Abstract
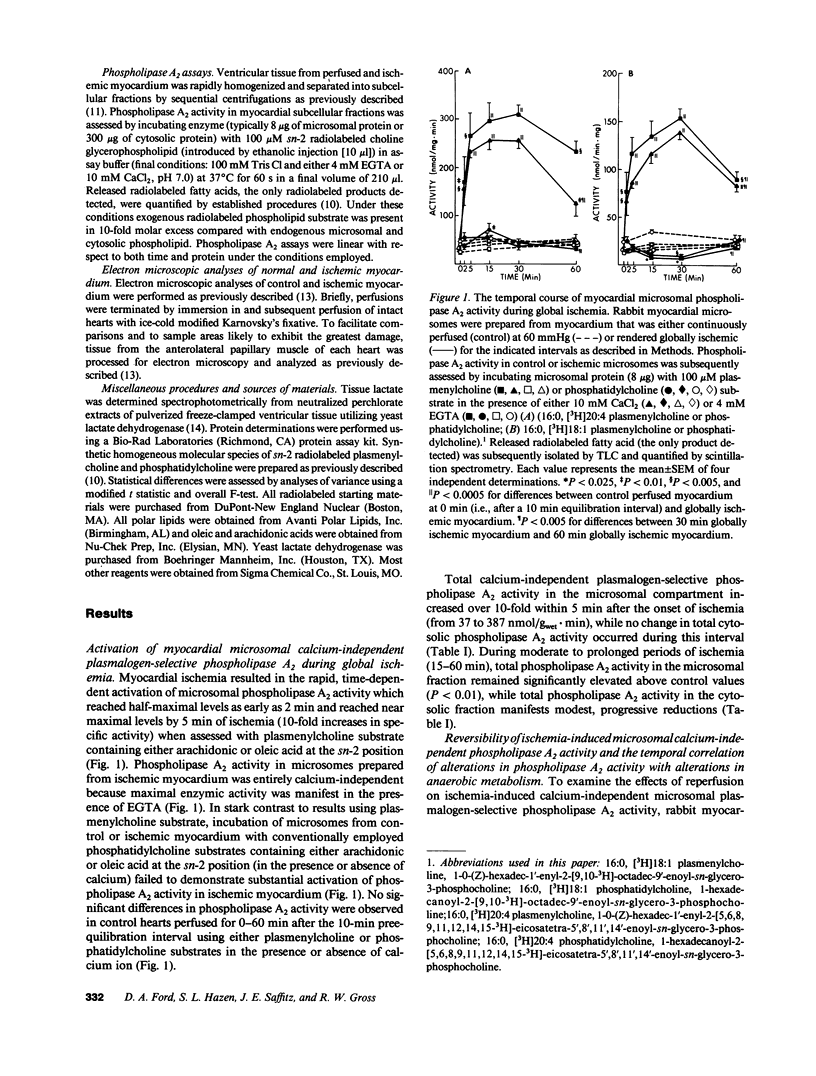
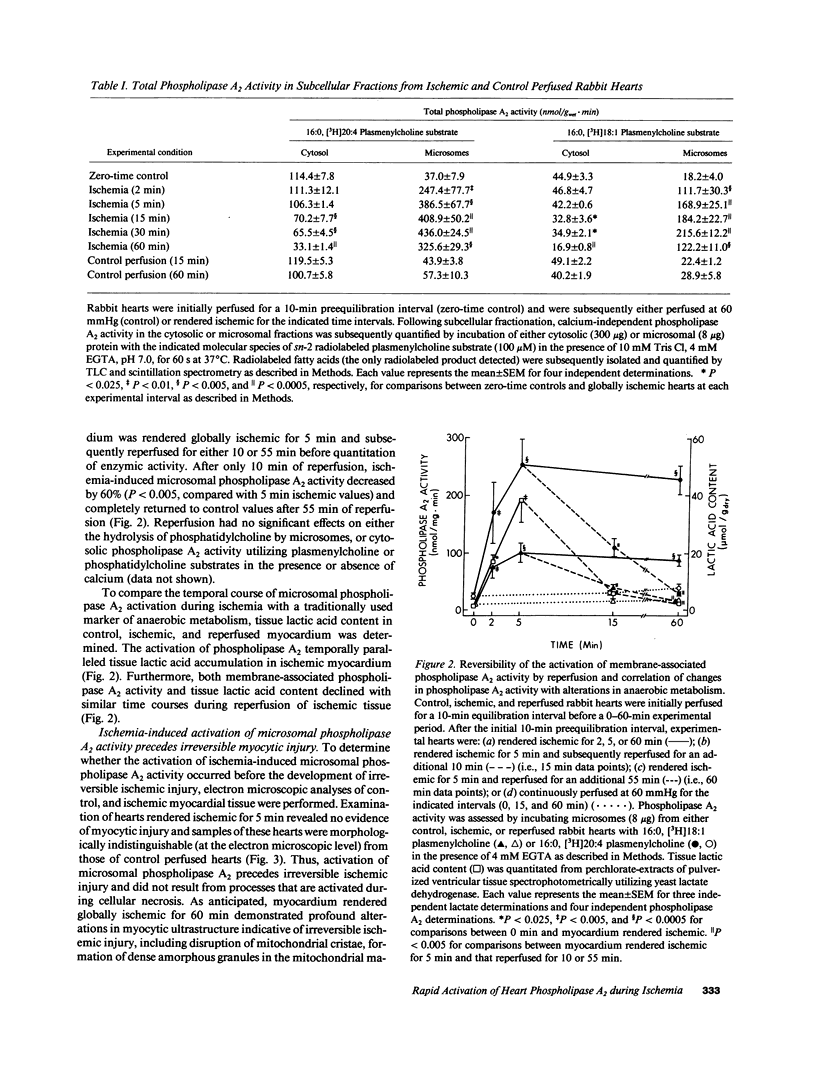
Recent studies have demonstrated the existence of two members of a novel family of calcium-independent plasmalogen-selective phospholipases A2 in mammalian myocardium (Wolf, R. A., and R. W. Gross. 1985. J. Biol. Chem. 260:7295-7303; and Hazen, S. L., D. A. Ford, and R. W. Gross. 1991. J. Biol. Chem. 266:5629-5633). To examine the potential role of these calcium-independent phospholipases A2 in mediating membrane dysfunction during early myocardial ischemia, the temporal course of alterations in phospholipase A2 activity during global ischemia in Langendorf perfused rabbit hearts was quantified and compared with traditionally accepted markers of myocytic ischemic injury and anaerobic metabolism. We now report that membrane-associated calcium-independent plasmalogen-selective phospholipase A2 activity increased over 400% during 2 min of global ischemia (P less than 0.01), was near maximally activated (greater than 10-fold) after only 5 min of ischemia, and remained activated throughout the entire ischemic interval examined (2-60 min). Activation of membrane-associated plasmalogen-selective phospholipase A2 after 5 min of myocardial ischemia was rapidly reversible during reperfusion of ischemic tissue. Both the activation of phospholipase A2 and its reversibility during reperfusion were temporally correlated to alterations in myocytic anaerobic metabolism. Furthermore, activation of membrane-associated phospholipase A2 was essentially complete before electron microscopic evidence of cellular damage. Collectively, these results identify dynamic alterations in calcium-independent plasmalogen-selective phospholipase A2 activity during myocardial ischemia which precede irreversible cellular injury and demonstrate that activation of plasmalogen-selective phospholipase A2 is amongst the earliest biochemical alterations in ischemic myocardium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentham J. M., Higgins A. J., Woodward B. The effects of ischaemia, lysophosphatidylcholine and palmitoylcarnitine on rat heart phospholipase A2 activity. Basic Res Cardiol. 1987;82 (Suppl 1):127–135. doi: 10.1007/978-3-662-08390-1_16. [DOI] [PubMed] [Google Scholar]
