Abstract
Dendritic cells (DCs) play crucial roles in innate and adaptive immune responses, rendering them critical targets for virus infections. Porcine circovirus type 2 (PCV2) is associated with the development of postweaning multisystemic wasting syndrome (PMWS) in piglets. We demonstrate here that 80 to 90% of monocyte-derived and bone marrow-derived DCs interact with PCV2 similar to the early stages of an infection. There was no evidence for virus replication, but the virus did persist in DCs without loss of infectivity nor the induction of cell death. This could reflect an abortive infection, but there was no evidence of virus uncoating—the infectivity remained intact for at least 5 days. Alternatively, the results may reflect DC endocytosis of antigenic material. However, there was no modulation of DC surface major histocompatibility complex class I and class II, CD80/86, CD25, CD16, or CD14. Furthermore, infected DC did not transmit virus to syngeneic T lymphocytes, even when the latter were activated. Such coculture did not induce PCV2 replication or death of the lymphocytes or DCs. These results demonstrate that PCV2 can persist in DCs in the absence of virus replication or degradation. Such a silent virus infection presents a novel mechanism of not only immune evasion but also escaping the DC degradation pathway. Because of their migratory capacity, infection of DCs thus provides a potent vehicle for transport of the virus throughout the host without the need for replication. In addition, the lymphopenia seen in PMWS is not a direct effect of the virus on lymphocytes but would require additional events, as proposed by others.
Dendritic cells (DCs) are the most potent antigen-presenting cells of the immune system, playing a pivotal role in inducing a protective immunity against viral infection (4). As sentinels of the immune system, DCs are an ideal target for immune evasion by viruses (23). A number of viruses infect DCs, modulating immune responsiveness after infection, with or without virus replication (23). The range of mechanisms leading to immune evasion by the virus include inhibition (vaccinia virus) (13), induction of DC maturation (Dengue virus) (21), modulation of cytokine production (murine cytomegalovirus) (3), impairment of antigen presentation capacity (measles virus) (16), and virus transmission to lymphocytes (human immunodeficiency virus) (41). Studies on virus life cycles in DCs has led to a greater appreciation of the role of DCs in both protective and pathogenic aspects of viral infection (6).
DC-tropic viruses can be found among most families of double-stranded DNA (dsDNA) and single-stranded RNA viruses. In contrast, little is known about circular single-stranded DNA (ssDNA) viruses, such as porcine circovirus type 2 (PCV2) (18, 36) and the human Teno Torque Virus (38). PCV2 is the causative agent of postweaning multisystemic wasting syndrome (PMWS), affecting 5- to 12-week-old piglets (2, 10, 12). Outbreaks of PMWS have now been reported worldwide and are associated with significant mortality rates in nursery and fattening pigs (2, 26, 46). Although Koch's postulates have been fulfilled, the development and the severity of PMWS are closely linked to other factors, including other infectious agents and the immune status of the animal (30, 31). PMWS is characterized by weight loss, dyspnea, and jaundice, combined with the pathological findings of interstitial pneumonia, generalized enlarged lymph nodes, hepatitis, and nephritis (1, 31, 32). Microscopically, the most distinctive lesions in affected pigs are the lymphocyte depletion and histiocytic infiltration in the lymphoid organs (31, 43, 45). Inclusion bodies containing PCV2 antigen can be identified histologically in the cytoplasm of histiocytes, particularly in macrophages within lesions of various organs from PMWS-affected pigs; virus antigen-positive cells of DC-like morphology and peripheral monocytes have also been reported (24, 25, 30, 42). Although the tropism of PCV2 appeared to be for cells of the monocytic lineage, only nonproductive infection of macrophages in vitro has been demonstrated (15). PCV2 can induced a lymphopenia, involving an early loss of B lymphocytes, concomitant with depletion of helper (both naive and memory/activated), cytotoxic, and γδ T cells, as well as natural killer cells (37). Despite these observations, virus antigen has not been clearly demonstrated in lymphocytes (11, 42, 45, 47). It has been reported that virus impairment of DC function can have consequences on T-lymphocyte survival and anergy (23).
Consequently, we sought to investigate the interaction of PCV2 with DCs, since this could have implications for the pathogenesis of PMWS. The susceptibility of DCs to PCV2 and the consequences of the infection on DC-T-lymphocyte interactions were studied. For this purpose, bone marrow-derived DCs (BMDCs) and monocyte-derived DCs (MoDCs) were infected with PCV2, and infection was monitored by confocal microscopy and flow cytometry. Kinetic studies were conducted for the detection of virus replication, through the analysis of virus progeny and dsDNA replicative intermediate production. Finally, infected DC were cocultured with syngeneic lymphocytes for transinfection and viability assays.
