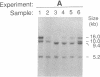
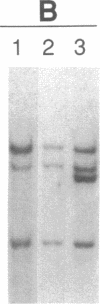
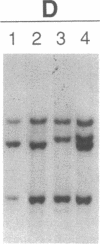
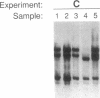
Abstract
Recently, combined serological and molecular studies of autoantibodies have revealed that these antibodies play an important role in the normal function of the immune system and in the development of the B cell repertoire. Accordingly, we hypothesized that a homozygous deletion of a critical autoantibody-associated Ig variable (V) gene may alter the immune system and thus predispose the host to autoimmune disorders. Initial experiments revealed several restriction fragment length polymorphisms (RFLP) of the Humhv3005 gene, that is likely to encode heavy chains of rheumatoid factors, and the closely related 1.9III gene. By probing EcoR1-digested DNA with the Humhv3005/P1 probe, we found that one of the four major hybridizing bands was missing in approximately 20% of patients with either rheumatoid arthritis or systemic lupus erythematosus, but only 2% of normal subjects. To delineate the genetic basis of this polymorphism, we have now employed the PCR to amplify and analyze hv3005, 1.9III, and homologous genes in individuals with characteristic RFLP genotypes. Our results indicate that the human Vh gene repertoire contains several hv3005- and 1.9III-like genes, and that a complete deletion of the hv3005-like genes is relatively restricted to a subset of autoimmune patients. These findings provide initial evidence for deletion of developmentally regulated autoreactive V genes in autoimmune diseases.
Full text
PDF


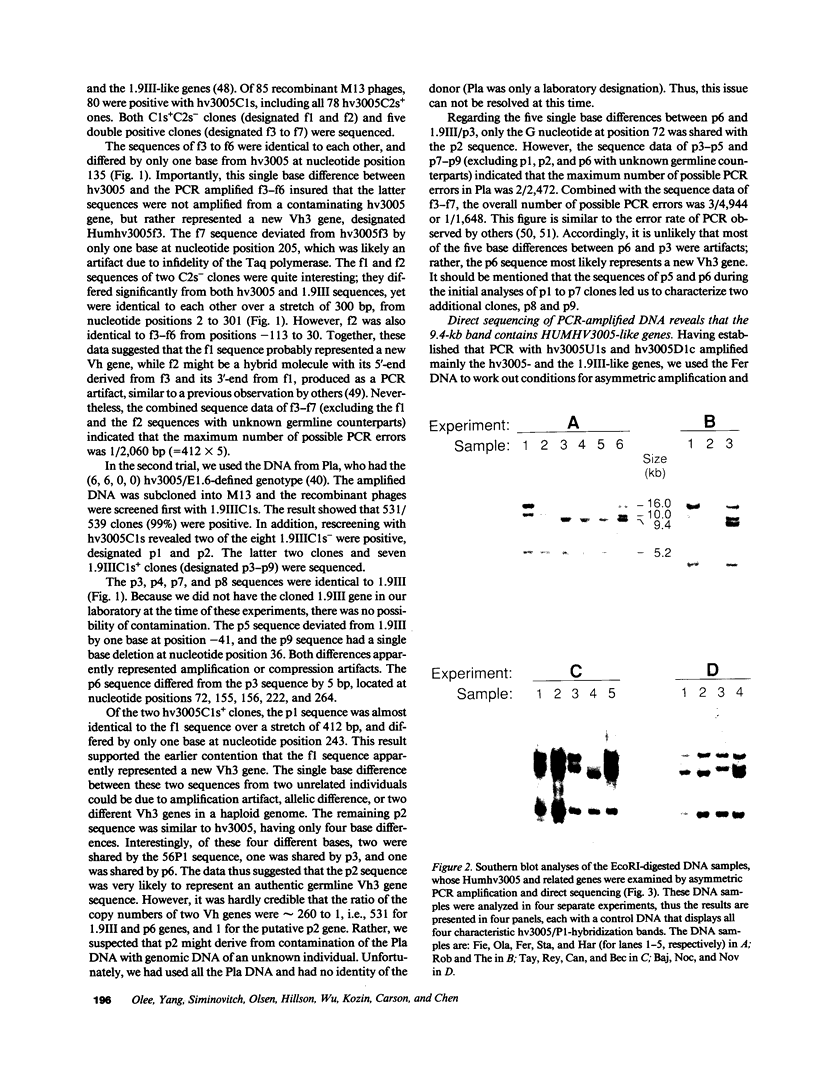
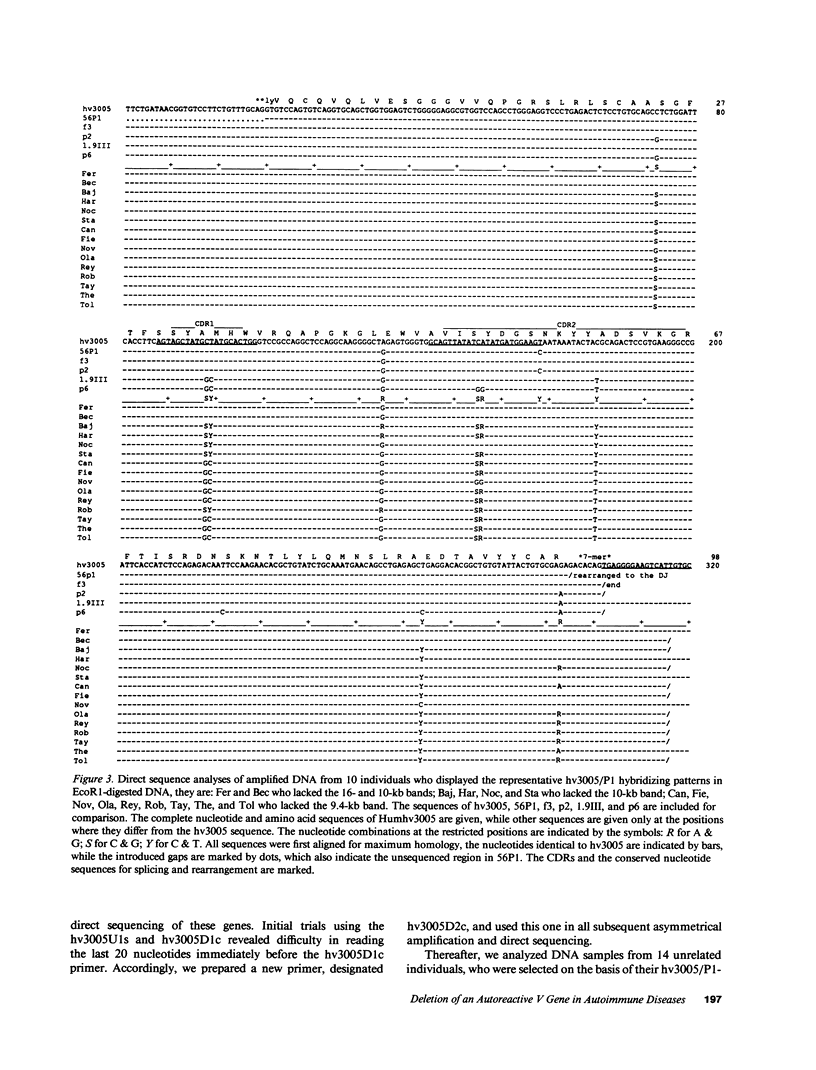
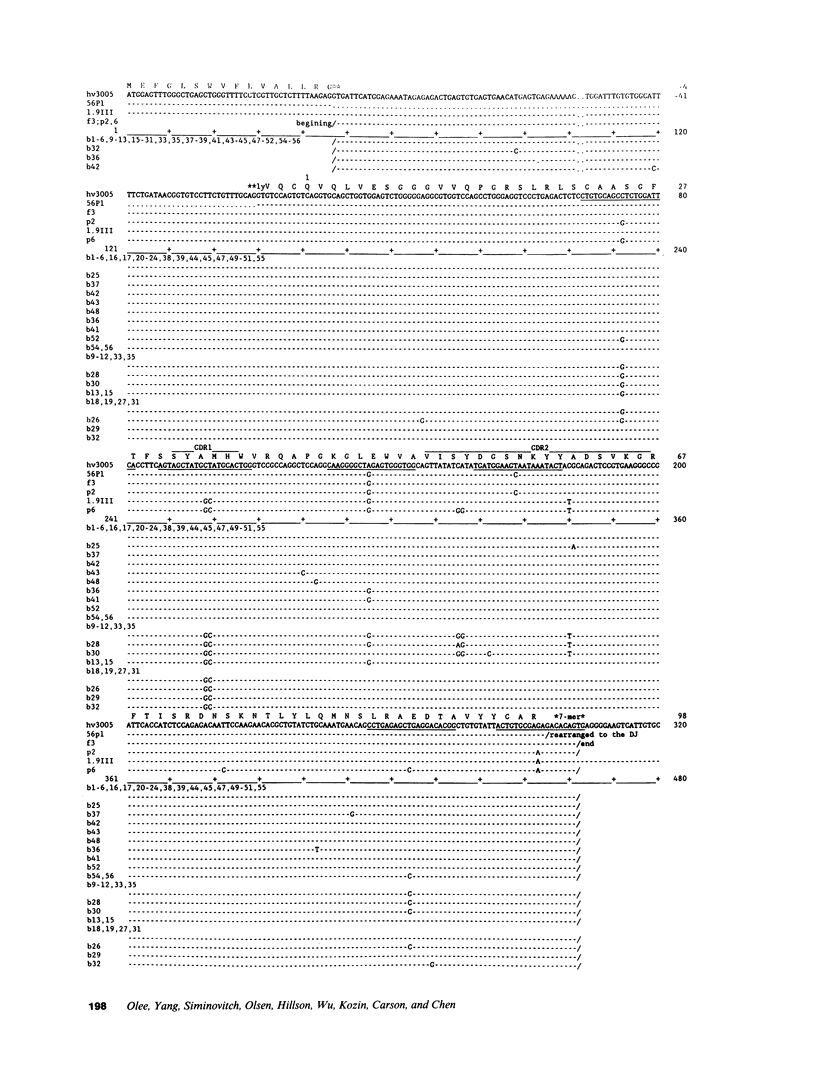

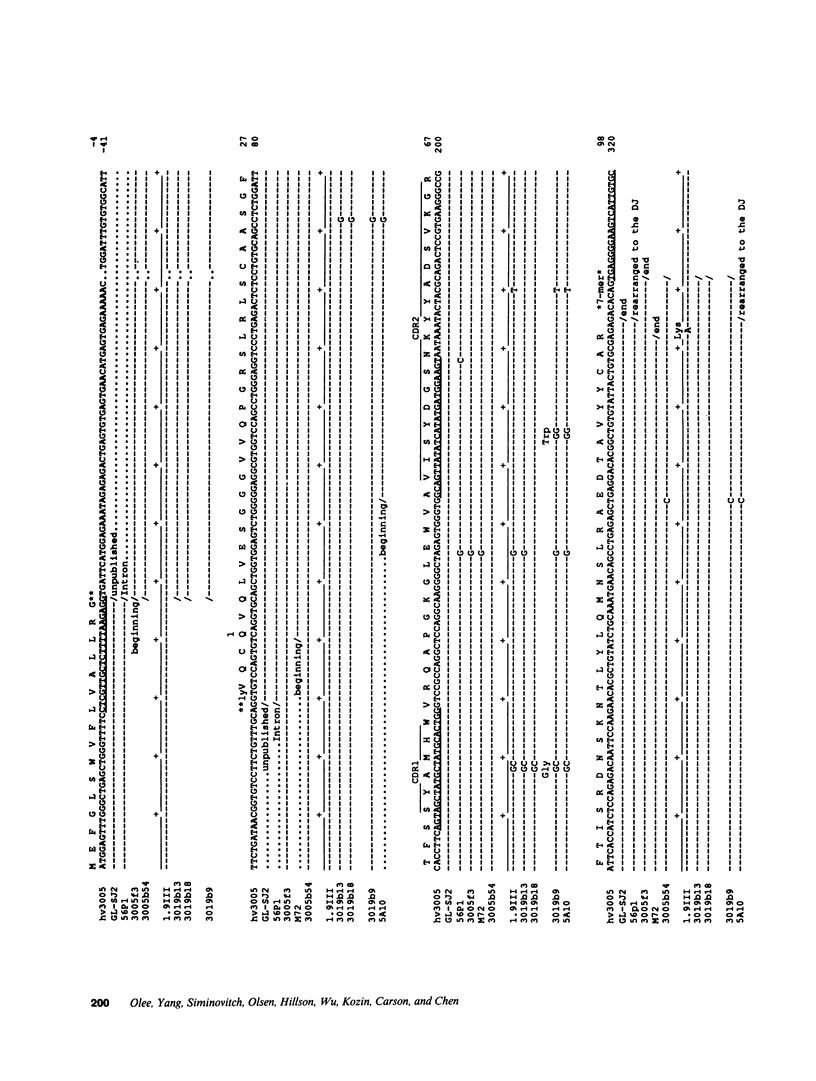



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Blackwell T. K., Yancopoulos G. D. Development of the primary antibody repertoire. Science. 1987 Nov 20;238(4830):1079–1087. doi: 10.1126/science.3317825. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Guilbert B., Mahana W., Matsiota P., Ternynck T. Recognition of self and non-self constituents by polyspecific autoreceptors. Int Rev Immunol. 1988 Mar;3(1-2):1–15. doi: 10.3109/08830188809051179. [DOI] [PubMed] [Google Scholar]