- Channon J. Y., Leslie C. C. A calcium-dependent mechanism for associating a soluble arachidonoyl-hydrolyzing phospholipase A2 with membrane in the macrophage cell line RAW 264.7. J Biol Chem. 1990 Apr 5;265(10):5409–5413. [PubMed] [Google Scholar]
- Chien K. R., Han A., Sen A., Buja L. M., Willerson J. T. Accumulation of unesterified arachidonic acid in ischemic canine myocardium. Relationship to a phosphatidylcholine deacylation-reacylation cycle and the depletion of membrane phospholipids. Circ Res. 1984 Mar;54(3):313–322. doi: 10.1161/01.res.54.3.313. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Gross R. W., Sobel B. E. Amphipathic metabolites and membrane dysfunction in ischemic myocardium. Circ Res. 1984 Aug;55(2):135–154. doi: 10.1161/01.res.55.2.135. [DOI] [PubMed] [Google Scholar]
- Downar E., Janse M. J., Durrer D. The effect of acute coronary artery occlusion on subepicardial transmembrane potentials in the intact porcine heart. Circulation. 1977 Aug;56(2):217–224. doi: 10.1161/01.cir.56.2.217. [DOI] [PubMed] [Google Scholar]
- Ford D. A., Gross R. W. Differential accumulation of diacyl and plasmalogenic diglycerides during myocardial ischemia. Circ Res. 1989 Jan;64(1):173–177. doi: 10.1161/01.res.64.1.173. [DOI] [PubMed] [Google Scholar]
- Franson R. C., Weir D. L., Thakkar J. Solubilization and characterization of a neutral-active, calcium-dependent, phospholipase A2 from rabbit heart and isolated chick embryo myocytes. J Mol Cell Cardiol. 1983 Mar;15(3):189–196. doi: 10.1016/0022-2828(83)90298-5. [DOI] [PubMed] [Google Scholar]
- Gronich J. H., Bonventre J. V., Nemenoff R. A. Identification and characterization of a hormonally regulated form of phospholipase A2 in rat renal mesangial cells. J Biol Chem. 1988 Nov 15;263(32):16645–16651. [PubMed] [Google Scholar]
- Gross R. W. High plasmalogen and arachidonic acid content of canine myocardial sarcolemma: a fast atom bombardment mass spectroscopic and gas chromatography-mass spectroscopic characterization. Biochemistry. 1984 Jan 3;23(1):158–165. doi: 10.1021/bi00296a026. [DOI] [PubMed] [Google Scholar]
