2 | 0.900 |
| O14—C22 | 1.244 (4) | C29—C30iii | 1.513 (5) |
| O14—Bi1i | 2.971 (3) | C29—H29A | 0.990 |
| O15—C28 | 1.260 (4) | C29—H29B | 0.990 |
| O16—C28 | 1.252 (4) | C30—C29iii | 1.513 (5) |
| N1—C6 | 1.328 (4) | C30—H30A | 0.990 |
| N1—C2 | 1.343 (4) | C30—H30B | 0.990 |
| N2—C13 | 1.336 (4) | N6—C32 | 1.488 (4) |
| N2—C9 | 1.336 (4) | N6—C31 | 1.505 (4) |
| N3—C20 | 1.328 (4) | N6—H6N1 | 0.900 |
| N3—C16 | 1.335 (4) | N6—H6N2 | 0.900 |
| N4—C27 | 1.333 (4) | C31—C32iv | 1.507 (5) |
| N4—C23 | 1.333 (4) | C31—H31A | 0.990 |
| C1—C2 | 1.523 (5) | C31—H31B | 0.990 |
| C2—C3 | 1.384 (5) | C32—C31iv | 1.507 (5) |
| C3—C4 | 1.378 (5) | C32—H32A | 0.990 |
| C3—H3A | 0.950 | C32—H32B | 0.990 |
| C4—C5 | 1.397 (5) | O1W—H1W1 | 0.850 |
| C4—H4A | 0.950 | O1W—H1W2 | 0.850 |
| C5—C6 | 1.394 (5) | O2W—H2W1 | 0.850 |
| C5—H5A | 0.950 | O2W—H2W2 | 0.850 |
| C6—C7 | 1.520 (5) | ||
| O7—Bi1—O3 | 91.67 (8) | C5—C6—C7 | 122.4 (3) |
| O7—Bi1—N2 | 69.13 (9) | O4—C7—O3 | 125.1 (3) |
| O3—Bi1—N2 | 71.64 (9) | O4—C7—C6 | 119.6 (3) |
| O7—Bi1—N1 | 74.33 (9) | O3—C7—C6 | 115.3 (3) |
| O3—Bi1—N1 | 67.54 (9) | O6—C8—O5 | 127.0 (3) |
| N2—Bi1—N1 | 123.21 (9) | O6—C8—C9 | 116.3 (3) |
| O7—Bi1—O9 | 75.63 (8) | O5—C8—C9 | 116.7 (3) |
| O3—Bi1—O9 | 150.67 (8) | N2—C9—C10 | 120.8 (3) |
| N2—Bi1—O9 | 79.11 (8) | N2—C9—C8 | 115.7 (3) |
| N1—Bi1—O9 | 130.88 (8) | C10—C9—C8 | 123.5 (3) |
| O7—Bi1—O5 | 133.72 (8) | C11—C10—C9 | 118.9 (3) |
| O3—Bi1—O5 | 80.95 (8) | C11—C10—H10A | 120.6 |
| N2—Bi1—O5 | 65.17 (8) | C9—C10—H10A | 120.6 |
| N1—Bi1—O5 | 139.27 (8) | C10—C11—C12 | 120.0 (3) |
| O9—Bi1—O5 | 88.88 (8) | C10—C11—H11A | 120.0 |
| O7—Bi1—O1 | 71.85 (8) | C12—C11—H11A | 120.0 |
| O3—Bi1—O1 | 131.37 (8) | C13—C12—C11 | 117.7 (3) |
| N2—Bi1—O1 | 134.97 (8) | C13—C12—H12A | 121.1 |
| N1—Bi1—O1 | 64.02 (8) | C11—C12—H12A | 121.1 |
| O9—Bi1—O1 | 70.14 (8) | N2—C13—C12 | 122.4 (3) |
| O5—Bi1—O1 | 142.67 (8) | N2—C13—C14 | 114.6 (3) |
| O7—Bi1—O14i | 142.66 (8) | C12—C13—C14 | 122.9 (3) |
| O3—Bi1—O14i | 75.43 (8) | O8—C14—O7 | 124.1 (3) |
