Abstract
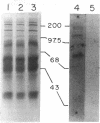
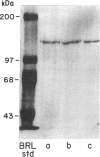
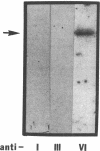
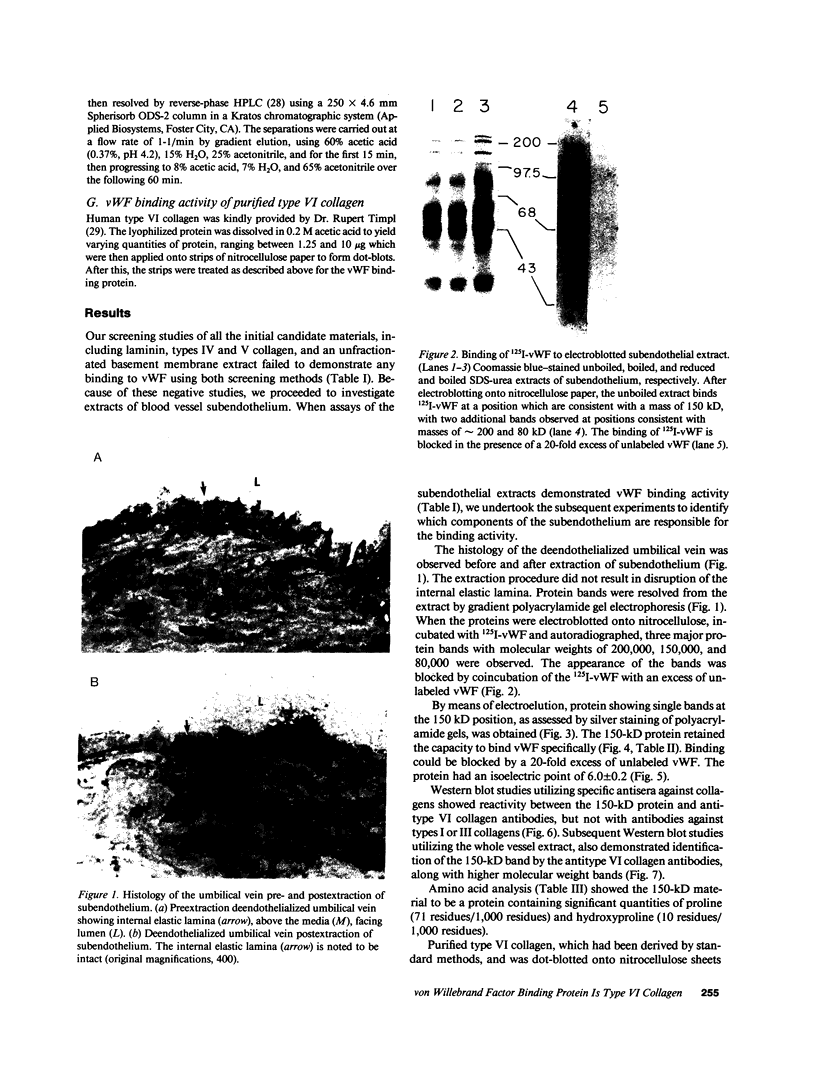
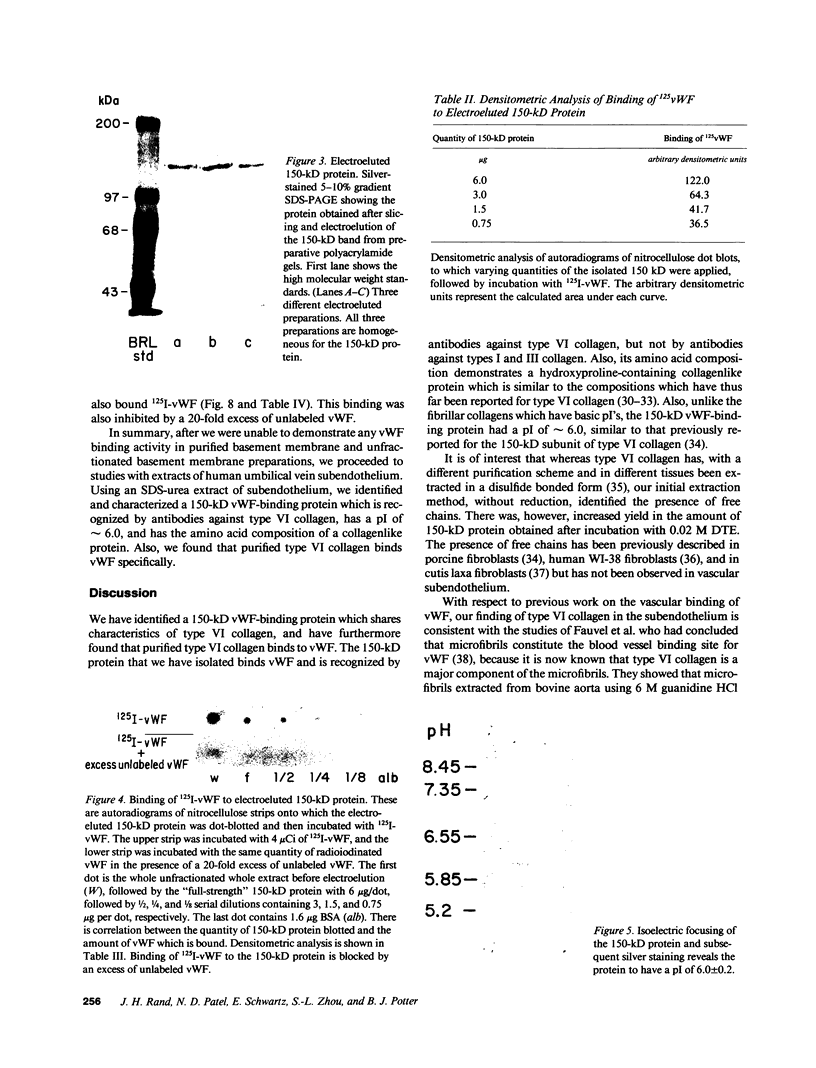
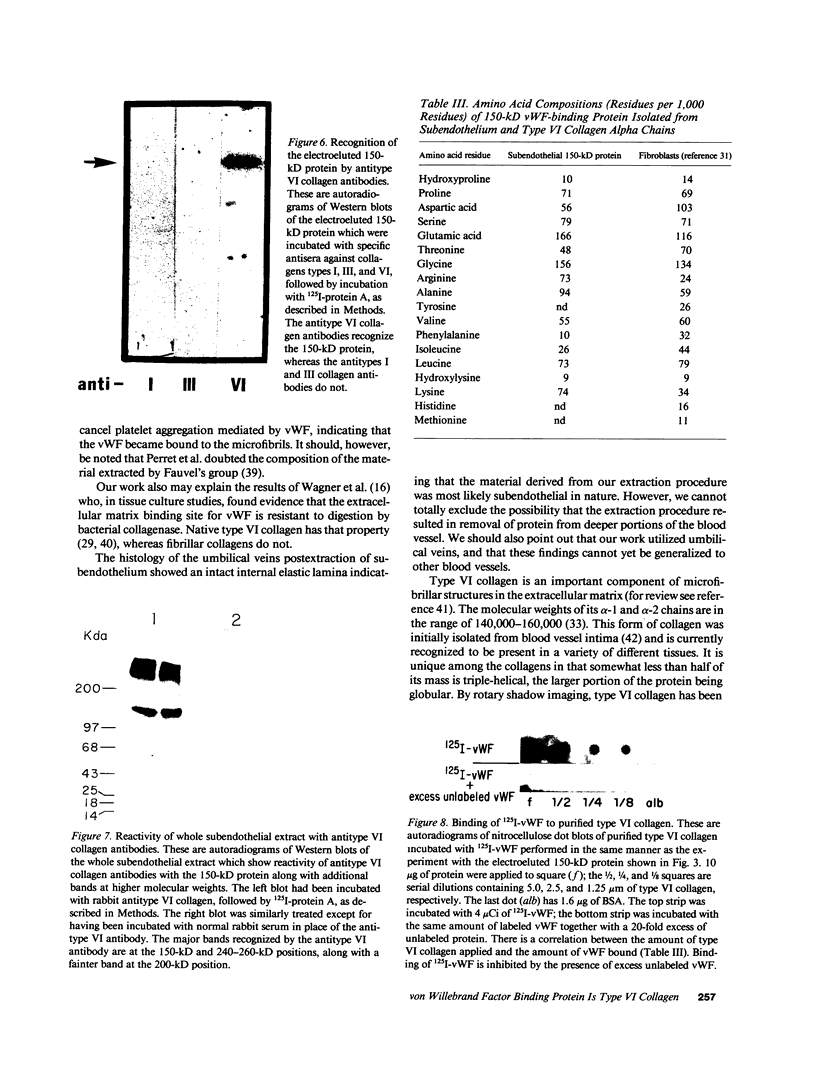
We have previously shown that von Willebrand factor (vWF), a glycoprotein which plays a critical role in the adhesion of platelets to injured blood vessels, is present within vascular subendothelium. We investigated the identity of the subendothelial binding site(s) for vWF by examining vWF binding to subendothelial constituents and solubilized a 150-kD protein with SDS-urea that bound vWF. This protein had an amino-acid composition similar to that of the type VI collagen alpha-1/alpha-2 chains, was recognized by specific polyclonal antibodies against type VI collagen, and had a similar acidic isoelectric point. Furthermore, we found that purified type VI collagen also bound vWF. Thus, we have identified the extracted 150-kD protein as type VI collagen. This protein may play a significant role in the binding of vWF to vascular subendothelium in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badimon L., Badimon J. J., Rand J., Turitto V. T., Fuster V. Platelet deposition on von Willebrand factor-deficient vessels. Extracorporeal perfusion studies in swine with von Willebrand's disease using native and heparinized blood. J Lab Clin Med. 1987 Nov;110(5):634–647. [PubMed] [Google Scholar]
- Bockenstedt P., McDonagh J., Handin R. I. Binding and covalent cross-linking of purified von Willebrand factor to native monomeric collagen. J Clin Invest. 1986 Aug;78(2):551–556. doi: 10.1172/JCI112608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaldo P., Colombatti A. The carboxyl terminus of the chicken alpha 3 chain of collagen VI is a unique mosaic structure with glycoprotein Ib-like, fibronectin type III, and Kunitz modules. J Biol Chem. 1989 Dec 5;264(34):20235–20239. [PubMed] [Google Scholar]
- Bonaldo P., Russo V., Bucciotti F., Bressan G. M., Colombatti A. Alpha 1 chain of chick type VI collagen. The complete cDNA sequence reveals a hybrid molecule made of one short collagen and three von Willebrand factor type A-like domains. J Biol Chem. 1989 Apr 5;264(10):5575–5580. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Carter W. G. The cooperative role of the transformation-sensitive glycoproteins, GP140 and fibronectin, in cell attachment and spreading. J Biol Chem. 1982 Mar 25;257(6):3249–3257. [PubMed] [Google Scholar]