MATERIALS AND METHODS
Animals.
Swiss White Landrace pigs, shown previously to be seronegative for anti-PCV2 antibody and kept under specific-pathogen-free (SPF) conditions at the institute, were used throughout the present study.
Bone marrow and monocytic cell preparation and culture. (i) BMHCs. Bone marrow hematopoietic cells (BMHCs) were isolated from the sternums of SPF pigs as previously described (49). Briefly, each sternum was flushed with phosphate-buffered saline (PBS)-0.03% (wt/vol) EDTA at 37°C, and BMHCs were obtained by centrifugation at 1,000 × g for 40 min at room temperature over Ficoll-Paque (1.077 g/liter; Amersham Pharmacia Biotech AG, Dübendorf, Switzerland). BMHCs were cultured in phenol red-free Dulbecco modified Eagle medium (Gibco-BRL/Life Technology AG, Basel, Switzerland), supplemented with 100 U of penicillin/ml, 100 μg of streptomycin/ml, and porcine serum (10% [vol/vol]; obtained from SPF pigs). BMDCs were generated from BMHCs as previously described (9). Briefly, BMHCs were cultured for 8 to 9 days with 25 ng of recombinant porcine granulocyte-macrophage colony-stimulating factor (GM-CSF; kindly provided by S. Inumaru [22])/ml in combination with 30 U of recombinant porcine tumor necrosis factor alpha (kindly provided by B. Von Niederhausern [52])/ml. The nonadherent derived cell population contained the DCs.
(ii) MoDCs.
Peripheral blood mononuclear cells (PBMC) were isolated from the buffy coat fraction of blood by using density centrifugation at 1,000 × g for 25 min over Ficoll-Paque (1.077 g/liter). Monocytes were harvested after permitting overnight adherence to plastic as previously described (34), from which the MoDCs were generated (9). Briefly, monocytes were cultured for 6 to 7 days in Dulbecco modified Eagle medium supplemented with 10% (vol/vol) porcine serum, 150 ng of recombinant porcine GM-CSF/ml, and 100 U of recombinant porcine interleukin-4 (IL-4)/ml. Again, the nonadherent cell population contained the DCs.
Isolation of T lymphocytes.
Lymphocyte purification was performed by magnetic cell sorting by using the MACS system (Miltenyi Biotec GmbH). These cells were purified from PBMCs by using one of two methods: (i) positive selection of T lymphocytes with a monoclonal antibody to CD6 (a38b2; kindly provided by A. Saalmüller, Bundesforschungsanstalt für Viruskranheiten der Tiere, Tübingen, Germany) (39) or (ii) negative selection of B and T lymphocytes by the removal of monocytic cells with a monoclonal antibody against the panmyeloid marker SWC3 (44). In both cases, a purity of 95 to 98% lymphocytes was obtained.
Preparation of PCV2 stock.
A previously characterized PCV2 isolate from Canada (2) was used to generate the virus stock pools required for experimental infections. Briefly, the continuous porcine kidney cell line PK-15A, free of PCV1 and PCV2, was cultured in minimal essential medium containing Earle's salts supplemented with 10% (vol/vol) fetal calf serum (FCS). The cell monolayer was dispersed by using trypsin-EDTA, and the cells then infected with PCV2 in minimal essential medium-10% (vol/vol) FCS or in medium alone for the “mock” production, followed by seeding into cell culture flasks. After 24 h of incubation at 37°C, the supernatant was discarded, and the monolayer treated with 300 mM d-glucosamine in Hanks balanced salt solution for 30 min. After removal of the glucosamine and a further 48 h of incubation, the infected or mock-treated cells were scraped from the flask surface into the medium, frozen and thawed three times, and then sonicated at a maximum amplitude for 15 s. The cell lysate was then clarified at 3,000 × g for 30 min at 4°C, and the resulting virus or mock stocks were divided into aliquots before storing them at −70°C. PCV2 stocks were titrated on PK-15A cells. Based on the immunofluorescent detection of PCV2 antigen by using an antibody directed against the ORF2-encoded capsid protein (7G5-G4-A1) (35), titers were calculated by using the Karber formula, and expressed in 50% tissue culture infectivity doses (TCID50) per milliliter.
Infection of DCs with PCV2.
DCs were either PCV2 infected at different multiplicities of infection (MOIs) or mock treated in six-well-plates for 2 h at 39°C. After five intensive washes, cells were seeded into fresh polypropylene tubes or six-well plates, as required for subsequent experimentation. For the studies of replication kinetics, the extracellular virus (ECV) was defined as cell-free virus in the culture medium after a clarification of the supernatant at 500 × g for 15 min. Cell-associated virus (CAV) was recovered from the cells by three cycles of freeze-thawing, followed by clarification at 3,000 × g for 30 min; the same number of cells was used at each time point postinfection (p.i.). The resulting CAV and the ECV were titrated on PK-15A cells.
