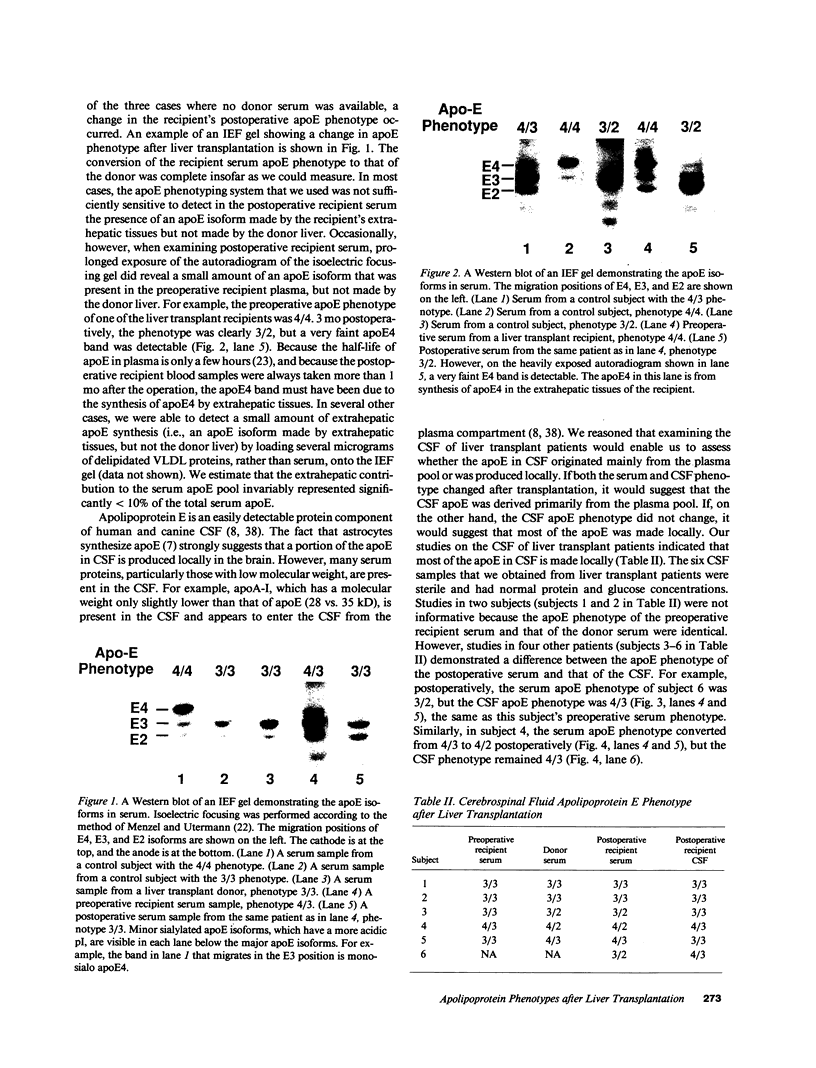
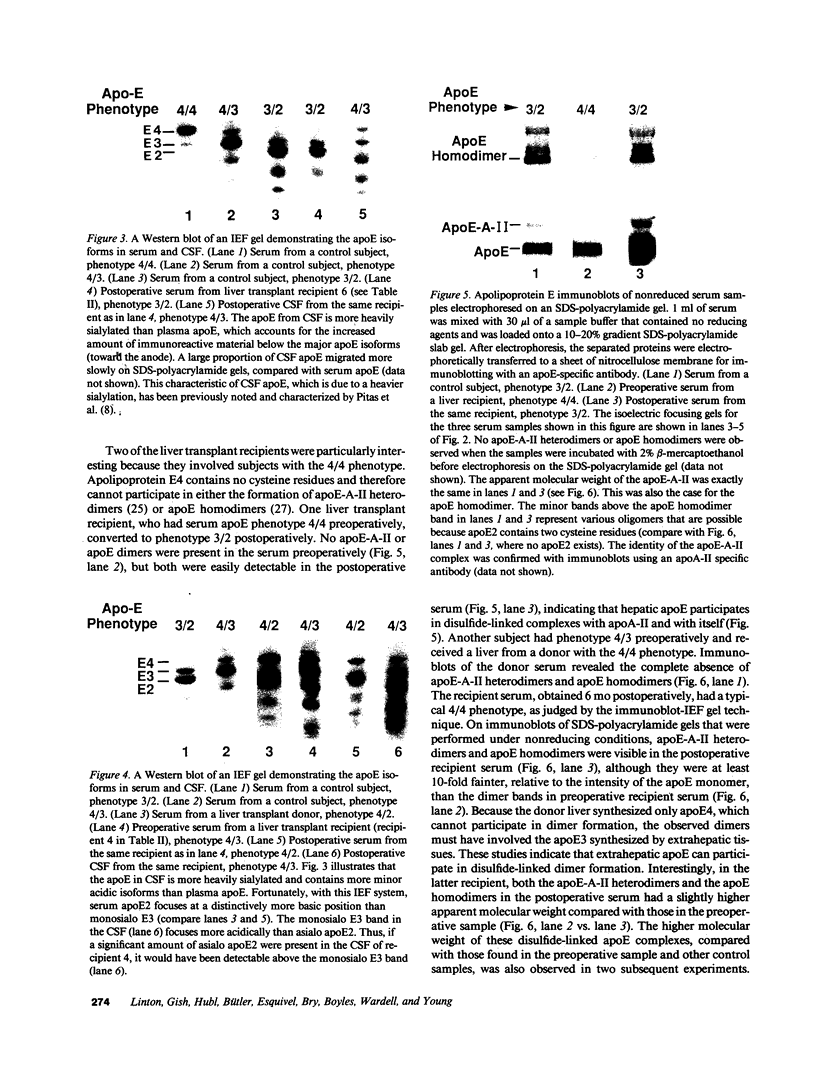
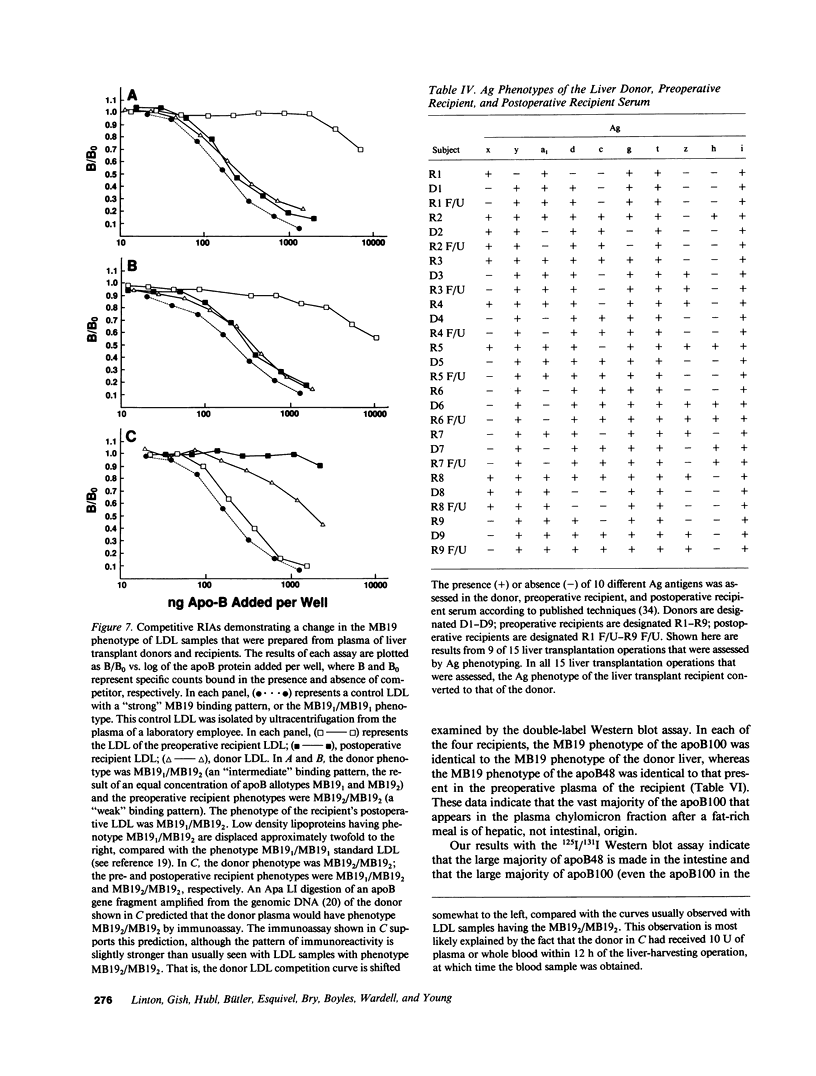
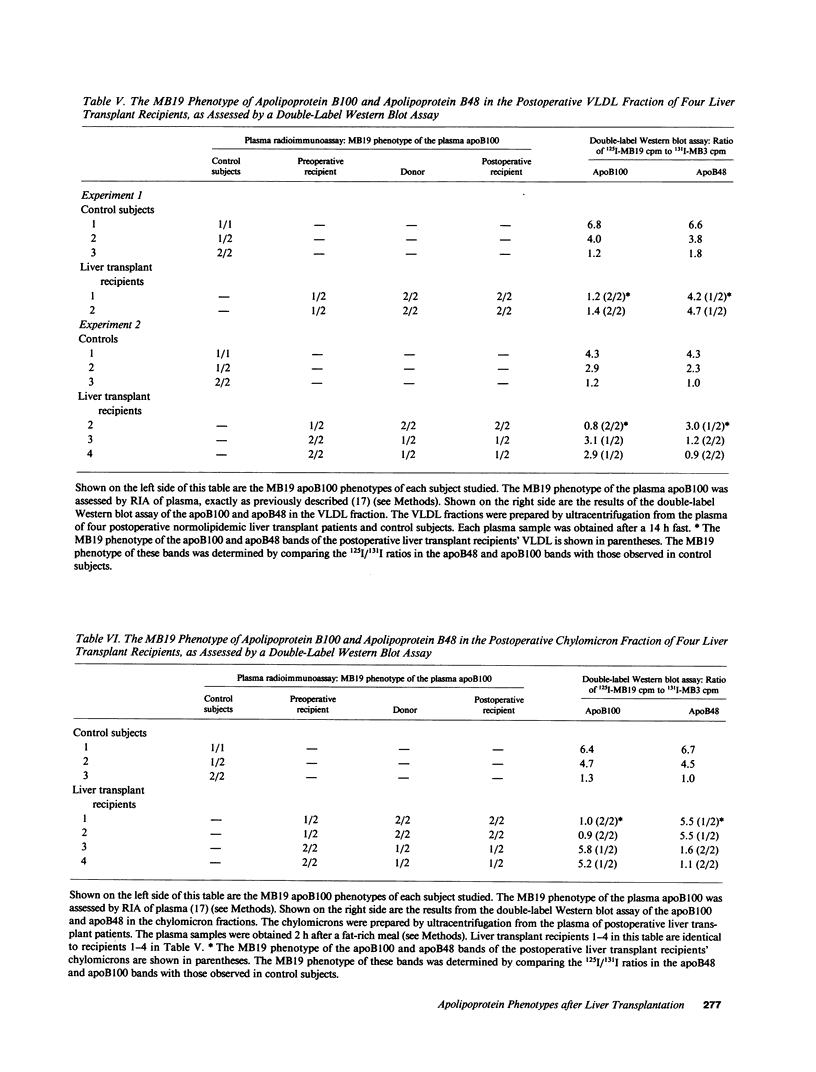
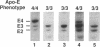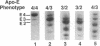Abstract
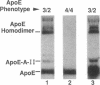
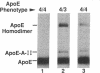
Apolipoprotein (apo) E and the two B apolipoproteins, apoB48 and apoB100, are important proteins in human lipoprotein metabolism. Commonly occurring polymorphisms in the genes for apoE and apoB result in amino acid substitutions that produce readily detectable phenotypic differences in these proteins. We studied changes in apoE and apoB phenotypes before and after liver transplantation to gain new insights into apolipoprotein physiology. In all 29 patients that we studied, the postoperative serum apoE phenotype of the recipient, as assessed by isoelectric focusing, converted virtually completely to that of the donor, providing evidence that greater than 90% of the apoE in the plasma is synthesized by the liver. In contrast, the cerebrospinal fluid apoE phenotype did not change to the donor's phenotype after liver transplantation, indicating that most of the apoE in CSF cannot be derived from the plasma pool and therefore must be synthesized locally. The apoB100 phenotype (assessed with immunoassays using monoclonal antibody MB19, an antibody that detects a two-allele polymorphism in apoB) invariably converted to the phenotype of the donor. In four normolipidemic patients, we determined the MB19 phenotype of both the apoB100 and apoB48 in the "chylomicron fraction" isolated from plasma 3 h after a fat-rich meal. Interestingly, the apoB100 in the chylomicron fraction invariably had the phenotype of the donor, indicating that the vast majority of the large, triglyceride-rich apoB100-containing lipoproteins that appear in the plasma after a fat-rich meal are actually VLDL of hepatic