- Carter W. G. The role of intermolecular disulfide bonding in deposition of GP140 in the extracellular matrix. J Cell Biol. 1984 Jul;99(1 Pt 1):105–114. doi: 10.1083/jcb.99.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W. G. Transformation-dependent alterations is glycoproteins of extracellular matrix of human fibroblasts. Characterization of GP250 and the collagen-like GP140. J Biol Chem. 1982 Nov 25;257(22):13805–13815. [PubMed] [Google Scholar]
- Chu M. L., Pan T. C., Conway D., Kuo H. J., Glanville R. W., Timpl R., Mann K., Deutzmann R. Sequence analysis of alpha 1(VI) and alpha 2(VI) chains of human type VI collagen reveals internal triplication of globular domains similar to the A domains of von Willebrand factor and two alpha 2(VI) chain variants that differ in the carboxy terminus. EMBO J. 1989 Jul;8(7):1939–1946. doi: 10.1002/j.1460-2075.1989.tb03598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E., Rhodes K., Miller E. J. Isolation of three collagenous components of probable basement membrane origin from several tissues. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1167–1174. doi: 10.1016/0006-291x(76)90776-2. [DOI] [PubMed] [Google Scholar]
- Colombatti A., Ainger K., Colizzi F. Type VI collagen: high yields of a molecule with multiple forms of alpha 3 chain from avian and human tissues. Matrix. 1989 Jun;9(3):177–185. doi: 10.1016/s0934-8832(89)80048-4. [DOI] [PubMed] [Google Scholar]
- Crawford S. W., Featherstone J. A., Holbrook K., Yong S. L., Bornstein P., Sage H. Characterization of a type VI collagen-related Mr-140 000 protein from cutis-laxa fibroblasts in culture. Biochem J. 1985 Apr 15;227(2):491–502. doi: 10.1042/bj2270491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauvel F., Grant M. E., Legrand Y. J., Souchon H., Tobelem G., Jackson D. S., Caen J. P. Interaction of blood platelets with a microfibrillar extract from adult bovine aorta: requirement for von Willebrand factor. Proc Natl Acad Sci U S A. 1983 Jan;80(2):551–554. doi: 10.1073/pnas.80.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furthmayr H., Wiedemann H., Timpl R., Odermatt E., Engel J. Electron-microscopical approach to a structural model of intima collagen. Biochem J. 1983 May 1;211(2):303–311. doi: 10.1042/bj2110303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson M. A., Cleary E. G. A collagen-like glycoprotein from elastin-rich tissues. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1288–1295. doi: 10.1016/0006-291x(82)90926-3. [DOI] [PubMed] [Google Scholar]
