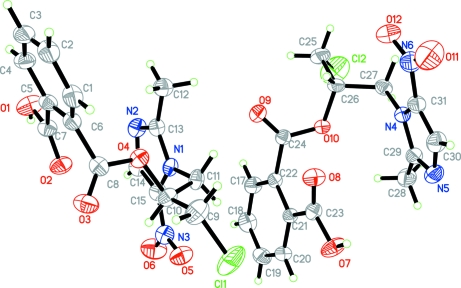
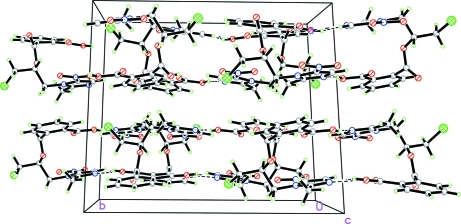
Abstract
The asymmetric unit of the title compound, C15H14ClN3O6, contains two independent molecules. The imidazole rings are oriented with respect to the benzene rings at dihedral angles of 19.66 (3) and 21.64 (3)°. In the crystal structure, intermolecular O—H⋯N hydrogen bonds link the molecules into infinite chains.
Related literature
For bond-length data, see: Allen et al. (1987 ▶).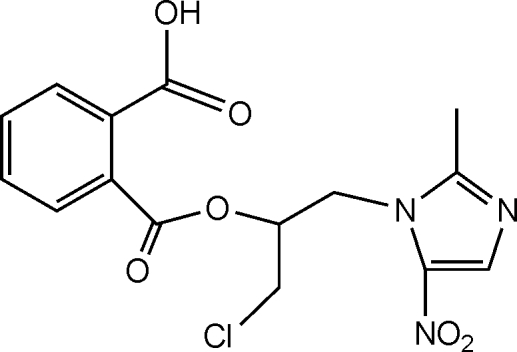
Experimental
Crystal data
C15H14ClN3O6
M r = 367.74
Monoclinic,
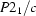
a = 15.214 (3) Å
b = 16.271 (3) Å
c = 15.069 (3) Å
β = 113.86 (3)°
V = 3411.5 (14) Å3
Z = 8
Mo Kα radiation
μ = 0.26 mm−1
T = 294 (2) K
0.40 × 0.30 × 0.20 mm
Data collection
Enraf–Nonius CAD-4 diffractometer
Absorption correction: ψ scan (North et al., 1968 ▶) T min = 0.903, T max = 0.950
6938 measured reflections
6682 independent reflections
3559 reflections with I > 2σ(I)
R int = 0.037
3 standard reflections frequency: 120 min intensity decay: none
Refinement
R[F 2 > 2σ(F 2)] = 0.066
wR(F 2) = 0.175
S = 1.02
6682 reflections
451 parameters
H-atom parameters constrained
Δρmax = 0.41 e Å−3
Δρmin = −0.36 e Å−3
Data collection: CAD-4 Software (Enraf–Nonius, 1989 ▶); cell refinement: CAD-4 Software; data reduction: XCAD4 (Harms & Wocadlo, 1995 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808001268/hk2413sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808001268/hk2413Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1B⋯N5i | 0.82 | 1.81 | 2.623 (3) | 172 |
| O7—H7A⋯N2ii | 0.82 | 1.82 | 2.621 (3) | 166 |
Symmetry codes: (i) 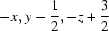 ; (ii)
; (ii) 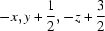 .
.
supplementary crystallographic information
Comment
As part of our ongoing studies, we synthesized the title compound, (I), and report herein its crystal structure.
The asymmetric unit of the title compound, (I), contains two independent molecules (Fig. 1), in which the bond lengths are within normal ranges (Allen et al., 1987).
Rings A (C1–C6), B (N1/N2/C13–C15), C (C17–C22) and D (N4/N5/C29–C31) are, of course, planar and the dihedral angles between them are A/B = 19.66 (3)° and C/D = 21.64 (3)°.
In the crystal structure, intermolecular O—H···N hydrogen bonds (Table 1) link the molecules into infinite chains (Fig. 2), in which they may be effective in the stabilization of the structure.
Experimental
For the preparation of the title conpound, ornidazole (14.6 g, 66 mmol), phthalic anhydride (11.8 g, 80 mmol), acetone (80 ml) and pyridine (6 ml) were added into a three-necked round-bottom flask (250 ml) fitted with a mechanical stirrer and a reflux condensing tube. The mixture was stirred until the solids were completely dissolved, and heated to reflux for about 7 h, and then the reaction was stopped and the mixture was cooled. After filtration of the mixture under vacuum, the colorless deposition was obtained (yield; 18 g, 74%). Suitable crystals for X-ray analysis were obtained by dissolving the title compound (0.1 g) in dry methanol (5 ml), and then allowing the solution to evaporate slowly at room temperature for about 12 d.