- Berman J. E., Mellis S. J., Pollock R., Smith C. L., Suh H., Heinke B., Kowal C., Surti U., Chess L., Cantor C. R. Content and organization of the human Ig VH locus: definition of three new VH families and linkage to the Ig CH locus. EMBO J. 1988 Mar;7(3):727–738. doi: 10.1002/j.1460-2075.1988.tb02869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bona C. A. V genes encoding autoantibodies: molecular and phenotypic characteristics. Annu Rev Immunol. 1988;6:327–358. doi: 10.1146/annurev.iy.06.040188.001551. [DOI] [PubMed] [Google Scholar]
- Cairns E., Kwong P. C., Misener V., Ip P., Bell D. A., Siminovitch K. A. Analysis of variable region genes encoding a human anti-DNA antibody of normal origin. Implications for the molecular basis of human autoimmune responses. J Immunol. 1989 Jul 15;143(2):685–691. [PubMed] [Google Scholar]
- Carroll W. L., Yu M., Link M. P., Korsmeyer S. J. Absence of Ig V region gene somatic hypermutation in advanced Burkitt's lymphoma. J Immunol. 1989 Jul 15;143(2):692–698. [PubMed] [Google Scholar]
- Carson D. A., Chen P. P., Fox R. I., Kipps T. J., Jirik F., Goldfien R. D., Silverman G., Radoux V., Fong S. Rheumatoid factor and immune networks. Annu Rev Immunol. 1987;5:109–126. doi: 10.1146/annurev.iy.05.040187.000545. [DOI] [PubMed] [Google Scholar]
- Casali P., Notkins A. L. Probing the human B-cell repertoire with EBV: polyreactive antibodies and CD5+ B lymphocytes. Annu Rev Immunol. 1989;7:513–535. doi: 10.1146/annurev.iy.07.040189.002501. [DOI] [PubMed] [Google Scholar]
- Chen P. P., Fong S., Carson D. A. Rheumatoid factor. Rheum Dis Clin North Am. 1987 Dec;13(3):545–568. [PubMed] [Google Scholar]
- Chen P. P., Fong S., Goni F., Silverman G. J., Fox R. I., Liu M. F., Frangione B., Carson D. A. Cross-reacting idiotypes on cryoprecipitating rheumatoid factor. Springer Semin Immunopathol. 1988;10(1):35–55. doi: 10.1007/BF02054022. [DOI] [PubMed] [Google Scholar]
- Chen P. P., Fong S., Houghten R. A., Carson D. A. Characterization of an epibody. An antiidiotype that reacts with both the idiotype of rheumatoid factors (RF) and the antigen recognized by RF. J Exp Med. 1985 Feb 1;161(2):323–331. doi: 10.1084/jem.161.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. P., Liu M. F., Sinha S., Carson D. A. A 16/6 idiotype-positive anti-DNA antibody is encoded by a conserved VH gene with no somatic mutation. Arthritis Rheum. 1988 Nov;31(11):1429–1431. doi: 10.1002/art.1780311113. [DOI] [PubMed] [Google Scholar]