- Hazen S. L., Ford D. A., Gross R. W. Activation of a membrane-associated phospholipase A2 during rabbit myocardial ischemia which is highly selective for plasmalogen substrate. J Biol Chem. 1991 Mar 25;266(9):5629–5633. [PubMed] [Google Scholar]
- Hazen S. L., Ford D. A., Gross R. W. Activation of a membrane-associated phospholipase A2 during rabbit myocardial ischemia which is highly selective for plasmalogen substrate. J Biol Chem. 1991 Mar 25;266(9):5629–5633. [PubMed] [Google Scholar]
- Hazen S. L., Stuppy R. J., Gross R. W. Purification and characterization of canine myocardial cytosolic phospholipase A2. A calcium-independent phospholipase with absolute f1-2 regiospecificity for diradyl glycerophospholipids. J Biol Chem. 1990 Jun 25;265(18):10622–10630. [PubMed] [Google Scholar]
- Hoyt R. H., Cohen M. L., Saffitz J. E. Distribution and three-dimensional structure of intercellular junctions in canine myocardium. Circ Res. 1989 Mar;64(3):563–574. doi: 10.1161/01.res.64.3.563. [DOI] [PubMed] [Google Scholar]
- Katz A. M., Messineo F. C. Lipid-membrane interactions and the pathogenesis of ischemic damage in the myocardium. Circ Res. 1981 Jan;48(1):1–16. doi: 10.1161/01.res.48.1.1. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y., Gross R. W., Sobel B. E., Saffitz J. E. Selective turnover of sarcolemmal phospholipids with lethal cardiac myocyte injury. Am J Physiol. 1990 Aug;259(2 Pt 1):C325–C331. doi: 10.1152/ajpcell.1990.259.2.C325. [DOI] [PubMed] [Google Scholar]
- Nalbone G., Hostetler K. Y. Subcellular localization of the phospholipases A of rat heart: evidence for a cytosolic phospholipase A1. J Lipid Res. 1985 Jan;26(1):104–114. [PubMed] [Google Scholar]
- Schön R. A simple and sensitive enzymic method for determination of L(+)-lactic acid. Anal Biochem. 1965 Sep;12(3):413–420. doi: 10.1016/0003-2697(65)90208-3. [DOI] [PubMed] [Google Scholar]
- Tam S. W., Man R. Y., Choy P. C. The hydrolysis of phosphatidylcholine by phospholipase A2 in hamster heart. Can J Biochem Cell Biol. 1984 Dec;62(12):1269–1274. doi: 10.1139/o84-161. [DOI] [PubMed] [Google Scholar]
- Wolf R. A., Gross R. W. Identification of neutral active phospholipase C which hydrolyzes choline glycerophospholipids and plasmalogen selective phospholipase A2 in canine myocardium. J Biol Chem. 1985 Jun 25;260(12):7295–7303. [PubMed] [Google Scholar]