Refinement
H atoms were positioned geometrically, with O00—H = 0.82 Å (for OH), C-00H = 0.93, 0.98, 0.97 and 0.96 Å for aromatic, methine, methylene and methyl H, and constrained to ride on their parent atoms, with Uiso(H) = xUeq(C,O), where x = 1.5 for OH and methyl H, and x = 1.2 for all other H atoms.
Figures
Fig. 1.
Ellipsoid plot.
Fig. 2.
Packing diagram.
Crystal data
| C15H14ClN3O6 | F000 = 1520 |
| Mr = 367.74 | Dx = 1.432 Mg m−3 |
| Monoclinic, P21/c | Melting point = 444–447 K |
| Hall symbol: -P 2ybc | Mo Kα radiation λ = 0.71073 Å |
| a = 15.214 (3) Å | Cell parameters from 25 reflections |
| b = 16.271 (3) Å | θ = 10–13º |
| c = 15.069 (3) Å | µ = 0.26 mm−1 |
| β = 113.86 (3)º | T = 294 (2) K |
| V = 3411.5 (14) Å3 | Block, colourless |
| Z = 8 | 0.40 × 0.30 × 0.20 mm |
Data collection
| Enraf–Nonius CAD-4 diffractometer | Rint = 0.037 |
| Radiation source: fine-focus sealed tube | θmax = 26.0º |
| Monochromator: graphite | θmin = 1.5º |
| T = 294(2) K | h = −18→0 |
| ω/2θ scans | k = 0→20 |
| Absorption correction: ψ scan(North et al., 1968) | l = −16→18 |
| Tmin = 0.903, Tmax = 0.950 | 3 standard reflections |
| 6938 measured reflections | every 120 min |
| 6682 independent reflections | intensity decay: none |
| 3559 reflections with I > 2σ(I) |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.066 | H-atom parameters constrained |
| wR(F2) = 0.175 | w = 1/[σ2(Fo2) + (0.07P)2 + 1.25P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.02 | (Δ/σ)max < 0.001 |
| 6682 reflections | Δρmax = 0.41 e Å−3 |
| 451 parameters | Δρmin = −0.36 e Å−3 |
| Primary atom site location: structure-invariant direct methods | Extinction correction: none |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | 0.11705 (11) | 0.43257 (8) | 0.72492 (11) | 0.1116 (6) | |
| Cl2 | −0.38901 (14) | 0.04807 (9) | 0.66545 (14) | 0.1333 (7) | |
| O1 | 0.3761 (2) | 0.03250 (17) | 1.0499 (2) | 0.0784 (9) | |
| H1B | 0.3710 | 0.0047 | 1.0026 | 0.118* | |
| O2 | 0.3562 (2) | 0.13615 (16) | 0.94718 (18) | 0.0613 (7) | |
| O3 | 0.3744 (2) | 0.32764 (19) | 0.96664 (19) | 0.0684 (8) | |
| O4 | 0.23091 (17) | 0.27469 (14) | 0.94386 (16) | 0.0485 (6) | |
| O5 | 0.1328 (2) | 0.2233 (2) | 0.6314 (2) | 0.0748 (8) | |
| O6 | 0.1459 (2) | 0.0990 (2) | 0.5886 (2) | 0.0887 (10) | |
| O7 | −0.1465 (2) | 0.39839 (15) | 0.55531 (19) | 0.0719 (9) | |
| H7A | −0.1519 | 0.4404 | 0.5828 | 0.108* | |
| O8 | −0.1401 (2) | 0.33919 (14) | 0.69075 (19) | 0.0596 (7) | |
| O9 | −0.11021 (18) | 0.16192 (17) | 0.77884 (18) | 0.0592 (7) | |
| O10 | −0.25765 (16) | 0.19193 (14) | 0.66714 (15) | 0.0453 (6) | |
| O11 | −0.3413 (3) | 0.4576 (2) | 0.8743 (2) | 0.1134 (14) | |
| O12 | −0.3404 (2) | 0.3246 (2) | 0.8740 (2) | 0.0832 (10) | |