origin. The MB19 phenotype of the apoB48 in the plasma chylomicron fraction did not change after liver transplantation, indicating that almost all of the apoB48 in plasma chylomicrons is derived from the intestine. These results were consistent with our immunocytochemical studies on intestinal biopsy specimens of organ donors; using apoB-specific monoclonal antibodies, we found evidence for apoB48, but not apoB100, in donor intestinal biopsy specimens.
Full text
PDF



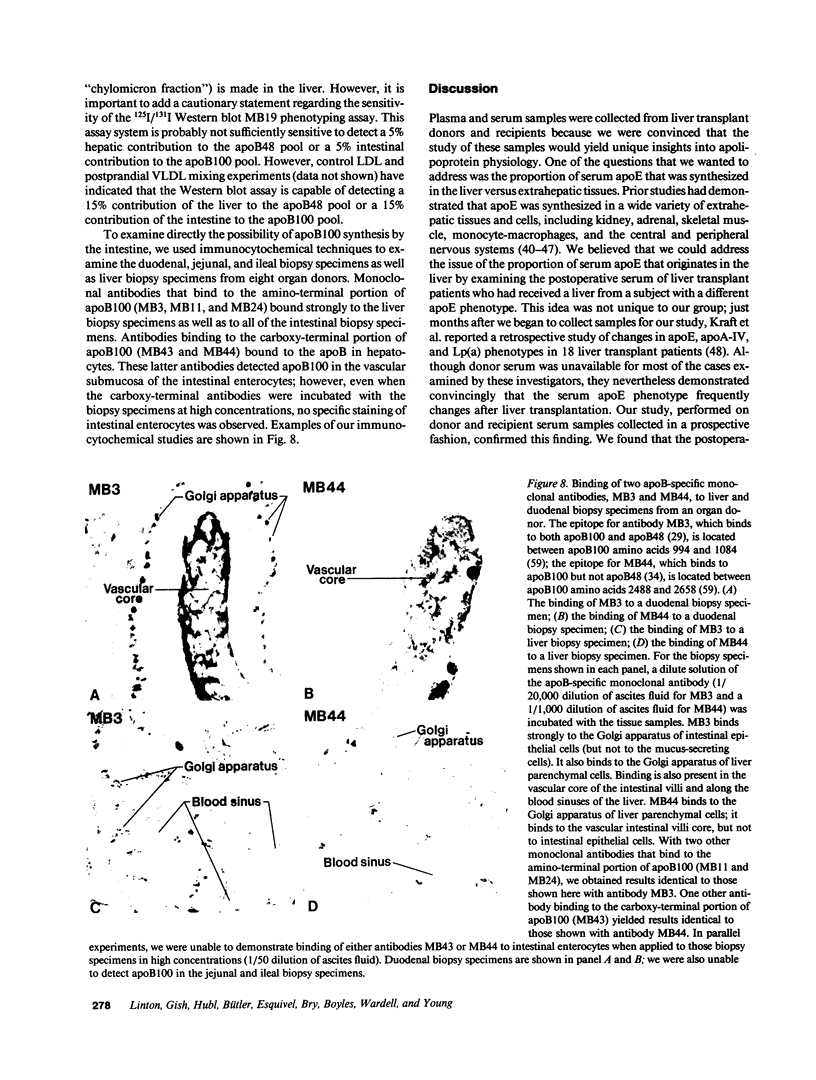



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu S. K., Ho Y. K., Brown M. S., Bilheimer D. W., Anderson R. G., Goldstein J. L. Biochemical and genetic studies of the apoprotein E secreted by mouse macrophages and human monocytes. J Biol Chem. 1982 Aug 25;257(16):9788–9795. [PubMed] [Google Scholar]
- Bilheimer D. W., Goldstein J. L., Grundy S. M., Starzl T. E., Brown M. S. Liver transplantation to provide low-density-lipoprotein receptors and lower plasma cholesterol in a child with homozygous familial hypercholesterolemia. N Engl J Med. 1984 Dec 27;311(26):1658–1664. doi: 10.1056/NEJM198412273112603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue M. L., Williams D. L., Zucker S., Khan S. A., Blum C. B. Apolipoprotein E synthesis in human kidney, adrenal gland, and liver. Proc Natl Acad Sci U S A. 1983 Jan;80(1):283–287. doi: 10.1073/pnas.80.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyles J. K., Pitas R. E., Wilson E., Mahley R. W., Taylor J. M. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest. 1985 Oct;76(4):1501–1513. doi: 10.1172/JCI112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyles J. K., Zoellner C. D., Anderson L. J., Kosik L. M., Pitas R. E., Weisgraber K. H., Hui D. Y., Mahley R. W., Gebicke-Haerter P. J., Ignatius M. J. A role for apolipoprotein E, apolipoprotein A-I, and low density lipoprotein receptors in cholesterol transport during regeneration and remyelination of the rat sciatic nerve. J Clin Invest. 