| N2—Bi1—O14i | 134.51 (8) | O8—C14—C13 | 120.0 (3) |
| N1—Bi1—O14i | 68.34 (8) | O7—C14—C13 | 115.8 (3) |
| O9—Bi1—O14i | 129.79 (8) | O10—C15—O9 | 125.5 (3) |
| O5—Bi1—O14i | 79.51 (7) | O10—C15—C16 | 118.7 (3) |
| O1—Bi1—O14i | 90.47 (7) | O9—C15—C16 | 115.8 (3) |
| O13—Bi2—O11 | 90.55 (9) | N3—C16—C17 | 121.9 (3) |
| O13—Bi2—N4 | 67.58 (9) | N3—C16—C15 | 117.2 (3) |
| O11—Bi2—N4 | 72.05 (9) | C17—C16—C15 | 120.8 (3) |
| O13—Bi2—N3 | 72.91 (9) | C16—C17—C18 | 118.6 (3) |
| O11—Bi2—N3 | 66.29 (9) | C16—C17—H17A | 120.7 |
| N4—Bi2—N3 | 121.00 (9) | C18—C17—H17A | 120.7 |
| O13—Bi2—O1 | 75.37 (8) | C17—C18—C19 | 119.1 (3) |
| O11—Bi2—O1 | 151.73 (8) | C17—C18—H18A | 120.5 |
| N4—Bi2—O1 | 79.89 (9) | C19—C18—H18A | 120.5 |
| N3—Bi2—O1 | 129.27 (8) | C20—C19—C18 | 118.1 (3) |
| O13—Bi2—O15 | 132.74 (8) | C20—C19—H19A | 120.9 |
| O11—Bi2—O15 | 79.38 (8) | C18—C19—H19A | 120.9 |
| N4—Bi2—O15 | 65.37 (8) | N3—C20—C19 | 122.5 (3) |
| N3—Bi2—O15 | 138.11 (8) | N3—C20—C21 | 115.3 (3) |
| O1—Bi2—O15 | 92.19 (7) | C19—C20—C21 | 122.1 (3) |
| O13—Bi2—O9 | 72.26 (8) | O12—C21—O11 | 124.5 (3) |
| O11—Bi2—O9 | 129.51 (8) | O12—C21—C20 | 119.4 (3) |
| N4—Bi2—O9 | 134.46 (8) | O11—C21—C20 | 116.1 (3) |
| N3—Bi2—O9 | 63.32 (8) | O14—C22—O13 | 124.5 (3) |
| O1—Bi2—O9 | 69.86 (8) | O14—C22—C23 | 119.0 (3) |
| O15—Bi2—O9 | 145.72 (8) | O13—C22—C23 | 116.4 (3) |
| O13—Bi2—O8ii | 135.52 (7) | N4—C23—C24 | 121.6 (3) |
| O11—Bi2—O8ii | 73.14 (8) | N4—C23—C22 | 114.2 (3) |
| N4—Bi2—O8ii | 137.85 (8) | C24—C23—C22 | 124.1 (3) |
| N3—Bi2—O8ii | 62.62 (8) | C23—C24—C25 | 118.5 (3) |
| O1—Bi2—O8ii | 133.56 (8) | C23—C24—H24A | 120.8 |
| O15—Bi2—O8ii | 85.53 (7) | C25—C24—H24A | 120.8 |
| O9—Bi2—O8ii | 86.61 (7) | C26—C25—C24 | 119.0 (3) |
| O13—Bi2—O1W | 139.60 (8) | C26—C25—H25A | 120.5 |
| O11—Bi2—O1W | 129.27 (8) | C24—C25—H25A | 120.5 |
| N4—Bi2—O1W | 125.24 (8) | C27—C26—C25 | 118.7 (3) |
| N3—Bi2—O1W | 113.37 (8) | C27—C26—H26A | 120.7 |
| O1—Bi2—O1W | 70.76 (8) | C25—C26—H26A | 120.7 |
| O15—Bi2—O1W | 70.62 (7) | N4—C27—C26 | 121.7 (3) |