- Girma J. P., Meyer D., Verweij C. L., Pannekoek H., Sixma J. J. Structure-function relationship of human von Willebrand factor. Blood. 1987 Sep;70(3):605–611. [PubMed] [Google Scholar]
- Hatamochi A., Aumailley M., Mauch C., Chu M. L., Timpl R., Krieg T. Regulation of collagen VI expression in fibroblasts. Effects of cell density, cell-matrix interactions, and chemical transformation. J Biol Chem. 1989 Feb 25;264(6):3494–3499. [PubMed] [Google Scholar]
- King I. A., Tabiowo A., Fryer P. R., Pope F. M. A type VI collagen-related glycopolypeptide is the major concanavalin A-binding component in pig skin. Biochem J. 1989 Jan 1;257(1):79–86. doi: 10.1042/bj2570079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Hassell J. R., Martin G. R. Formation of a supramolecular complex is involved in the reconstitution of basement membrane components. Biochemistry. 1983 Oct 11;22(21):4969–4974. doi: 10.1021/bi00290a014. [DOI] [PubMed] [Google Scholar]
- Koller E., Winterhalter K. H., Trueb B. The globular domains of type VI collagen are related to the collagen-binding domains of cartilage matrix protein and von Willebrand factor. EMBO J. 1989 Apr;8(4):1073–1077. doi: 10.1002/j.1460-2075.1989.tb03475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leiderman I. Z., Greenberg M. L., Adelsberg B. R., Siegal F. P. A glycoprotein inhibitor of in vitro granulopoiesis associated with AIDS. Blood. 1987 Nov;70(5):1267–1272. [PubMed] [Google Scholar]
- Madri J. A., Dreyer B., Pitlick F. A., Furthmayr H. The collagenous components of the subendothelium. Correlation of structure and function. Lab Invest. 1980 Oct;43(4):303–315. [PubMed] [Google Scholar]
- Marton L. S., Arnason B. G. A basement membrane-associated glycoprotein from skeletal muscle. J Cell Biochem. 1982;19(4):363–381. doi: 10.1002/jcb.240190406. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Switzer R. C., Van Keuren M. L. Trace polypeptides in cellular extracts and human body fluids detected by two-dimensional electrophoresis and a highly sensitive silver stain. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4335–4339. doi: 10.1073/pnas.76.9.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohri H., Fujimura Y., Shima M., Yoshioka A., Houghten R. A., Ruggeri Z. M., Zimmerman T. S. Structure of the von Willebrand factor domain interacting with glycoprotein Ib. J Biol Chem. 1988 Dec 5;263(34):17901–17904. [PubMed] [Google Scholar]