1989 Mar;83(3):1015–1031. doi: 10.1172/JCI113943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. H., Habib G., Yang C. Y., Gu Z. W., Lee B. R., Weng S. A., Silberman S. R., Cai S. J., Deslypere J. P., Rosseneu M. Apolipoprotein B-48 is the product of a messenger RNA with an organ-specific in-frame stop codon. Science. 1987 Oct 16;238(4825):363–366. doi: 10.1126/science.3659919. [DOI] [PubMed] [Google Scholar]
- Cohn J. S., McNamara J. R., Cohn S. D., Ordovas J. M., Schaefer E. J. Plasma apolipoprotein changes in the triglyceride-rich lipoprotein fraction of human subjects fed a fat-rich meal. J Lipid Res. 1988 Jul;29(7):925–936. [PubMed] [Google Scholar]
- Craig W. Y., Nutik R., Cooper A. D. Regulation of apoprotein synthesis and secretion in the human hepatoma Hep G2. The effect of exogenous lipoprotein. J Biol Chem. 1988 Sep 25;263(27):13880–13890. [PubMed] [Google Scholar]
- Curtiss L. K., Edgington T. S. Immunochemical heterogeneity of human plasma apolipoprotein B. I. Apolipoprotein B binding of mouse hybridoma antibodies. J Biol Chem. 1982 Dec 25;257(24):15213–15221. [PubMed] [Google Scholar]
- Driscoll D. M., Getz G. S. Extrahepatic synthesis of apolipoprotein E. J Lipid Res. 1984 Dec 1;25(12):1368–1379. [PubMed] [Google Scholar]
- Dullaart R. P., Speelberg B., Schuurman H. J., Milne R. W., Havekes L. M., Marcel Y. L., Geuze H. J., Hulshof M. M., Erkelens D. W. Epitopes of apolipoprotein B-100 and B-48 in both liver and intestine. Expression and evidence for local synthesis in recessive abetalipoproteinemia. J Clin Invest. 1986 Nov;78(5):1397–1404. doi: 10.1172/JCI112727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshourbagy N. A., Liao W. S., Mahley R. W., Taylor J. M. Apolipoprotein E mRNA is abundant in the brain and adrenals, as well as in the liver, and is present in other peripheral tissues of rats and marmosets. Proc Natl Acad Sci U S A. 1985 Jan;82(1):203–207. doi: 10.1073/pnas.82.