| O9—Bi2—O1W | 75.85 (7) | N4—C27—C28 | 115.2 (3) |
| O8ii—Bi2—O1W | 64.79 (7) | C26—C27—C28 | 123.1 (3) |
| C1—O1—Bi2 | 127.3 (2) | O16—C28—O15 | 125.9 (3) |
| C1—O1—Bi1 | 121.4 (2) | O16—C28—C27 | 117.2 (3) |
| Bi2—O1—Bi1 | 110.39 (9) | O15—C28—C27 | 116.9 (3) |
| C7—O3—Bi1 | 124.1 (2) | C30—N5—C29 | 111.4 (3) |
| C8—O5—Bi1 | 118.5 (2) | C30—N5—H5N1 | 106.3 |
| C14—O7—Bi1 | 122.1 (2) | C29—N5—H5N1 | 110.5 |
| C14—O8—Bi2ii | 140.2 (2) | C30—N5—H5N2 | 111.7 |
| C15—O9—Bi1 | 122.0 (2) | C29—N5—H5N2 | 111.4 |
| C15—O9—Bi2 | 120.3 (2) | H5N1—N5—H5N2 | 105.1 |
| Bi1—O9—Bi2 | 108.23 (9) | N5—C29—C30iii | 110.0 (3) |
| C21—O11—Bi2 | 123.5 (2) | N5—C29—H29A | 109.7 |
| C22—O13—Bi2 | 122.5 (2) | C30iii—C29—H29A | 109.7 |
| C22—O14—Bi1i | 147.0 (2) | N5—C29—H29B | 109.7 |
| C28—O15—Bi2 | 119.9 (2) | C30iii—C29—H29B | 109.7 |
| C6—N1—C2 | 120.1 (3) | H29A—C29—H29B | 108.2 |
| C6—N1—Bi1 | 117.1 (2) | N5—C30—C29iii | 109.5 (3) |
| C2—N1—Bi1 | 122.8 (2) | N5—C30—H30A | 109.8 |
| C13—N2—C9 | 120.1 (3) | C29iii—C30—H30A | 109.8 |
| C13—N2—Bi1 | 116.9 (2) | N5—C30—H30B | 109.8 |
| C9—N2—Bi1 | 122.7 (2) | C29iii—C30—H30B | 109.8 |
| C20—N3—C16 | 119.7 (3) | H30A—C30—H30B | 108.2 |
| C20—N3—Bi2 | 116.9 (2) | C32—N6—C31 | 110.7 (3) |
| C16—N3—Bi2 | 122.9 (2) | C32—N6—H6N1 | 116.6 |
| C27—N4—C23 | 120.3 (3) | C31—N6—H6N1 | 105.6 |
| C27—N4—Bi2 | 121.4 (2) | C32—N6—H6N2 | 111.5 |
| C23—N4—Bi2 | 117.3 (2) | C31—N6—H6N2 | 114.5 |
| O2—C1—O1 | 126.6 (3) | H6N1—N6—H6N2 | 97.4 |
| O2—C1—C2 | 118.3 (3) | N6—C31—C32iv | 109.6 (3) |
| O1—C1—C2 | 115.1 (3) | N6—C31—H31A | 109.7 |
| N1—C2—C3 | 121.2 (3) | C32iv—C31—H31A | 109.8 |
| N1—C2—C1 | 116.5 (3) | N6—C31—H31B | 109.7 |
| C3—C2—C1 | 122.3 (3) | C32iv—C31—H31B | 109.7 |
| C4—C3—C2 | 119.3 (3) | H31A—C31—H31B | 108.2 |
| C4—C3—H3A | 120.4 | N6—C32—C31iv | 110.3 (3) |
| C2—C3—H3A | 120.4 | N6—C32—H32A | 109.6 |
| C3—C4—C5 | 119.5 (3) | C31iv—C32—H32A | 109.6 |
| C3—C4—H4A | 120.3 | N6—C32—H32B | 109.6 |
| C5—C4—H4A | 120.3 | C31iv—C32—H32B | 109.6 |