| N1 | 0.11477 (19) | 0.16366 (16) | 0.7989 (2) | 0.0426 (7) | |
| N2 | 0.1320 (2) | 0.03336 (18) | 0.8448 (2) | 0.0554 (8) | |
| N3 | 0.1364 (2) | 0.1490 (2) | 0.6445 (2) | 0.0592 (9) | |
| N4 | −0.37734 (19) | 0.32555 (17) | 0.6748 (2) | 0.0438 (7) | |
| N5 | −0.3757 (2) | 0.4369 (2) | 0.5904 (2) | 0.0597 (9) | |
| N6 | −0.3466 (3) | 0.3912 (3) | 0.8340 (2) | 0.0684 (10) | |
| C1 | 0.3666 (3) | 0.3017 (3) | 1.1689 (3) | 0.0580 (11) | |
| H1A | 0.3550 | 0.3577 | 1.1582 | 0.070* | |
| C2 | 0.3935 (3) | 0.2701 (3) | 1.2607 (3) | 0.0673 (13) | |
| H2B | 0.3988 | 0.3047 | 1.3117 | 0.081* | |
| C3 | 0.4129 (3) | 0.1871 (3) | 1.2777 (3) | 0.0676 (12) | |
| H3A | 0.4320 | 0.1663 | 1.3402 | 0.081* | |
| C4 | 0.4037 (3) | 0.1351 (3) | 1.2018 (3) | 0.0580 (11) | |
| H4A | 0.4166 | 0.0793 | 1.2133 | 0.070* | |
| C5 | 0.3755 (2) | 0.1661 (2) | 1.1088 (2) | 0.0464 (9) | |
| C6 | 0.3568 (2) | 0.2497 (2) | 1.0920 (2) | 0.0462 (9) | |
| C7 | 0.3675 (3) | 0.1105 (2) | 1.0262 (3) | 0.0498 (9) | |
| C8 | 0.3245 (3) | 0.2879 (2) | 0.9941 (3) | 0.0475 (9) | |
| C9 | 0.1664 (3) | 0.3935 (2) | 0.8436 (3) | 0.0749 (13) | |
| H9A | 0.2255 | 0.4225 | 0.8812 | 0.090* | |
| H9B | 0.1219 | 0.4027 | 0.8740 | 0.090* | |
| C10 | 0.1872 (3) | 0.3018 (2) | 0.8441 (2) | 0.0476 (9) | |
| H10A | 0.2298 | 0.2909 | 0.8110 | 0.057* | |
| C11 | 0.0955 (2) | 0.2518 (2) | 0.7990 (3) | 0.0491 (9) | |
| H11A | 0.0560 | 0.2615 | 0.8349 | 0.059* | |
| H11B | 0.0596 | 0.2701 | 0.7328 | 0.059* | |
| C12 | 0.1003 (3) | 0.1285 (3) | 0.9559 (3) | 0.0616 (11) | |
| H12A | 0.1046 | 0.0788 | 0.9918 | 0.092* | |
| H12B | 0.0378 | 0.1523 | 0.9380 | 0.092* | |
| H12C | 0.1484 | 0.1666 | 0.9954 | 0.092* | |
| C13 | 0.1158 (2) | 0.1095 (2) | 0.8677 (3) | 0.0465 (9) | |
| C14 | 0.1408 (3) | 0.0381 (2) | 0.7593 (3) | 0.0569 (10) | |
| H14A | 0.1517 | −0.0061 | 0.7259 | 0.068* | |
| C15 | 0.1311 (3) | 0.1176 (2) | 0.7298 (3) | 0.0477 (9) | |
| C17 | −0.1188 (3) | 0.1073 (2) | 0.5763 (3) | 0.0552 (10) | |
| H17A | −0.1214 | 0.0583 | 0.6069 | 0.066* | |
| C18 | −0.0988 (3) | 0.1060 (3) | 0.4947 (3) | 0.0643 (11) | |
| H18A | −0.0887 | 0.0560 | 0.4703 | 0.077* | |
| C19 | −0.0939 (3) | 0.1783 (3) | 0.4494 (3) | 0.0594 (11) | |
| H19A | −0.0811 | 0.1773 | 0.3940 | 0.071* | |
| C20 | −0.1079 (2) | 0.2521 (2) | 0.4867 (2) | 0.0477 (9) | |
| H20A | −0.1032 | 0.3009 | 0.4568 | 0.057* | |
| C21 | −0.1288 (2) | 0.2548 (2) | 0.5676 (2) | 0.0389 (8) | |
| C22 | −0.1352 (2) | 0.1814 (2) | 0.6130 (2) | 0.0399 (8) | |
| C23 | −0.1395 (3) | 0.3347 (2) | 0.6110 (3) | 0.0459 (9) | |
| C24 | −0.1631 (3) | 0.1788 (2) | 0.6968 (3) | 0.0428 (8) | |
| C25 | −0.3119 (3) | 0.1052 (3) | 0.7666 (3) | 0.0737 (13) | |
