Abstract
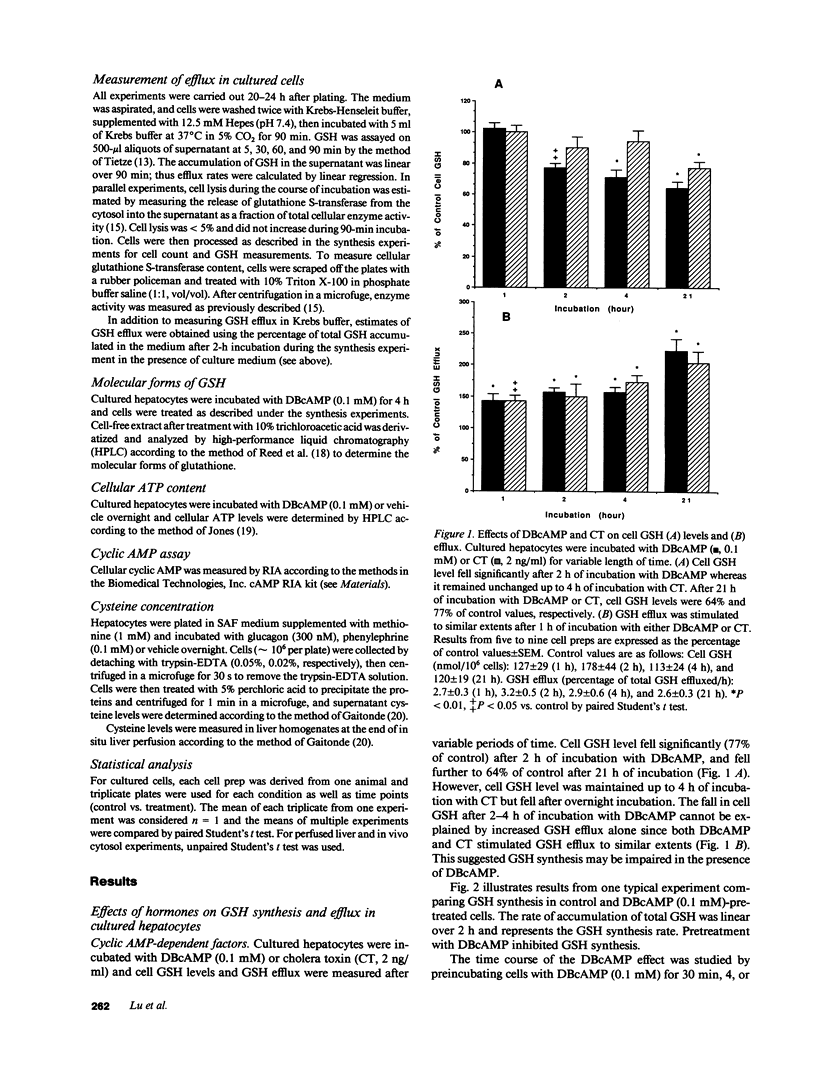
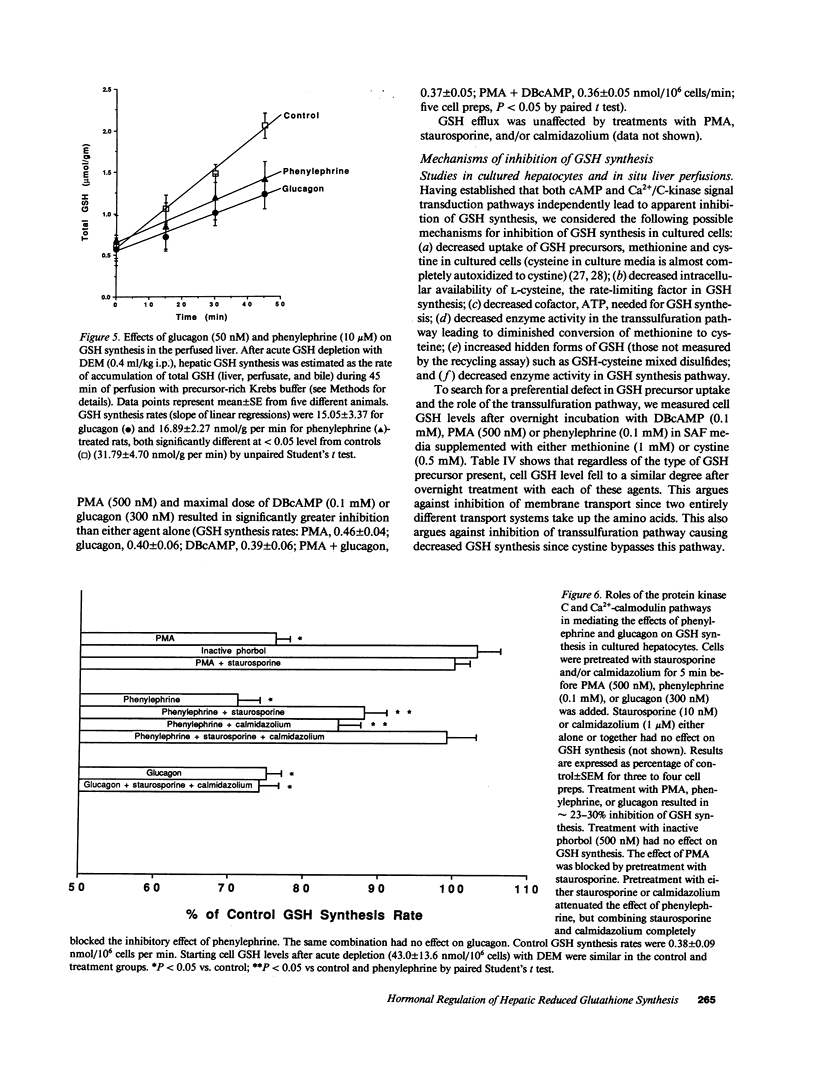
Our present work characterized the role of hormone-mediated signal transduction pathways in regulating hepatic reduced glutathione (GSH) synthesis. Cholera toxin, dibutyryl cAMP (DBcAMP), and glucagon inhibited GSH synthesis in cultured hepatocytes by 25-43%. Cellular cAMP levels exhibited a lower threshold for stimulation of the GSH efflux than inhibition of its synthesis. The effect of DBcAMP was independent of the type of sulfur amino acid precursor and cellular ATP levels and unassociated with increased GSH mixed disulfide formation or altered GSH/oxidized glutathione ratio. In liver cytosols, addition of DBcAMP and cAMP-dependent protein kinase (A-kinase) inhibited GSH synthesis from substrates (cysteine, ATP, glutamate, and glycine) by approximately 20% which was prevented by the A-kinase inhibitor. However, if only substrates of the second step in GSH synthesis were used (gamma-glutamylcysteine, glycine, and ATP), DBcAMP and A-kinase exerted no inhibitory effect. Phenylephrine, vasopressin, and phorbol ester also inhibited GSH synthesis in cultured cells by approximately 20%, and depleted cell GSH independent of the type of sulfur amino acid precursor. Cellular cysteine level was unchanged despite the significant fall in GSH after glucagon or phenylephrine treatment. Pretreatment with either staurosporine, C-kinase inhibitor, or calmidazolium, a calmodulin inhibitor, partially prevented but, together, completely prevented the inhibitory effect of phenylephrine. The same combination had no effect on the inhibitory effect of glucagon. The effects of hormones were confirmed in both the intact perfused liver and after in vivo administration. Thus, two classes of hormones acting through distinct signal transduction pathways may down-regulate hepatic GSH synthesis by phosphorylation of gamma-glutamylcysteine synthetase.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. D., Jr, Lauterburg B. H., Mitchell J. R. Plasma glutathione and glutathione disulfide in the rat: regulation and response to oxidative stress. J Pharmacol Exp Ther. 1983 Dec;227(3):749–754. [PubMed] [Google Scholar]
- BECK L. V., LINKENHEIMER W. H., MARRACCINI A. Effects of tumbling trauma, scalding and hemorrhage on rat tissue non-protein sulfhydryl. Proc Soc Exp Biol Med. 1954 Aug-Sep;86(4):823–827. doi: 10.3181/00379727-86-21243. [DOI] [PubMed] [Google Scholar]