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman R. M., Rogers M., Glickman J. N. Apolipoprotein B synthesis by human liver and intestine in vitro. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5296–5300. doi: 10.1073/pnas.83.14.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotto A. M., Jr, Pownall H. J., Havel R. J. Introduction to the plasma lipoproteins. Methods Enzymol. 1986;128:3–41. doi: 10.1016/0076-6879(86)28061-1. [DOI] [PubMed] [Google Scholar]
- Gregg R. E., Zech L. A., Schaefer E. J., Stark D., Wilson D., Brewer H. B., Jr Abnormal in vivo metabolism of apolipoprotein E4 in humans. J Clin Invest. 1986 Sep;78(3):815–821. doi: 10.1172/JCI112645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi K., Hospattankar A. V., Law S. W., Meglin N., Cortright J., Brewer H. B., Jr Human apolipoprotein B (apoB) mRNA: identification of two distinct apoB mRNAs, an mRNA with the apoB-100 sequence and an apoB mRNA containing a premature in-frame translational stop codon, in both liver and intestine. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1772–1776. doi: 10.1073/pnas.85.6.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeg J. M., Sviridov D. D., Tennyson G. E., Demosky S. J., Jr, Meng M. S., Bojanovski D., Safonova I. G., Repin V. S., Kuberger M. B., Smirnov V. N. Both apolipoproteins B-48 and B-100 are synthesized and secreted by the human intestine. J Lipid Res. 1990 Oct;31(10):1761–1769. [PubMed] [Google Scholar]
- Hussain M. M., Zannis V. I. Intracellular modification of human apolipoprotein AII (apoAII) and sites of apoAII mRNA synthesis: comparison of apoAII with apoCII and apoCIII isoproteins. Biochemistry. 1990 Jan 9;29(1):209–217. doi: 10.1021/bi00453a029. [DOI] [PubMed] [Google Scholar]