- Mohri H., Yoshioka A., Zimmerman T. S., Ruggeri Z. M. Isolation of the von Willebrand factor domain interacting with platelet glycoprotein Ib, heparin, and collagen and characterization of its three distinct functional sites. J Biol Chem. 1989 Oct 15;264(29):17361–17367. [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Nyman D. Interaction of collagen with the factor VIII antigen-activity - von Willebrand factor complex. Thromb Res. 1977 Sep;11(3):433–438. doi: 10.1016/0049-3848(77)90196-7. [DOI] [PubMed] [Google Scholar]
- Pareti F. I., Niiya K., McPherson J. M., Ruggeri Z. M. Isolation and characterization of two domains of human von Willebrand factor that interact with fibrillar collagen types I and III. J Biol Chem. 1987 Oct 5;262(28):13835–13841. [PubMed] [Google Scholar]
- Perret B. A., Furlan M., Jenö P., Beck E. A. Von Willebrand factor-dependent agglutination of washed fixed human platelets by insoluble collagen isolated from bovine aorta. J Lab Clin Med. 1986 Mar;107(3):244–252. [PubMed] [Google Scholar]
- Piétu G., Meulien P., Cherel G., Diaz J., Baruch D., Courtney M., Meyer D. Production in Escherichia coli of a biologically active subfragment of von Willebrand factor corresponding to the platelet glycoprotein Ib, collagen and heparin binding domains. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1339–1347. doi: 10.1016/0006-291x(89)91816-0. [DOI] [PubMed] [Google Scholar]
- Rand J. H., Sussman I. I., Gordon R. E., Chu S. V., Solomon V. Localization of factor-VIII-related antigen in human vascular subendothelium. Blood. 1980 May;55(5):752–756. [PubMed] [Google Scholar]
- Roth G. J., Titani K., Hoyer L. W., Hickey M. J. Localization of binding sites within human von Willebrand factor for monomeric type III collagen. Biochemistry. 1986 Dec 30;25(26):8357–8361. doi: 10.1021/bi00374a004. [DOI] [PubMed] [Google Scholar]
- Sakariassen K. S., Bolhuis P. A., Sixma J. J. Human blood platelet adhesion to artery subendothelium is mediated by factor VIII-Von Willebrand factor bound to the subendothelium. Nature. 1979 Jun 14;279(5714):636–638. doi: 10.1038/279636a0. [DOI] [PubMed] [Google Scholar]
- Salacinski P. R., McLean C., Sykes J. E., Clement-Jones V. V., Lowry P. J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen). Anal Biochem. 1981 Oct;117(1):136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- Santoro S. A., Cowan J. F. Adsorption of von Willebrand factor by fibrillar collagen--implications concerning the adhesion of platelets to collagen. Coll Relat Res. 1982 Jan;2(1):31–43. doi: 10.1016/s0174-173x(82)80039-3. [DOI] [PubMed] [Google Scholar]
- Shekhonin B. V., Domogatsky S. P., Muzykantov V. R., Idelson G. L., Rukosuev V. S. Distribution of type I, III, IV and V collagen in normal and atherosclerotic human arterial wall: immunomorphological characteristics. Coll Relat Res. 1985 Sep;5(4):355–368. doi: 10.1016/s0174-173x(85)80024-8. [DOI] [PubMed] [Google Scholar]