| C6—C5—C4 | 117.8 (3) | H32A—C32—H32B | 108.1 |
| C6—C5—H5A | 121.1 | Bi2—O1W—H1W1 | 99.6 |
| C4—C5—H5A | 121.1 | Bi2—O1W—H1W2 | 130.1 |
| N1—C6—C5 | 122.2 (3) | H1W1—O1W—H1W2 | 106.1 |
| N1—C6—C7 | 115.4 (3) | H2W1—O2W—H2W2 | 119.0 |
| O13—Bi2—O1—C1 | 124.0 (3) | O13—Bi2—N3—C16 | 79.3 (3) |
| O11—Bi2—O1—C1 | 61.7 (3) | O11—Bi2—N3—C16 | 177.7 (3) |
| N4—Bi2—O1—C1 | 54.7 (3) | N4—Bi2—N3—C16 | 128.7 (2) |
| N3—Bi2—O1—C1 | 176.7 (3) | O1—Bi2—N3—C16 | 25.6 (3) |
| O15—Bi2—O1—C1 | −9.8 (3) | O15—Bi2—N3—C16 | −144.6 (2) |
| O9—Bi2—O1—C1 | −159.9 (3) | O9—Bi2—N3—C16 | 1.0 (2) |
| O8ii—Bi2—O1—C1 | −95.7 (3) | O8ii—Bi2—N3—C16 | −99.7 (3) |
| O1W—Bi2—O1—C1 | −78.4 (3) | O1W—Bi2—N3—C16 | −58.0 (3) |
| O13—Bi2—O1—Bi1 | −67.02 (10) | O13—Bi2—N4—C27 | 179.2 (3) |
| O11—Bi2—O1—Bi1 | −129.36 (14) | O11—Bi2—N4—C27 | 80.9 (3) |
| N4—Bi2—O1—Bi1 | −136.30 (11) | N3—Bi2—N4—C27 | 127.4 (2) |
| N3—Bi2—O1—Bi1 | −14.30 (15) | O1—Bi2—N4—C27 | −102.5 (3) |
| O15—Bi2—O1—Bi1 | 159.20 (10) | O15—Bi2—N4—C27 | −5.4 (2) |
| O9—Bi2—O1—Bi1 | 9.11 (8) | O9—Bi2—N4—C27 | −150.8 (2) |
| O8ii—Bi2—O1—Bi1 | 73.28 (12) | O8ii—Bi2—N4—C27 | 45.2 (3) |
| O1W—Bi2—O1—Bi1 | 90.61 (10) | O1W—Bi2—N4—C27 | −44.9 (3) |
| O7—Bi1—O1—C1 | 79.5 (3) | O13—Bi2—N4—C23 | 10.5 (2) |
| O3—Bi1—O1—C1 | 3.8 (3) | O11—Bi2—N4—C23 | −87.8 (2) |
| N2—Bi1—O1—C1 | 110.5 (3) | N3—Bi2—N4—C23 | −41.3 (3) |
| N1—Bi1—O1—C1 | −1.5 (2) | O1—Bi2—N4—C23 | 88.7 (2) |
| O9—Bi1—O1—C1 | 160.3 (3) | O15—Bi2—N4—C23 | −174.1 (3) |
| O5—Bi1—O1—C1 | −140.2 (2) | O9—Bi2—N4—C23 | 40.4 (3) |
| O14i—Bi1—O1—C1 | −67.0 (3) | O8ii—Bi2—N4—C23 | −123.5 (2) |
| O7—Bi1—O1—Bi2 | −90.28 (10) | O1W—Bi2—N4—C23 | 146.3 (2) |
| O3—Bi1—O1—Bi2 | −165.93 (9) | Bi2—O1—C1—O2 | −6.5 (5) |
| N2—Bi1—O1—Bi2 | −59.29 (15) | Bi1—O1—C1—O2 | −174.4 (3) |
| N1—Bi1—O1—Bi2 | −171.27 (13) | Bi2—O1—C1—C2 | 172.4 (2) |
| O9—Bi1—O1—Bi2 | −9.44 (9) | Bi1—O1—C1—C2 | 4.5 (4) |
| O5—Bi1—O1—Bi2 | 50.04 (16) | C6—N1—C2—C3 | 1.8 (5) |
| O14i—Bi1—O1—Bi2 | 123.22 (9) | Bi1—N1—C2—C3 | −174.4 (2) |
| O7—Bi1—O3—C7 | −79.5 (3) | C6—N1—C2—C1 | −178.4 (3) |