- Kane J. P., Hardman D. A., Paulus H. E. Heterogeneity of apolipoprotein B: isolation of a new species from human chylomicrons. Proc Natl Acad Sci U S A. 1980 May;77(5):2465–2469. doi: 10.1073/pnas.77.5.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft H. G., Menzel H. J., Hoppichler F., Vogel W., Utermann G. Changes of genetic apolipoprotein phenotypes caused by liver transplantation. Implications for apolipoprotein synthesis. J Clin Invest. 1989 Jan;83(1):137–142. doi: 10.1172/JCI113849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levy E., Rochette C., Londono I., Roy C. C., Milne R. W., Marcel Y. L., Bendayan M. Apolipoprotein B-100: immunolocalization and synthesis in human intestinal mucosa. J Lipid Res. 1990 Nov;31(11):1937–1946. [PubMed] [Google Scholar]
- Lewis J. H., Bontempo F. A., Spero J. A., Ragni M. V., Starzl T. E. Liver transplantation in a hemophiliac. N Engl J Med. 1985 May 2;312(18):1189–1190. doi: 10.1056/NEJM198505023121812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Lee Y. C., Kao F. T., Cheung P., Chan L. Apolipoprotein E gene mapping and expression: localization of the structural gene to human chromosome 19 and expression of ApoE mRNA in lipoprotein- and non-lipoprotein-producing tissues. Biochemistry. 1985 Jul 2;24(14):3751–3756. doi: 10.1021/bi00335a050. [DOI] [PubMed] [Google Scholar]
- Lin C. T., Xu Y. F., Wu J. Y., Chan L. Immunoreactive apolipoprotein E is a widely distributed cellular protein. Immunohistochemical localization of apolipoprotein E in baboon tissues. J Clin Invest. 1986 Oct;78(4):947–958. doi: 10.1172/JCI112685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merion R. M., Delius R. E., Campbell D. A., Jr, Turcotte J. G. Orthotopic liver transplantation totally corrects factor IX deficiency in hemophilia B. Surgery. 1988 Nov;104(5):929–931. [PubMed] [Google Scholar]
- Milne R., Théolis R., Jr, Maurice R., Pease R. J., Weech P. K., Rassart E., Fruchart J. C., Scott J., Marcel Y. L. The use of monoclonal antibodies to localize the low density lipoprotein receptor-binding domain of apolipoprotein B. J Biol Chem. 1989 Nov 25;264(33):19754–19760. [PubMed] [Google Scholar]