- Chen P. P., Olsen N. J., Yang P. M., Soto-Gil R. W., Olee T., Siminovitch K. A., Carson D. A. From human autoantibodies to the fetal antibody repertoire to B cell malignancy: it's a small world after all. Int Rev Immunol. 1990;5(3-4):239–251. doi: 10.3109/08830189009056732. [DOI] [PubMed] [Google Scholar]
- Chen P. P., Siminovitch K. A., Olsen N. J., Erger R. A., Carson D. A. A highly informative probe for two polymorphic Vh gene regions that contain one or more autoantibody-associated Vh genes. J Clin Invest. 1989 Aug;84(2):706–710. doi: 10.1172/JCI114218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. P. Structural analyses of human developmentally regulated Vh3 genes. Scand J Immunol. 1990 Mar;31(3):257–267. doi: 10.1111/j.1365-3083.1990.tb02767.x. [DOI] [PubMed] [Google Scholar]
- Coutinho A. Beyond clonal selection and network. Immunol Rev. 1989 Aug;110:63–87. doi: 10.1111/j.1600-065x.1989.tb00027.x. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Grandien A., Faro-Rivas J., Mota-Santos T. A. Idiotypes, tailors and networks. Ann Inst Pasteur Immunol. 1988 Nov-Dec;139(6):599–607. doi: 10.1016/0769-2625(88)90049-9. [DOI] [PubMed] [Google Scholar]
- Crowley J. J., Mageed R. A., Silverman G. J., Chen P. P., Kozin F., Erger R. A., Jefferis R., Carson D. A. The incidence of a new human cross-reactive idiotype linked to subgroup VHIII heavy chains. Mol Immunol. 1990 Jan;27(1):87–94. doi: 10.1016/0161-5890(90)90063-6. [DOI] [PubMed] [Google Scholar]
- Cuisinier A. M., Guigou V., Boubli L., Fougereau M., Tonnelle C. Preferential expression of VH5 and VH6 immunoglobulin genes in early human B-cell ontogeny. Scand J Immunol. 1989 Oct;30(4):493–497. doi: 10.1111/j.1365-3083.1989.tb02455.x. [DOI] [PubMed] [Google Scholar]
- Dersimonian H., Schwartz R. S., Barrett K. J., Stollar B. D. Relationship of human variable region heavy chain germ-line genes to genes encoding anti-DNA autoantibodies. J Immunol. 1987 Oct 1;139(7):2496–2501. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dighiero G., Lymberi P., Holmberg D., Lundquist I., Coutinho A., Avrameas S. High frequency of natural autoantibodies in normal newborn mice. J Immunol. 1985 Feb;134(2):765–771. [PubMed] [Google Scholar]
- French D. L., Laskov R., Scharff M. D. The role of somatic hypermutation in the generation of antibody diversity. Science. 1989 Jun 9;244(4909):1152–1157. doi: 10.1126/science.2658060. [DOI] [PubMed] [Google Scholar]
- Glotz D., Sollazzo M., Riley S., Zanetti M. Isotype, VH genes, and antigen-binding analysis of hybridomas from newborn normal BALB/c mice. J Immunol. 1988 Jul 15;141(2):383–390. [PubMed] [Google Scholar]