- Bannai S., Tateishi N. Role of membrane transport in metabolism and function of glutathione in mammals. J Membr Biol. 1986;89(1):1–8. doi: 10.1007/BF01870891. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Robinson G. S., Bucher N. L., Farmer S. R. Cell-cell and cell-matrix interactions differentially regulate the expression of hepatic and cytoskeletal genes in primary cultures of rat hepatocytes. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2161–2165. doi: 10.1073/pnas.85.7.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth J., Boyland E., Sims P. An enzyme from rat liver catalysing conjugations with glutathione. Biochem J. 1961 Jun;79(3):516–524. doi: 10.1042/bj0790516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragt P. C., Bonta I. L. Oxidant stress during inflammation: anti-inflammatory effects of antioxidants. Agents Actions. 1980 Dec;10(6):536–539. doi: 10.1007/BF02024159. [DOI] [PubMed] [Google Scholar]
- Estrela J. M., Gil F., Vila J. M., Viña J. Alpha-adrenergic modulation of glutathione metabolism in isolated rat hepatocytes. Am J Physiol. 1988 Dec;255(6 Pt 1):E801–E805. doi: 10.1152/ajpendo.1988.255.6.E801. [DOI] [PubMed] [Google Scholar]
- Fernández-Checa J. C., Kaplowitz N. The use of monochlorobimane to determine hepatic GSH levels and synthesis. Anal Biochem. 1990 Nov 1;190(2):212–219. doi: 10.1016/0003-2697(90)90183-a. [DOI] [PubMed] [Google Scholar]
- Gaitonde M. K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J. 1967 Aug;104(2):627–633. doi: 10.1042/bj1040627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso J. A., Bruno M., Yates A. A., Wei L. T., Epstein P. M. Calmodulin dependence of transferrin receptor recycling in rat reticulocytes. Biochem J. 1990 Feb 15;266(1):261–272. doi: 10.1042/bj2660261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi T., Tateishi N., Naruse A., Sakamoto Y. Decrease of glutathione and induction of gamma-glutamyltransferase by dibutyryl-3', 5'-cyclic AMP in rat liver. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1280–1286. doi: 10.1016/0006-291x(76)90335-1. [DOI] [PubMed] [Google Scholar]
- Hirota M., Inoue M., Ando Y., Hirayama K., Morino Y., Sakamoto K., Mori K., Akagi M. Inhibition of stress-induced gastric injury in the rat by glutathione. Gastroenterology. 1989 Oct;97(4):853–859. doi: 10.1016/0016-5085(89)91488-1. [DOI] [PubMed] [Google Scholar]