- Newman T. C., Dawson P. A., Rudel L. L., Williams D. L. Quantitation of apolipoprotein E mRNA in the liver and peripheral tissues of nonhuman primates. J Biol Chem. 1985 Feb 25;260(4):2452–2457. [PubMed] [Google Scholar]
- Pitas R. E., Boyles J. K., Lee S. H., Foss D., Mahley R. W. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim Biophys Acta. 1987 Jan 13;917(1):148–161. doi: 10.1016/0005-2760(87)90295-5. [DOI] [PubMed] [Google Scholar]
- Pitas R. E., Boyles J. K., Lee S. H., Hui D., Weisgraber K. H. Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B,E(LDL) receptors in the brain. J Biol Chem. 1987 Oct 15;262(29):14352–14360. [PubMed] [Google Scholar]
- Powell L. M., Wallis S. C., Pease R. J., Edwards Y. H., Knott T. J., Scott J. A novel form of tissue-specific RNA processing produces apolipoprotein-B48 in intestine. Cell. 1987 Sep 11;50(6):831–840. doi: 10.1016/0092-8674(87)90510-1. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D., Albers J. J., Saunders D. R., Fainaru M. Apoprotein synthesis by human duodenojejunal mucosa. Gastroenterology. 1978 Oct;75(4):677–682. [PubMed] [Google Scholar]
- Roheim P. S., Carey M., Forte T., Vega G. L. Apolipoproteins in human cerebrospinal fluid. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4646–4649. doi: 10.1073/pnas.76.9.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl T. E., Demetris A. J., Van Thiel D. Liver transplantation (2). N Engl J Med. 1989 Oct 19;321(16):1092–1099. doi: 10.1056/NEJM198910193211606. [DOI] [PubMed] [Google Scholar]