| N2—Bi1—O3—C7 | −146.9 (3) | Bi1—N1—C2—C1 | 5.3 (4) |
| N1—Bi1—O3—C7 | −7.2 (2) | O2—C1—C2—N1 | 172.7 (3) |
| O9—Bi1—O3—C7 | −142.5 (2) | O1—C1—C2—N1 | −6.3 (5) |
| O5—Bi1—O3—C7 | 146.4 (3) | O2—C1—C2—C3 | −7.6 (5) |
| O1—Bi1—O3—C7 | −12.4 (3) | O1—C1—C2—C3 | 173.5 (3) |
| O14i—Bi1—O3—C7 | 65.0 (3) | N1—C2—C3—C4 | −0.7 (5) |
| O7—Bi1—O5—C8 | −18.4 (3) | C1—C2—C3—C4 | 179.5 (3) |
| O3—Bi1—O5—C8 | 65.2 (2) | C2—C3—C4—C5 | −1.0 (5) |
| N2—Bi1—O5—C8 | −8.7 (2) | C3—C4—C5—C6 | 1.7 (5) |
| N1—Bi1—O5—C8 | 104.1 (3) | C2—N1—C6—C5 | −1.1 (5) |
| O9—Bi1—O5—C8 | −87.2 (2) | Bi1—N1—C6—C5 | 175.4 (3) |
| O1—Bi1—O5—C8 | −141.3 (2) | C2—N1—C6—C7 | 179.3 (3) |
| O14i—Bi1—O5—C8 | 141.9 (3) | Bi1—N1—C6—C7 | −4.3 (4) |
| O3—Bi1—O7—C14 | −73.4 (2) | C4—C5—C6—N1 | −0.7 (5) |
| N2—Bi1—O7—C14 | −3.8 (2) | C4—C5—C6—C7 | 178.9 (3) |
| N1—Bi1—O7—C14 | −139.5 (3) | Bi1—O3—C7—O4 | −172.1 (3) |
| O9—Bi1—O7—C14 | 79.8 (2) | Bi1—O3—C7—C6 | 7.7 (4) |
| O5—Bi1—O7—C14 | 5.6 (3) | N1—C6—C7—O4 | 178.1 (3) |
| O1—Bi1—O7—C14 | 153.2 (3) | C5—C6—C7—O4 | −1.5 (5) |
| O14i—Bi1—O7—C14 | −141.3 (2) | N1—C6—C7—O3 | −1.6 (4) |
| O7—Bi1—O9—C15 | −129.3 (3) | C5—C6—C7—O3 | 178.7 (3) |
| O3—Bi1—O9—C15 | −62.6 (3) | Bi1—O5—C8—O6 | −174.0 (3) |
| N2—Bi1—O9—C15 | −58.3 (3) | Bi1—O5—C8—C9 | 6.9 (4) |
| N1—Bi1—O9—C15 | 176.9 (2) | C13—N2—C9—C10 | −2.6 (5) |
| O5—Bi1—O9—C15 | 6.6 (3) | Bi1—N2—C9—C10 | 170.4 (2) |
| O1—Bi1—O9—C15 | 155.1 (3) | C13—N2—C9—C8 | 176.3 (3) |
| O14i—Bi1—O9—C15 | 81.9 (3) | Bi1—N2—C9—C8 | −10.7 (4) |
| O7—Bi1—O9—Bi2 | 84.45 (9) | O6—C8—C9—N2 | −177.4 (3) |
| O3—Bi1—O9—Bi2 | 151.22 (13) | O5—C8—C9—N2 | 1.8 (4) |
| N2—Bi1—O9—Bi2 | 155.47 (11) | O6—C8—C9—C10 | 1.5 (5) |
| N1—Bi1—O9—Bi2 | 30.64 (15) | O5—C8—C9—C10 | −179.3 (3) |
| O5—Bi1—O9—Bi2 | −139.62 (9) | N2—C9—C10—C11 | 0.5 (5) |
| O1—Bi1—O9—Bi2 | 8.88 (8) | C8—C9—C10—C11 | −178.2 (3) |
| O14i—Bi1—O9—Bi2 | −64.28 (12) | C9—C10—C11—C12 | 1.6 (5) |
| O13—Bi2—O9—C15 | −75.6 (2) | C10—C11—C12—C13 | −1.7 (5) |
| O11—Bi2—O9—C15 | −0.1 (3) | C9—N2—C13—C12 | 2.5 (5) |