- Jones D. P. Determination of pyridine dinucleotides in cell extracts by high-performance liquid chromatography. J Chromatogr. 1981 Oct 9;225(2):446–449. doi: 10.1016/s0378-4347(00)80293-5. [DOI] [PubMed] [Google Scholar]
- Kaplowitz N., Aw T. Y., Ookhtens M. The regulation of hepatic glutathione. Annu Rev Pharmacol Toxicol. 1985;25:715–744. doi: 10.1146/annurev.pa.25.040185.003435. [DOI] [PubMed] [Google Scholar]
- Kilberg M. S. Amino acid transport in isolated rat hepatocytes. J Membr Biol. 1982;69(1):1–12. doi: 10.1007/BF01871236. [DOI] [PubMed] [Google Scholar]
- Lauterburg B. H., Adams J. D., Mitchell J. R. Hepatic glutathione homeostasis in the rat: efflux accounts for glutathione turnover. Hepatology. 1984 Jul-Aug;4(4):586–590. doi: 10.1002/hep.1840040402. [DOI] [PubMed] [Google Scholar]
- Lew H., Pyke S., Quintanilha A. Changes in the glutathione status of plasma, liver and muscle following exhaustive exercise in rats. FEBS Lett. 1985 Jun 17;185(2):262–266. doi: 10.1016/0014-5793(85)80919-4. [DOI] [PubMed] [Google Scholar]
- Lu S. C., Garcia-Ruiz C., Kuhlenkamp J., Ookhtens M., Salas-Prato M., Kaplowitz N. Hormonal regulation of glutathione efflux. J Biol Chem. 1990 Sep 25;265(27):16088–16095. [PubMed] [Google Scholar]
- Mauger J. P., Claret M. Mobilization of intracellular calcium by glucagon and cyclic AMP analogues in isolated rat hepatocytes. FEBS Lett. 1986 Jan 20;195(1-2):106–110. doi: 10.1016/0014-5793(86)80140-5. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Mine T., Kojima I., Ogata E. Evidence of cyclic AMP-independent action of glucagon on calcium mobilization in rat hepatocytes. Biochim Biophys Acta. 1988 Jun 30;970(2):166–171. doi: 10.1016/0167-4889(88)90175-9. [DOI] [PubMed] [Google Scholar]
- Moldéus P., Högberg J., Orrenius S. Isolation and use of liver cells. Methods Enzymol. 1978;52:60–71. doi: 10.1016/s0076-6879(78)52006-5. [DOI] [PubMed] [Google Scholar]
- Ookhtens M., Hobdy K., Corvasce M. C., Aw T. Y., Kaplowitz N. Sinusoidal efflux of glutathione in the perfused rat liver. Evidence for a carrier-mediated process. J Clin Invest. 1985 Jan;75(1):258–265. doi: 10.1172/JCI111682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed D. J., Babson J. R., Beatty P. W., Brodie A. E., Ellis W. W., Potter D. W. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem. 1980 Jul 15;106(1):55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- Reichard S. M., Bailey N. M., Galvin M. J., Jr Alterations in tissue glutathione levels following traumatic shock. Adv Shock Res. 1981;5:37–45. [PubMed] [Google Scholar]