- Starzl T. E., Zitelli B. J., Shaw B. W., Jr, Iwatsuki S., Gartner J. C., Gordon R. D., Malatuck J. J., Fox I. J., Urbach A. H., Van Thiel D. H. Changing concepts: liver replacement for hereditary tyrosinemia and hepatoma. J Pediatr. 1985 Apr;106(4):604–606. doi: 10.1016/s0022-3476(85)80081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz A., Jakobs C., Motzny S., Kaffarnik H. Differential distribution of apolipoprotein E isoforms in human plasma lipoproteins. Arteriosclerosis. 1989 May-Jun;9(3):405–411. doi: 10.1161/01.atv.9.3.405. [DOI] [PubMed] [Google Scholar]
- Tikkanen M. J., Ehnholm C., Kovanen P. T., Bütler R., Young S. G., Curtiss L. K., Witztum J. L. Detection of two apolipoprotein B species (apoBc and apoBg) by a monoclonal antibody. Atherosclerosis. 1987 Jun;65(3):247–256. doi: 10.1016/0021-9150(87)90040-2. [DOI] [PubMed] [Google Scholar]
- Weisgraber K. H. Apolipoprotein E distribution among human plasma lipoproteins: role of the cysteine-arginine interchange at residue 112. J Lipid Res. 1990 Aug;31(8):1503–1511. [PubMed] [Google Scholar]
- Weisgraber K. H., Mahley R. W. Apoprotein (E--A-II) complex of human plasma lipoproteins. I. Characterization of this mixed disulfide and its identification in a high density lipoprotein subfraction. J Biol Chem. 1978 Sep 10;253(17):6281–6288. [PubMed] [Google Scholar]
- Wernette-Hammond M. E., Lauer S. J., Corsini A., Walker D., Taylor J. M., Rall S. C., Jr Glycosylation of human apolipoprotein E. The carbohydrate attachment site is threonine 194. J Biol Chem. 1989 May 25;264(15):9094–9101. [PubMed] [Google Scholar]
- Williams D. L., Dawson P. A., Newman T. C., Rudel L. L. Apolipoprotein E synthesis in peripheral tissues of nonhuman primates. J Biol Chem. 1985 Feb 25;260(4):2444–2451. [PubMed] [Google Scholar]
- Young S. G., Bertics S. J., Curtiss L. K., Casal D. C., Witztum J. L. Monoclonal antibody MB19 detects genetic polymorphism in human apolipoprotein B. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1101–1105. doi: 10.1073/pnas.83.