| H25A | −0.3391 | 0.1072 | 0.8147 | 0.088* | |
| H25B | −0.2503 | 0.0777 | 0.7955 | 0.088* | |
| C26 | −0.2968 (3) | 0.1925 (2) | 0.7394 (3) | 0.0491 (9) | |
| H26A | −0.2528 | 0.2216 | 0.7972 | 0.059* | |
| C27 | −0.3904 (2) | 0.2392 (2) | 0.6947 (3) | 0.0490 (9) | |
| H27A | −0.4314 | 0.2125 | 0.6345 | 0.059* | |
| H27B | −0.4229 | 0.2368 | 0.7384 | 0.059* | |
| C28 | −0.4060 (3) | 0.3050 (3) | 0.5004 (3) | 0.0655 (11) | |
| H28A | −0.4093 | 0.3398 | 0.4476 | 0.098* | |
| H28B | −0.4660 | 0.2767 | 0.4832 | 0.098* | |
| H28C | −0.3553 | 0.2656 | 0.5136 | 0.098* | |
| C29 | −0.3866 (3) | 0.3556 (2) | 0.5877 (3) | 0.0494 (9) | |
| C30 | −0.3610 (3) | 0.4604 (3) | 0.6814 (3) | 0.0618 (11) | |
| H30A | −0.3517 | 0.5142 | 0.7039 | 0.074* | |
| C31 | −0.3617 (3) | 0.3934 (2) | 0.7348 (3) | 0.0508 (9) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.1335 (12) | 0.0557 (8) | 0.1106 (11) | 0.0032 (8) | 0.0131 (9) | 0.0320 (7) |
| Cl2 | 0.1632 (15) | 0.0625 (9) | 0.1512 (15) | −0.0410 (9) | 0.0400 (12) | 0.0081 (9) |
| O1 | 0.128 (3) | 0.0514 (19) | 0.0574 (18) | −0.0074 (17) | 0.0396 (18) | −0.0063 (14) |
| O2 | 0.085 (2) | 0.0620 (18) | 0.0400 (15) | 0.0030 (15) | 0.0285 (14) | −0.0037 (13) |
| O3 | 0.0697 (19) | 0.086 (2) | 0.0548 (17) | −0.0269 (16) | 0.0310 (15) | −0.0091 (15) |
| O4 | 0.0524 (16) | 0.0461 (14) | 0.0426 (14) | −0.0039 (12) | 0.0146 (12) | −0.0052 (11) |
| O5 | 0.099 (2) | 0.060 (2) | 0.0677 (19) | −0.0005 (17) | 0.0358 (17) | 0.0091 (16) |
| O6 | 0.124 (3) | 0.083 (2) | 0.078 (2) | −0.011 (2) | 0.059 (2) | −0.0271 (19) |
| O7 | 0.126 (3) | 0.0319 (15) | 0.0609 (17) | 0.0040 (15) | 0.0406 (17) | 0.0059 (13) |
| O8 | 0.093 (2) | 0.0387 (15) | 0.0592 (17) | −0.0026 (13) | 0.0427 (16) | −0.0068 (13) |
| O9 | 0.0560 (16) | 0.0704 (19) | 0.0476 (16) | 0.0104 (14) | 0.0173 (13) | 0.0095 (14) |
| O10 | 0.0485 (15) | 0.0482 (14) | 0.0430 (13) | 0.0016 (12) | 0.0225 (12) | 0.0073 (11) |
| O11 | 0.165 (4) | 0.093 (3) | 0.071 (2) | 0.024 (3) | 0.037 (2) | −0.026 (2) |
| O12 | 0.098 (2) | 0.098 (3) | 0.0586 (19) | 0.004 (2) | 0.0372 (18) | 0.0143 (18) |
| N1 | 0.0440 (17) | 0.0327 (16) | 0.0447 (17) | 0.0032 (13) | 0.0115 (13) | −0.0036 (14) |
| N2 | 0.066 (2) | 0.0311 (17) | 0.066 (2) | −0.0015 (15) | 0.0234 (17) | −0.0005 (15) |
| N3 | 0.061 (2) | 0.056 (2) | 0.056 (2) | −0.0040 (18) | 0.0196 (17) | −0.0071 (19) |
| N4 | 0.0451 (17) | 0.0426 (18) | 0.0454 (17) | 0.0005 (14) | 0.0199 (14) | 0.0039 (14) |
| N5 | 0.067 (2) | 0.050 (2) | 0.061 (2) | 0.0025 (17) | 0.0253 (18) | 0.0116 (17) |
| N6 | 0.071 (2) | 0.082 (3) | 0.049 (2) | 0.007 (2) | 0.0225 (18) | −0.006 (2) |
| C1 | 0.053 (2) | 0.070 (3) | 0.049 (2) | 0.003 (2) | 0.0197 (19) | −0.023 (2) |