| N4—Bi2—O9—C15 | −104.6 (3) | Bi1—N2—C13—C12 | −170.9 (3) |
| N3—Bi2—O9—C15 | 3.8 (2) | C9—N2—C13—C14 | −176.1 (3) |
| O1—Bi2—O9—C15 | −156.1 (3) | Bi1—N2—C13—C14 | 10.5 (4) |
| O15—Bi2—O9—C15 | 141.7 (2) | C11—C12—C13—N2 | −0.3 (5) |
| O8ii—Bi2—O9—C15 | 64.7 (2) | C11—C12—C13—C14 | 178.2 (3) |
| O1W—Bi2—O9—C15 | 129.5 (3) | Bi2ii—O8—C14—O7 | 128.7 (3) |
| O13—Bi2—O9—Bi1 | 71.32 (10) | Bi2ii—O8—C14—C13 | −51.2 (5) |
| O11—Bi2—O9—Bi1 | 146.80 (9) | Bi1—O7—C14—O8 | −169.4 (2) |
| N4—Bi2—O9—Bi1 | 42.35 (15) | Bi1—O7—C14—C13 | 10.5 (4) |
| N3—Bi2—O9—Bi1 | 150.68 (12) | N2—C13—C14—O8 | 166.2 (3) |
| O1—Bi2—O9—Bi1 | −9.18 (8) | C12—C13—C14—O8 | −12.4 (5) |
| O15—Bi2—O9—Bi1 | −71.42 (16) | N2—C13—C14—O7 | −13.7 (4) |
| O8ii—Bi2—O9—Bi1 | −148.38 (9) | C12—C13—C14—O7 | 167.7 (3) |
| O1W—Bi2—O9—Bi1 | −83.55 (9) | Bi1—O9—C15—O10 | 32.0 (5) |
| O13—Bi2—O11—C21 | 83.7 (3) | Bi2—O9—C15—O10 | 174.4 (3) |
| N4—Bi2—O11—C21 | 149.8 (3) | Bi1—O9—C15—C16 | −149.7 (2) |
| N3—Bi2—O11—C21 | 12.7 (2) | Bi2—O9—C15—C16 | −7.4 (4) |
| O1—Bi2—O11—C21 | 142.7 (2) | C20—N3—C16—C17 | 1.3 (5) |
| O15—Bi2—O11—C21 | −142.8 (3) | Bi2—N3—C16—C17 | 173.0 (3) |
| O9—Bi2—O11—C21 | 16.4 (3) | C20—N3—C16—C15 | −176.7 (3) |
| O8ii—Bi2—O11—C21 | −54.3 (2) | Bi2—N3—C16—C15 | −4.9 (4) |
| O1W—Bi2—O11—C21 | −88.9 (3) | O10—C15—C16—N3 | −173.6 (3) |
| O11—Bi2—O13—C22 | 67.3 (3) | O9—C15—C16—N3 | 8.1 (5) |
| N4—Bi2—O13—C22 | −3.0 (2) | O10—C15—C16—C17 | 8.5 (5) |
| N3—Bi2—O13—C22 | 132.2 (3) | O9—C15—C16—C17 | −169.9 (3) |
| O1—Bi2—O13—C22 | −87.9 (3) | N3—C16—C17—C18 | −2.6 (5) |
| O15—Bi2—O13—C22 | −8.7 (3) | C15—C16—C17—C18 | 175.3 (3) |
| O9—Bi2—O13—C22 | −161.0 (3) | C16—C17—C18—C19 | 1.9 (5) |
| O8ii—Bi2—O13—C22 | 133.4 (2) | C17—C18—C19—C20 | −0.1 (5) |
| O1W—Bi2—O13—C22 | −121.6 (3) | C16—N3—C20—C19 | 0.7 (5) |
| O13—Bi2—O15—C28 | 3.6 (3) | Bi2—N3—C20—C19 | −171.5 (3) |
| O11—Bi2—O15—C28 | −77.2 (2) | C16—N3—C20—C21 | −179.6 (3) |
| N4—Bi2—O15—C28 | −2.2 (2) | Bi2—N3—C20—C21 | 8.1 (4) |
| N3—Bi2—O15—C28 | −111.9 (3) | C18—C19—C20—N3 | −1.3 (5) |