- Guillaume T., Rubinstein D. B., Young F., Tucker L., Logtenberg T., Schwartz R. S., Barrett K. J. Individual VH genes detected with oligonucleotide probes from the complementarity-determining regions. J Immunol. 1990 Sep 15;145(6):1934–1945. [PubMed] [Google Scholar]
- Hardy R. R., Carmack C. E., Shinton S. A., Riblet R. J., Hayakawa K. A single VH gene is utilized predominantly in anti-BrMRBC hybridomas derived from purified Ly-1 B cells. Definition of the VH11 family. J Immunol. 1989 May 15;142(10):3643–3651. [PubMed] [Google Scholar]
- Holmberg D., Freitas A. A., Portnoï D., Jacquemart F., Avrameas S., Coutinho A. Antibody repertoires of normal BALB/c mice: B lymphocyte populations defined by state of activation. Immunol Rev. 1986 Oct;93:147–169. doi: 10.1111/j.1600-065x.1986.tb01506.x. [DOI] [PubMed] [Google Scholar]
- Holmberg D. High connectivity, natural antibodies preferentially use 7183 and QUPC 52 VH families. Eur J Immunol. 1987 Mar;17(3):399–403. doi: 10.1002/eji.1830170315. [DOI] [PubMed] [Google Scholar]
- Humphries C. G., Shen A., Kuziel W. A., Capra J. D., Blattner F. R., Tucker P. W. A new human immunoglobulin VH family preferentially rearranged in immature B-cell tumours. Nature. 1988 Feb 4;331(6155):446–449. doi: 10.1038/331446a0. [DOI] [PubMed] [Google Scholar]
- Innis M. A., Myambo K. B., Gelfand D. H., Brow M. A. DNA sequencing with Thermus aquaticus DNA polymerase and direct sequencing of polymerase chain reaction-amplified DNA. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9436–9440. doi: 10.1073/pnas.85.24.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. J., Natali A. M., Cann H. M., Honjo T., Cavalli-Sforza L. L. Polymorphisms of a human variable heavy chain gene show linkage with constant heavy chain genes. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7840–7844. doi: 10.1073/pnas.81.24.7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Solvason N., Bloem A., Vakil M. Idiotypes and B cell development. Chem Immunol. 1990;48:1–13. [PubMed] [Google Scholar]
- Kodaira M., Kinashi T., Umemura I., Matsuda F., Noma T., Ono Y., Honjo T. Organization and evolution of variable region genes of the human immunoglobulin heavy chain. J Mol Biol. 1986 Aug 20;190(4):529–541. doi: 10.1016/0022-2836(86)90239-1. [DOI] [PubMed] [Google Scholar]