| C2 | 0.056 (3) | 0.103 (4) | 0.042 (2) | −0.002 (3) | 0.0194 (19) | −0.029 (2) |
| C3 | 0.058 (3) | 0.109 (4) | 0.037 (2) | −0.006 (3) | 0.0205 (19) | −0.007 (2) |
| C4 | 0.062 (3) | 0.073 (3) | 0.042 (2) | −0.013 (2) | 0.0236 (19) | −0.003 (2) |
| C5 | 0.043 (2) | 0.060 (3) | 0.038 (2) | −0.0115 (18) | 0.0194 (16) | −0.0109 (18) |
| C6 | 0.042 (2) | 0.057 (2) | 0.040 (2) | −0.0054 (18) | 0.0172 (16) | −0.0101 (18) |
| C7 | 0.057 (2) | 0.052 (2) | 0.043 (2) | −0.0065 (19) | 0.0218 (18) | −0.0092 (19) |
| C8 | 0.051 (2) | 0.047 (2) | 0.047 (2) | −0.0061 (18) | 0.0213 (19) | −0.0142 (18) |
| C9 | 0.098 (3) | 0.039 (2) | 0.080 (3) | 0.005 (2) | 0.027 (3) | −0.001 (2) |
| C10 | 0.061 (2) | 0.0322 (19) | 0.047 (2) | 0.0016 (17) | 0.0196 (19) | −0.0002 (16) |
| C11 | 0.047 (2) | 0.034 (2) | 0.058 (2) | 0.0078 (16) | 0.0130 (18) | −0.0016 (17) |
| C12 | 0.068 (3) | 0.061 (3) | 0.059 (3) | 0.001 (2) | 0.029 (2) | 0.001 (2) |
| C13 | 0.046 (2) | 0.040 (2) | 0.052 (2) | −0.0008 (17) | 0.0182 (18) | −0.0039 (18) |
| C14 | 0.066 (3) | 0.040 (2) | 0.063 (3) | −0.0031 (19) | 0.025 (2) | −0.0110 (19) |
| C15 | 0.053 (2) | 0.036 (2) | 0.052 (2) | −0.0026 (17) | 0.0184 (18) | −0.0069 (18) |
| C17 | 0.071 (3) | 0.035 (2) | 0.063 (3) | 0.0029 (19) | 0.031 (2) | −0.0004 (18) |
| C18 | 0.077 (3) | 0.047 (2) | 0.072 (3) | 0.003 (2) | 0.033 (2) | −0.019 (2) |
| C19 | 0.064 (3) | 0.069 (3) | 0.055 (2) | −0.001 (2) | 0.033 (2) | −0.010 (2) |
| C20 | 0.054 (2) | 0.048 (2) | 0.045 (2) | −0.0027 (18) | 0.0246 (18) | 0.0026 (17) |
| C21 | 0.0421 (19) | 0.0359 (19) | 0.0389 (19) | 0.0000 (16) | 0.0165 (16) | 0.0000 (15) |
| C22 | 0.041 (2) | 0.0353 (19) | 0.044 (2) | −0.0007 (16) | 0.0175 (16) | −0.0013 (15) |
| C23 | 0.052 (2) | 0.034 (2) | 0.051 (2) | −0.0003 (16) | 0.0193 (18) | −0.0002 (17) |
| C24 | 0.048 (2) | 0.0344 (19) | 0.047 (2) | −0.0010 (17) | 0.0209 (18) | 0.0019 (16) |
| C25 | 0.091 (3) | 0.064 (3) | 0.079 (3) | 0.007 (3) | 0.048 (3) | 0.028 (2) |
| C26 | 0.056 (2) | 0.050 (2) | 0.050 (2) | −0.0022 (18) | 0.0307 (19) | 0.0088 (18) |
| C27 | 0.049 (2) | 0.047 (2) | 0.057 (2) | −0.0090 (18) | 0.0280 (19) | 0.0007 (18) |
| C28 | 0.074 (3) | 0.073 (3) | 0.047 (2) | 0.008 (2) | 0.022 (2) | −0.002 (2) |
| C29 | 0.046 (2) | 0.059 (3) | 0.042 (2) | 0.0018 (19) | 0.0163 (17) | 0.0036 (18) |
| C30 | 0.070 (3) | 0.045 (2) | 0.071 (3) | 0.001 (2) | 0.028 (2) | 0.000 (2) |
| C31 | 0.054 (2) | 0.055 (2) | 0.044 (2) | 0.0006 (19) | 0.0203 (18) | −0.0033 (19) |
Geometric parameters (Å, °)
| Cl1—C9 | 1.755 (4) | C9—C10 | 1.524 (5) |
| Cl2—C25 | 1.765 (5) | C9—H9A | 0.9700 |
| O1—C7 | 1.311 (4) | C9—H9B | 0.9700 |
| O1—H1B | 0.8200 | C10—C11 | 1.517 (5) |
| O2—C7 | 1.207 (4) | C10—H10A | 0.9800 |