- Richman P. G., Meister A. Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem. 1975 Feb 25;250(4):1422–1426. [PubMed] [Google Scholar]
- Ruiz M. B., Ochoa B., Lacort M. Glucagon- and dibutyryl cyclic AMP-produced inhibition of cholesterol ester hydrolase in isolated rat hepatocytes: role of calcium. J Biochem. 1990 Mar;107(3):476–479. doi: 10.1093/oxfordjournals.jbchem.a123070. [DOI] [PubMed] [Google Scholar]
- Schleifer L. S., Black I. B., Reid L. M. Regulation of beta-adrenergic receptor expression in rat liver. J Cell Physiol. 1989 Jul;140(1):52–58. doi: 10.1002/jcp.1041400107. [DOI] [PubMed] [Google Scholar]
- Staddon J. M., Hansford R. G. 4 beta-Phorbol 12-myristate 13-acetate attenuates the glucagon-induced increase in cytoplasmic free Ca2+ concentration in isolated rat hepatocytes. Biochem J. 1986 Sep 15;238(3):737–743. doi: 10.1042/bj2380737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada A., Bannai S. Transport of cystine in isolated rat hepatocytes in primary culture. J Biol Chem. 1984 Feb 25;259(4):2441–2445. [PubMed] [Google Scholar]
- Tateishi N., Higashi T., Naruse A., Nakashima K., Shiozaki H. Rat liver glutathione: possible role as a reservoir of cysteine. J Nutr. 1977 Jan;107(1):51–60. doi: 10.1093/jn/107.1.51. [DOI] [PubMed] [Google Scholar]
- Thor H., Moldéus P., Orrenius S. Metabolic activation and hepatotoxicity. Effect of cysteine, N-acetylcysteine, and methionine on glutathione biosynthesis and bromobenzene toxicity in isolated rat hepatocytes. Arch Biochem Biophys. 1979 Feb;192(2):405–413. doi: 10.1016/0003-9861(79)90109-7. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Verme T. B., Velarde R. T., Cunningham R. M., Hootman S. R. Effects of staurosporine on protein kinase C and amylase secretion from pancreatic acini. Am J Physiol. 1989 Oct;257(4 Pt 1):G548–G553. doi: 10.1152/ajpgi.1989.257.4.G548. [DOI] [PubMed] [Google Scholar]
- Yamada H. [Anti-shock effect of reduced glutathione. Effects on survival rate and metabolism in traumatic shock rats]. Masui. 1977 Jun;26(6):640–645. [PubMed] [Google Scholar]