| O1—Bi2—O15—C28 | 75.6 (2) | C18—C19—C20—C21 | 179.1 (3) |
| O9—Bi2—O15—C28 | 131.8 (2) | Bi2—O11—C21—O12 | 167.7 (3) |
| O8ii—Bi2—O15—C28 | −150.9 (2) | Bi2—O11—C21—C20 | −13.3 (4) |
| O1W—Bi2—O15—C28 | 144.3 (3) | N3—C20—C21—O12 | −178.5 (3) |
| O7—Bi1—N1—C6 | 104.3 (3) | C19—C20—C21—O12 | 1.1 (5) |
| O3—Bi1—N1—C6 | 5.7 (2) | N3—C20—C21—O11 | 2.4 (4) |
| N2—Bi1—N1—C6 | 53.0 (3) | C19—C20—C21—O11 | −177.9 (3) |
| O9—Bi1—N1—C6 | 158.5 (2) | Bi1i—O14—C22—O13 | −149.2 (3) |
| O5—Bi1—N1—C6 | −36.5 (3) | Bi1i—O14—C22—C23 | 31.2 (6) |
| O1—Bi1—N1—C6 | −178.6 (3) | Bi2—O13—C22—O14 | 176.5 (3) |
| O14i—Bi1—N1—C6 | −76.9 (2) | Bi2—O13—C22—C23 | −3.9 (4) |
| O7—Bi1—N1—C2 | −79.4 (3) | C27—N4—C23—C24 | −4.3 (5) |
| O3—Bi1—N1—C2 | −178.0 (3) | Bi2—N4—C23—C24 | 164.6 (3) |
| N2—Bi1—N1—C2 | −130.7 (2) | C27—N4—C23—C22 | 175.3 (3) |
| O9—Bi1—N1—C2 | −25.1 (3) | Bi2—N4—C23—C22 | −15.9 (4) |
| O5—Bi1—N1—C2 | 139.9 (2) | O14—C22—C23—N4 | −167.1 (3) |
| O1—Bi1—N1—C2 | −2.3 (2) | O13—C22—C23—N4 | 13.3 (4) |
| O14i—Bi1—N1—C2 | 99.4 (3) | O14—C22—C23—C24 | 12.5 (5) |
| O7—Bi1—N2—C13 | −4.3 (2) | O13—C22—C23—C24 | −167.2 (3) |
| O3—Bi1—N2—C13 | 94.9 (2) | N4—C23—C24—C25 | 4.0 (5) |
| N1—Bi1—N2—C13 | 49.2 (3) | C22—C23—C24—C25 | −175.5 (3) |
| O9—Bi1—N2—C13 | −82.9 (2) | C23—C24—C25—C26 | −0.6 (5) |
| O5—Bi1—N2—C13 | −176.8 (3) | C24—C25—C26—C27 | −2.4 (5) |
| O1—Bi1—N2—C13 | −35.8 (3) | C23—N4—C27—C26 | 1.1 (5) |
| O14i—Bi1—N2—C13 | 140.6 (2) | Bi2—N4—C27—C26 | −167.3 (3) |
| O7—Bi1—N2—C9 | −177.5 (3) | C23—N4—C27—C28 | 179.7 (3) |
| O3—Bi1—N2—C9 | −78.3 (2) | Bi2—N4—C27—C28 | 11.3 (4) |
| N1—Bi1—N2—C9 | −124.0 (2) | C25—C26—C27—N4 | 2.3 (5) |
| O9—Bi1—N2—C9 | 103.9 (3) | C25—C26—C27—C28 | −176.2 (3) |
| O5—Bi1—N2—C9 | 10.0 (2) | Bi2—O15—C28—O16 | −172.0 (3) |
| O1—Bi1—N2—C9 | 150.9 (2) | Bi2—O15—C28—C27 | 8.5 (4) |
| O14i—Bi1—N2—C9 | −32.6 (3) | N4—C27—C28—O16 | 167.6 (3) |
| O13—Bi2—N3—C20 | −108.7 (3) | C26—C27—C28—O16 | −13.8 (5) |
| O11—Bi2—N3—C20 | −10.3 (2) | N4—C27—C28—O15 | −12.9 (4) |
| N4—Bi2—N3—C20 | −59.3 (3) | C26—C27—C28—O15 | 165.7 (3) |