| O3—C8 | 1.191 (4) | C11—H11A | 0.9700 |
| O4—C8 | 1.333 (4) | C11—H11B | 0.9700 |
| O4—C10 | 1.446 (4) | C12—C13 | 1.474 (5) |
| O5—N3 | 1.222 (4) | C12—H12A | 0.9600 |
| O6—N3 | 1.221 (4) | C12—H12B | 0.9600 |
| O7—C23 | 1.310 (4) | C12—H12C | 0.9600 |
| O7—H7A | 0.8200 | C14—C15 | 1.357 (5) |
| O8—C23 | 1.207 (4) | C14—H14A | 0.9300 |
| O9—C24 | 1.203 (4) | C15—N3 | 1.416 (5) |
| O10—C24 | 1.340 (4) | C17—C18 | 1.381 (5) |
| O10—C26 | 1.436 (4) | C17—C22 | 1.390 (5) |
| O11—N6 | 1.226 (5) | C17—H17A | 0.9300 |
| O12—N6 | 1.225 (4) | C18—C19 | 1.378 (6) |
| N1—C13 | 1.356 (4) | C18—H18A | 0.9300 |
| N1—C15 | 1.383 (4) | C19—C20 | 1.379 (5) |
| N1—C11 | 1.465 (4) | C19—H19A | 0.9300 |
| N2—C13 | 1.336 (4) | C20—C21 | 1.379 (5) |
| N2—C14 | 1.351 (5) | C20—H20A | 0.9300 |
| N4—C29 | 1.353 (4) | C21—C22 | 1.398 (4) |
| N4—C31 | 1.385 (4) | C21—C23 | 1.494 (5) |
| N4—C27 | 1.468 (4) | C22—C24 | 1.486 (5) |
| N5—C29 | 1.331 (5) | C25—C26 | 1.522 (5) |
| N5—C30 | 1.351 (5) | C25—H25A | 0.9700 |
| C1—C2 | 1.373 (6) | C25—H25B | 0.9700 |
| C1—C6 | 1.394 (5) | C26—C27 | 1.511 (5) |
| C1—H1A | 0.9300 | C26—H26A | 0.9800 |
| C2—C3 | 1.384 (6) | C27—H27A | 0.9700 |
| C2—H2B | 0.9300 | C27—H27B | 0.9700 |
| C3—C4 | 1.384 (5) | C28—C29 | 1.478 (5) |
| C3—H3A | 0.9300 | C28—H28A | 0.9600 |
| C4—C5 | 1.383 (5) | C28—H28B | 0.9600 |
| C4—H4A | 0.9300 | C28—H28C | 0.9600 |
| C5—C6 | 1.392 (5) | C30—C31 | 1.357 (5) |
| C5—C7 | 1.503 (5) | C30—H30A | 0.9300 |
| C6—C8 | 1.489 (5) | C31—N6 | 1.419 (5) |
| C7—O1—H1B | 109.5 | N2—C13—N1 | 110.6 (3) |
| C8—O4—C10 | 118.6 (3) | N2—C13—C12 | 122.7 (3) |
| C23—O7—H7A | 109.5 | N1—C13—C12 | 126.7 (3) |
| C24—O10—C26 | 117.8 (3) | N2—C14—C15 | 109.1 (3) |
| C13—N1—C15 | 105.9 (3) | N2—C14—H14A | 125.5 |
| C13—N1—C11 | 125.1 (3) | C15—C14—H14A | 125.5 |
| C15—N1—C11 | 128.9 (3) | C14—C15—N1 | 107.4 (3) |
| C13—N2—C14 | 107.0 (3) | C14—C15—N3 | 127.0 (3) |
| O6—N3—O5 | 123.8 (4) | N1—C15—N3 | 125.6 (3) |
| O6—N3—C15 | 116.9 (3) | C18—C17—C22 | 120.5 (4) |
| O5—N3—C15 | 119.2 (3) | C18—C17—H17A | 119.8 |
| C29—N4—C31 | 105.5 (3) | C22—C17—H17A | 119.8 |
| C29—N4—C27 | 125.1 (3) | C19—C18—C17 | 120.3 (4) |
| C31—N4—C27 | 129.1 (3) | C19—C18—H18A | 119.9 |
| C29—N5—C30 | 106.3 (3) | C17—C18—H18A | 119.9 |
| O12—N6—O11 | 124.0 (4) | C18—C19—C20 | 119.5 (3) |
| O12—N6—C31 | 119.2 (4) | C18—C19—H19A | 120.2 |
| O11—N6—C31 | 116.8 (4) | C20—C19—H19A | 120.2 |
| C2—C1—C6 | 119.9 (4) | C19—C20—C21 | 121.1 (3) |
| C2—C1—H1A | 120.1 | C19—C20—H20A | 119.4 |
| C6—C1—H1A | 120.1 | C21—C20—H20A | 119.4 |
| C1—C2—C3 | 120.4 (4) | C20—C21—C22 | 119.5 (3) |
| C1—C2—H2B | 119.8 | C20—C21—C23 | 121.3 (3) |
| C3—C2—H2B | 119.8 | C22—C21—C23 | 119.2 (3) |
| C4—C3—C2 | 120.0 (4) | C17—C22—C21 | 119.1 (3) |