- Lee K. H., Matsuda F., Kinashi T., Kodaira M., Honjo T. A novel family of variable region genes of the human immunoglobulin heavy chain. J Mol Biol. 1987 Jun 20;195(4):761–768. doi: 10.1016/0022-2836(87)90482-7. [DOI] [PubMed] [Google Scholar]
- Levy S., Mendel E., Kon S., Avnur Z., Levy R. Mutational hot spots in Ig V region genes of human follicular lymphomas. J Exp Med. 1988 Aug 1;168(2):475–489. doi: 10.1084/jem.168.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logtenberg T., Young F. M., Van Es J. H., Gmelig-Meyling F. H., Alt F. W. Autoantibodies encoded by the most Jh-proximal human immunoglobulin heavy chain variable region gene. J Exp Med. 1989 Oct 1;170(4):1347–1355. doi: 10.1084/jem.170.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthyssens G., Rabbitts T. H. Structure and multiplicity of genes for the human immunoglobulin heavy chain variable region. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6561–6565. doi: 10.1073/pnas.77.11.6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Monestier M., Manheimer-Lory A., Bellon B., Painter C., Dang H., Talal N., Zanetti M., Schwartz R., Pisetsky D., Kuppers R. Shared idiotypes and restricted immunoglobulin variable region heavy chain genes characterize murine autoantibodies of various specificities. J Clin Invest. 1986 Sep;78(3):753–759. doi: 10.1172/JCI112637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naparstek Y., André-Schwartz J., Manser T., Wysocki L. J., Breitman L., Stollar B. D., Gefter M., Schwartz R. S. A single germline VH gene segment of normal A/J mice encodes autoantibodies characteristic of systemic lupus erythematosus. J Exp Med. 1986 Aug 1;164(2):614–626. doi: 10.1084/jem.164.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual V., Randen I., Thompson K., Sioud M., Forre O., Natvig J., Capra J. D. The complete nucleotide sequences of the heavy chain variable regions of six monospecific rheumatoid factors derived from Epstein-Barr virus-transformed B cells isolated from the synovial tissue of patients with rheumatoid arthritis. Further evidence that some autoantibodies are unmutated copies of germ line genes. J Clin Invest. 1990 Oct;86(4):1320–1328. doi: 10.1172/JCI114841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter R. M. Programmed development of the antibody repertoire. Curr Top Microbiol Immunol. 1987;135:95–109. doi: 10.1007/978-3-642-71851-9_7. [DOI] [PubMed] [Google Scholar]