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. G., Bertics S. J., Curtiss L. K., Dubois B. W., Witztum J. L. Genetic analysis of a kindred with familial hypobetalipoproteinemia. Evidence for two separate gene defects: one associated with an abnormal apolipoprotein B species, apolipoprotein B-37; and a second associated with low plasma concentrations of apolipoprotein B-100. J Clin Invest. 1987 Jun;79(6):1842–1851. doi: 10.1172/JCI113026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. G., Bertics S. J., Curtiss L. K., Witztum J. L. Characterization of an abnormal species of apolipoprotein B, apolipoprotein B-37, associated with familial hypobetalipoproteinemia. J Clin Invest. 1987 Jun;79(6):1831–1841. doi: 10.1172/JCI113025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. G., Bertics S. J., Scott T. M., Dubois B. W., Beltz W. F., Curtiss L. K., Witztum J. L. Apolipoprotein B allotypes MB19(1) and MB19(2) in subjects with coronary artery disease and hypercholesterolemia. Arteriosclerosis. 1987 Jan-Feb;7(1):61–65. doi: 10.1161/01.atv.7.1.61. [DOI] [PubMed] [Google Scholar]
- Young S. G., Bertics S. J., Scott T. M., Dubois B. W., Curtiss L. K., Witztum J. L. Parallel expression of the MB19 genetic polymorphism in apoprotein B-100 and apoprotein B-48. Evidence that both apoproteins are products of the same gene. J Biol Chem. 1986 Mar 5;261(7):2995–2998. [PubMed] [Google Scholar]
- Young S. G., Hubl S. T. An ApaLI restriction site polymorphism is associated with the MB19 polymorphism in apolipoprotein B. J Lipid Res. 1989 Mar;30(3):443–449. [PubMed] [Google Scholar]
- Young S. G., Peralta F. P., Dubois B. W., Curtiss L. K., Boyles J. K., Witztum J. L. Lipoprotein B37, a naturally occurring lipoprotein containing the amino-terminal portion of apolipoprotein B100, does not bind to the apolipoprotein B,E (low density lipoprotein) receptor. J Biol Chem. 1987 Dec 5;262(34):16604–16611. [PubMed] [Google Scholar]
- Young S. G. Recent progress in understanding apolipoprotein B. Circulation. 1990 Nov;82(5):1574–1594. doi: 10.1161/01.cir.82.5.1574. [DOI] [PubMed] [Google Scholar]