| C4—C3—H3A | 120.0 | C17—C22—C24 | 118.1 (3) |
| C2—C3—H3A | 120.0 | C21—C22—C24 | 122.7 (3) |
| C5—C4—C3 | 120.0 (4) | O8—C23—O7 | 123.9 (3) |
| C5—C4—H4A | 120.0 | O8—C23—C21 | 122.3 (3) |
| C3—C4—H4A | 120.0 | O7—C23—C21 | 113.8 (3) |
| C4—C5—C6 | 119.8 (3) | O9—C24—O10 | 124.1 (3) |
| C4—C5—C7 | 120.5 (4) | O9—C24—C22 | 125.3 (3) |
| C6—C5—C7 | 119.7 (3) | O10—C24—C22 | 110.4 (3) |
| C5—C6—C1 | 119.8 (3) | C26—C25—Cl2 | 112.3 (3) |
| C5—C6—C8 | 123.3 (3) | C26—C25—H25A | 109.1 |
| C1—C6—C8 | 116.9 (4) | Cl2—C25—H25A | 109.1 |
| O2—C7—O1 | 124.2 (3) | C26—C25—H25B | 109.1 |
| O2—C7—C5 | 122.8 (4) | Cl2—C25—H25B | 109.1 |
| O1—C7—C5 | 113.0 (3) | H25A—C25—H25B | 107.9 |
| O3—C8—O4 | 125.1 (4) | O10—C26—C27 | 105.7 (3) |
| O3—C8—C6 | 124.7 (3) | O10—C26—C25 | 110.6 (3) |
| O4—C8—C6 | 110.1 (3) | C27—C26—C25 | 111.7 (3) |
| C10—C9—Cl1 | 111.2 (3) | O10—C26—H26A | 109.6 |
| C10—C9—H9A | 109.4 | C27—C26—H26A | 109.6 |
| Cl1—C9—H9A | 109.4 | C25—C26—H26A | 109.6 |
| C10—C9—H9B | 109.4 | N4—C27—C26 | 113.0 (3) |
| Cl1—C9—H9B | 109.4 | N4—C27—H27A | 109.0 |
| H9A—C9—H9B | 108.0 | C26—C27—H27A | 109.0 |
| O4—C10—C11 | 104.8 (3) | N4—C27—H27B | 109.0 |
| O4—C10—C9 | 108.1 (3) | C26—C27—H27B | 109.0 |
| C11—C10—C9 | 111.8 (3) | H27A—C27—H27B | 107.8 |
| O4—C10—H10A | 110.7 | C29—C28—H28A | 109.5 |
| C11—C10—H10A | 110.7 | C29—C28—H28B | 109.5 |
| C9—C10—H10A | 110.7 | H28A—C28—H28B | 109.5 |
| N1—C11—C10 | 112.1 (3) | C29—C28—H28C | 109.5 |
| N1—C11—H11A | 109.2 | H28A—C28—H28C | 109.5 |
| C10—C11—H11A | 109.2 | H28B—C28—H28C | 109.5 |
| N1—C11—H11B | 109.2 | N5—C29—N4 | 111.5 (3) |
| C10—C11—H11B | 109.2 | N5—C29—C28 | 124.0 (4) |
| H11A—C11—H11B | 107.9 | N4—C29—C28 | 124.5 (4) |
| C13—C12—H12A | 109.5 | N5—C30—C31 | 109.7 (4) |
| C13—C12—H12B | 109.5 | N5—C30—H30A | 125.2 |
| H12A—C12—H12B | 109.5 | C31—C30—H30A | 125.2 |
| C13—C12—H12C | 109.5 | C30—C31—N4 | 107.1 (3) |
| H12A—C12—H12C | 109.5 | C30—C31—N6 | 127.4 (4) |
| H12B—C12—H12C | 109.5 | N4—C31—N6 | 125.5 (4) |
| C10—O4—C8—O3 | 7.7 (5) | C6—C5—C7—O2 | 8.8 (5) |
| C10—O4—C8—C6 | −175.5 (3) | C4—C5—C7—O1 | 9.1 (5) |
| C8—O4—C10—C11 | 159.8 (3) | C6—C5—C7—O1 | −172.6 (3) |
| C8—O4—C10—C9 | −80.9 (4) | C5—C6—C8—O3 | −104.2 (5) |
| C26—O10—C24—O9 | 5.3 (5) | C1—C6—C8—O3 | 76.8 (5) |
| C26—O10—C24—C22 | −179.2 (3) | C5—C6—C8—O4 | 78.9 (4) |
| C24—O10—C26—C27 | 158.9 (3) | C1—C6—C8—O4 | −100.0 (4) |
| C24—O10—C26—C25 | −80.0 (4) | Cl1—C9—C10—O4 | 177.8 (3) |
| C13—N1—C11—C10 | 94.9 (4) | Cl1—C9—C10—C11 | −67.4 (4) |
| C15—N1—C11—C10 | −88.2 (4) | O4—C10—C11—N1 | −63.4 (4) |
| C15—N1—C13—N2 | 0.1 (4) | C9—C10—C11—N1 | 179.8 (3) |
| C11—N1—C13—N2 | 177.6 (3) | N2—C14—C15—N1 | −0.6 (4) |