- Rechavi G., Ram D., Glazer L., Zakut R., Givol D. Evolutionary aspects of immunoglobulin heavy chain variable region (VH) gene subgroups. Proc Natl Acad Sci U S A. 1983 Feb;80(3):855–859. doi: 10.1073/pnas.80.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reininger L., Ollier P., Poncet P., Kaushik A., Jaton J. C. Novel V genes encode virtually identical variable regions of six murine monoclonal anti-bromelain-treated red blood cell autoantibodies. J Immunol. 1987 Jan 1;138(1):316–323. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanz I., Dang H., Takei M., Talal N., Capra J. D. VH sequence of a human anti-Sm autoantibody. Evidence that autoantibodies can be unmutated copies of germline genes. J Immunol. 1989 Feb 1;142(3):883–887. [PubMed] [Google Scholar]
- Sasso E. H., Van Dijk K. W., Milner E. C. Prevalence and polymorphism of human VH3 genes. J Immunol. 1990 Oct 15;145(8):2751–2757. [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Hillson J. L., Perlmutter R. M. Early restriction of the human antibody repertoire. Science. 1987 Nov 6;238(4828):791–793. doi: 10.1126/science.3118465. [DOI] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Wang J. Y. Preferential utilization of conserved immunoglobulin heavy chain variable gene segments during human fetal life. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6146–6150. doi: 10.1073/pnas.87.16.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuldiner A. R., Nirula A., Roth J. Hybrid DNA artifact from PCR of closely related target sequences. Nucleic Acids Res. 1989 Jun 12;17(11):4409–4409. doi: 10.1093/nar/17.11.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminovitch K. A., Chen P. P. The biologic significance of human natural autoimmune responses: relationship to the germline, early immune and malignant B cell variable gene repertoire. Int Rev Immunol. 1990;5(3-4):265–277. doi: 10.3109/08830189009056734. [DOI] [PubMed] [Google Scholar]
- Siminovitch K. A., Misener V., Kwong P. C., Song Q. L., Chen P. P. A natural autoantibody is encoded by germline heavy and lambda light chain variable region genes without somatic mutation. J Clin Invest. 1989 Nov;84(5):1675–1678. doi: 10.1172/JCI114347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollazzo M., Hasemann C. A., Meek K. D., Glotz D., Capra J. D., Zanetti M. Molecular characterization of the VH region of murine autoantibodies from neonatal and adult BALB/c mice. Eur J Immunol. 1989 Mar;19(3):453–457. doi: 10.1002/eji.1830190307. [DOI] [PubMed] [Google Scholar]