| O1—Bi2—N3—C20 | −162.4 (2) | C30—N5—C29—C30iii | −58.4 (4) |
| O15—Bi2—N3—C20 | 27.4 (3) | C29—N5—C30—C29iii | 58.0 (4) |
| O9—Bi2—N3—C20 | 172.9 (3) | C32—N6—C31—C32iv | −58.1 (4) |
| O8ii—Bi2—N3—C20 | 72.2 (2) | C31—N6—C32—C31iv | 58.5 (4) |
| O1W—Bi2—N3—C20 | 114.0 (2) |
Symmetry codes: (i) −x, −y+1, −z+1; (ii) −x, −y+1, −z; (iii) −x, −y+2, −z+1; (iv) −x−2, −y, −z.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N5—H5N1···O2Wiii | 0.90 | 2.00 | 2.761 (4) | 141 |
| N5—H5N2···O6 | 0.90 | 1.86 | 2.722 (4) | 160 |
| N6—H6N1···O16v | 0.90 | 1.78 | 2.676 (4) | 176 |
| N6—H6N2···O12 | 0.90 | 1.95 | 2.762 (4) | 149 |
| O1W—H1W1···O7 | 0.85 | 2.08 | 2.891 (3) | 160 |
| O1W—H1W2···O9ii | 0.85 | 2.53 | 3.337 (4) | 158 |
| O2W—H2W1···O14 | 0.85 | 2.04 | 2.887 (4) | 176 |
| O2W—H2W2···O5 | 0.85 | 2.35 | 3.162 (4) | 159 |
Symmetry codes: (iii) −x, −y+2, −z+1; (v) −x−1, −y, −z; (ii) −x, −y+1, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BI2264).
References
- Aghabozorg, H., Attar Gharamaleki, J., Ghadermazi, M., Ghasemikhah, P. & Soleimannejad, J. (2007). Acta Cryst. E63, m1803–m1804.
- Aghabozorg, H., Attar Gharamaleki, J., Ghasemikhah, P., Ghadermazi, M. & Soleimannejad, J. (2007). Acta Cryst. E63, m1710–m1711.
- Aghabozorg, H., Daneshvar, S., Motyeian, E., Ghadermazi, M. & Attar Gharamaleki, J. (2007). Acta Cryst. E63, m2468–m2469. [DOI] [PMC free article] [PubMed]
- Aghabozorg, H., Motyeian, E., Aghajani, Z., Ghadermazi, M. & Attar Gharamaleki, J. (2007). Acta Cryst. E63, m1754–m1755. [DOI] [PMC free article] [PubMed]
- Bruker (2005). APEX2 Bruker AXS Inc., Madison, Wisconsin, USA.
- Sharif, M. A., Aghabozorg, H. & Moghimi, A. (2007). Acta Cryst. E63, m1599–m1601.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheshmani, S., Ghadermazi, M. & Aghabozorg, H. (2006). Acta Cryst. E62, o3620–o3622.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S160053680800161X/bi2264sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053680800161X/bi2264Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report