| C15—N1—C13—C12 | −179.0 (3) | N2—C14—C15—N3 | 179.4 (3) |
| C11—N1—C13—C12 | −1.5 (6) | C14—C15—N3—O6 | 4.0 (6) |
| C13—N1—C15—C14 | 0.3 (4) | N1—C15—N3—O6 | −176.0 (3) |
| C11—N1—C15—C14 | −177.1 (3) | C14—C15—N3—O5 | −174.9 (4) |
| C13—N1—C15—N3 | −179.7 (3) | N1—C15—N3—O5 | 5.1 (6) |
| C11—N1—C15—N3 | 3.0 (6) | C22—C17—C18—C19 | 0.7 (6) |
| C14—N2—C13—N1 | −0.5 (4) | C18—C17—C22—C21 | −1.6 (5) |
| C14—N2—C13—C12 | 178.7 (3) | C18—C17—C22—C24 | 175.8 (3) |
| C13—N2—C14—C15 | 0.7 (4) | C17—C18—C19—C20 | 0.8 (6) |
| C29—N4—C27—C26 | 97.5 (4) | C18—C19—C20—C21 | −1.3 (6) |
| C31—N4—C27—C26 | −88.5 (4) | C19—C20—C21—C22 | 0.4 (5) |
| C31—N4—C29—N5 | 1.3 (4) | C19—C20—C21—C23 | 177.1 (3) |
| C27—N4—C29—N5 | 176.5 (3) | C20—C21—C22—C17 | 1.0 (5) |
| C31—N4—C29—C28 | −178.8 (3) | C23—C21—C22—C17 | −175.7 (3) |
| C27—N4—C29—C28 | −3.6 (5) | C20—C21—C22—C24 | −176.3 (3) |
| C29—N4—C31—C30 | −0.9 (4) | C23—C21—C22—C24 | 7.0 (5) |
| C27—N4—C31—C30 | −175.8 (3) | C20—C21—C23—O8 | −166.0 (3) |
| C29—N4—C31—N6 | −179.1 (3) | C22—C21—C23—O8 | 10.7 (5) |
| C27—N4—C31—N6 | 5.9 (6) | C20—C21—C23—O7 | 12.9 (5) |
| C30—N5—C29—N4 | −1.3 (4) | C22—C21—C23—O7 | −170.4 (3) |
| C30—N5—C29—C28 | 178.9 (4) | C17—C22—C24—O9 | 72.7 (5) |
| C29—N5—C30—C31 | 0.7 (4) | C21—C22—C24—O9 | −110.0 (4) |
| C6—C1—C2—C3 | 1.3 (6) | C17—C22—C24—O10 | −102.8 (4) |
| C2—C1—C6—C5 | −0.8 (5) | C21—C22—C24—O10 | 74.6 (4) |
| C2—C1—C6—C8 | 178.2 (3) | Cl2—C25—C26—O10 | −58.1 (4) |
| C1—C2—C3—C4 | −1.0 (6) | Cl2—C25—C26—C27 | 59.4 (4) |
| C2—C3—C4—C5 | 0.2 (6) | O10—C26—C27—N4 | −64.1 (4) |
| C3—C4—C5—C6 | 0.3 (5) | C25—C26—C27—N4 | 175.5 (3) |
| C3—C4—C5—C7 | 178.6 (3) | N5—C30—C31—N4 | 0.1 (4) |
| C4—C5—C6—C1 | 0.0 (5) | N5—C30—C31—N6 | 178.3 (3) |
| C7—C5—C6—C1 | −178.3 (3) | C30—C31—N6—O12 | −172.9 (4) |
| C4—C5—C6—C8 | −178.9 (3) | N4—C31—N6—O12 | 5.0 (6) |
| C7—C5—C6—C8 | 2.8 (5) | C30—C31—N6—O11 | 7.5 (6) |
| C4—C5—C7—O2 | −169.5 (4) | N4—C31—N6—O11 | −174.5 (4) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1B···N5i | 0.82 | 1.81 | 2.623 (3) | 172 |
| O7—H7A···N2ii | 0.82 | 1.82 | 2.621 (3) | 166 |
Symmetry codes: (i) −x, y−1/2, −z+3/2; (ii) −x, y+1/2, −z+3/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HK2413).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Enraf–Nonius (1989). CAD-4 Software Version 5. Enraf–Nonius, Delft, The Netherlands.
- Harms, K. & Wocadlo, S. (1995). XCAD4 University of Marburg, Germany.
- North, A. C. T., Phillips, D. C. & Mathews, F. S. (1968). Acta Cryst. A24, 351–359.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808001268/hk2413sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808001268/hk2413Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report