- Stel H. V., Sakariassen K. S., de Groot P. G., van Mourik J. A., Sixma J. J. Von Willebrand factor in the vessel wall mediates platelet adherence. Blood. 1985 Jan;65(1):85–90. [PubMed] [Google Scholar]
- Sussman I. I., Rand J. H. Subendothelial deposition of von Willebrand's factor requires the presence of endothelial cells. J Lab Clin Med. 1982 Oct;100(4):526–532. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trüeb B., Winterhalter K. H. Type VI collagen is composed of a 200 kd subunit and two 140 kd subunits. EMBO J. 1986 Nov;5(11):2815–2819. doi: 10.1002/j.1460-2075.1986.tb04573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp T. B., Weiss H. J., Baumgartner H. R. Decreased adhesion of platelets to subendothelium in von Willebrand's disease. J Lab Clin Med. 1974 Feb;83(2):296–300. [PubMed] [Google Scholar]
- Turitto V. T., Weiss H. J., Baumgartner H. R. Platelet interaction with rabbit subendothelium in von Willebrand's disease: altered thrombus formation distinct from defective platelet adhesion. J Clin Invest. 1984 Nov;74(5):1730–1741. doi: 10.1172/JCI111591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turitto V. T., Weiss H. J., Zimmerman T. S., Sussman I. I. Factor VIII/von Willebrand factor in subendothelium mediates platelet adhesion. Blood. 1985 Apr;65(4):823–831. [PubMed] [Google Scholar]
- Voss B., Rauterberg J. Localization of collagen types I, III, IV and V, fibronectin and laminin in human arteries by the indirect immunofluorescence method. Pathol Res Pract. 1986 Oct;181(5):568–575. doi: 10.1016/S0344-0338(86)80151-0. [DOI] [PubMed] [Google Scholar]
- Wagner D. D., Urban-Pickering M., Marder V. J. Von Willebrand protein binds to extracellular matrices independently of collagen. Proc Natl Acad Sci U S A. 1984 Jan;81(2):471–475. doi: 10.1073/pnas.81.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot P. G., Ottenhof-Rovers M., van Mourik J. A., Sixma J. J. Evidence that the primary binding site of von Willebrand factor that mediates platelet adhesion on subendothelium is not collagen. J Clin Invest. 1988 Jul;82(1):65–73. doi: 10.1172/JCI113602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Mark H., Aumailley M., Wick G., Fleischmajer R., Timpl R. Immunochemistry, genuine size and tissue localization of collagen VI. Eur J Biochem. 1984 Aug 1;142(3):493–502. doi: 10.1111/j.1432-1033.1984.tb08313.x. [DOI] [PubMed] [Google Scholar]