- Souroujon M. C., Rubinstein D. B., Schwartz R. S., Barrett K. J. Polymorphisms in human H chain V region genes from the VHIII gene family. J Immunol. 1989 Jul 15;143(2):706–711. [PubMed] [Google Scholar]
- Souroujon M., White-Scharf M. E., Andreschwartz J., Gefter M. L., Schwartz R. S. Preferential autoantibody reactivity of the preimmune B cell repertoire in normal mice. J Immunol. 1988 Jun 15;140(12):4173–4179. [PubMed] [Google Scholar]
- Steinberg A. D., Klinman D. M. Pathogenesis of systemic lupus erythematosus. Rheum Dis Clin North Am. 1988 Apr;14(1):25–41. [PubMed] [Google Scholar]
- Vakil M., Kearney J. F. Functional characterization of monoclonal auto-anti-idiotype antibodies isolated from the early B cell repertoire of BALB/c mice. Eur J Immunol. 1986 Sep;16(9):1151–1158. doi: 10.1002/eji.1830160920. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Miyada C. G. Oligonucleotide probes for the screening of recombinant DNA libraries. Methods Enzymol. 1987;152:432–442. doi: 10.1016/0076-6879(87)52050-x. [DOI] [PubMed] [Google Scholar]
- Walter M. A., Cox D. W. Analysis of genetic variation reveals human immunoglobulin VH-region gene organization. Am J Hum Genet. 1988 Mar;42(3):446–451. [PMC free article] [PubMed] [Google Scholar]
- Willems van Dijk K., Schroeder H. W., Jr, Perlmutter R. M., Milner E. C. Heterogeneity in the human Ig VH locus. J Immunol. 1989 Apr 1;142(7):2547–2554. [PubMed] [Google Scholar]
- Wysocki L. J., Gefter M. L. Gene conversion and the generation of antibody diversity. Annu Rev Biochem. 1989;58:509–531. doi: 10.1146/annurev.bi.58.070189.002453. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Malynn B. A., Alt F. W. Developmentally regulated and strain-specific expression of murine VH gene families. J Exp Med. 1988 Jul 1;168(1):417–435. doi: 10.1084/jem.168.1.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P. M., Olsen N. J., Siminovitch K. A., Olee T., Kozin F., Carson D. A., Chen P. P. Possible deletion of a developmentally regulated heavy-chain variable region gene in autoimmune diseases. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7907–7911. doi: 10.1073/pnas.87.20.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouali M., Stollar B. D., Schwartz R. S. Origin and diversification of anti-DNA antibodies. Immunol Rev. 1988 Oct;105:137–159. doi: 10.1111/j.1600-065x.1988.tb00770.x. [DOI] [PubMed] [Google Scholar]