When the DCs were cocultured with T lymphocytes (at ratios of 1:10, 1:100, and 1:1,000), the DCs were infected prior to the coculture so as not to interfere with the MOI calculated for the DCs. The microbial superantigen staphylococcal enterotoxin B (SEB) (Toxin Technology, Sarasota, Fla.) was applied at 1 μg/ml in the coculture, either by pulsing the DCs for an hour prior to addition of the lymphocytes or by direct addition to the coculture. Within additional experimental cocultures, concanavalin A (ConA; Sigma) was also used, at 1 μg/ml, to activate the T lymphocytes.
DC precursors (i.e., BMHCs or monocytes) were also mock treated or PCV2 infected at the same time as the addition of the differentiation cytokines to induce DC differentiation. Infection was performed for 4 h, followed by five washings, and the timing of the cytokine addition was as described previously (9).
Flow cytometric analysis.
Phenotyping was performed with the following monoclonal antibodies: anti-SWC3 porcine panmyeloid cell marker (74-22-15) (44), anti-major histocompatibility complex class I (MHC-I; 74-11-10) (40) and II (MHC-II; MSA3) (19), anti-CD14 (MIL2) (50), anti-CD16 (G7) (17), and anti-CD25 (231.3B2; Serotec, Oxford, United Kingdom). CD80/86 was detected by using the hCTLA4-mouse immunoglobulin fusion protein (Alexis) (51), and PCV2 antigen was detected by using an anti-PCV2 capsid protein (open reading frame 2 [ORF2]) monoclonal antibody. Briefly, cells were incubated with the antibodies, and reactivity was detected by using fluorescein isothiocyanate (FITC)-, phycoerythrin-, or biotin-conjugated goat F(ab′)2 anti-mouse isotype-specific immunoglobulins (Southern Technology, Birmingham, United Kingdom). Spectral red-conjugated streptavidin (Southern Technology) was finally added to detect the biotinylated conjugate. For the intracellular staining (PCV2 antigen), the DCs were first incubated with antibodies against the cell surface markers. The cells were fixed and permeabilized by using a cell permeabilization kit (Harlan Ser-Lab, Crawley Down, United Kingdom) and then incubated with the anti-PCV2 antibody.
Cellular viability and apoptosis analysis.
Discrimination between apoptotic and necrotic cells in mock-treated and PCV2-infected samples was determined by dual-parameter analysis of annexin V-FITC (ANN; Bender Medical Systems, Vienna, Austria) staining and propidium iodide (PI; Sigma) uptake, as determined by flow cytometry. The cells were labeled with 2 μg of annexin V-FITC/ml in buffer containing 140 mM NaCl-2.5 mM CaCl2-10 mM HEPES (pH 7.4) for 15 min. After FL1/FL2 compensation, PI (100 ng/ml) was added, and the sample was acquired. The percentage of viable DCs was evaluated by gating the ANN−/PI− cells, and ANN+/PI− cells were gated as the early apoptotic cells.
When DCs were cocultured with T lymphocytes, the samples were acquired twice, with gating separately on the DCs and the lymphocytes, due to their differences in autofluorescence levels.
Detection of viral replicative intermediate forms.
Total DNA was extracted from PCV2-infected DCs at different time points p.i., by using a Qiagen DNeasy tissue kit in accordance with the manufacturer's instructions. After elution (100 μl), 25-μl aliquots of DNA extracts were separated by gel electrophoresis overnight at 20 V on a 2% (wt/vol) agarose gel. The gels were subsequently denatured prior to nucleic acid transfer on to a nitrocellulose membrane by Southern blotting by using standard techniques (8). Radiolabeled probes were generated by using a gel-purified whole genomic 1.7-kbp PCV2 replicative form (RF) DNA fragment as the target for randomly primed probe production (36). This DNA fragment was from the same reference Canadian PCV2 virus used for the infection of DCs. The PCV2-specific probes were labeled with [α-32P]dATP (Redivue; Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom) by using the MegaPrime DNA labeling kit (Amersham) in accordance with the manufacturer's instructions. To detect PCV2 DNAs, the nitrocellulose membrane was prehybridized for 30 min at 65°C in Hyperhyb solution (ResGen; Invitrogen) prior to the addition of radiolabeled PCV2 probe and subsequent hybridization for 1 h 30 min at 65°C. After hybridization, the blots were subjected to three rounds of duplicate washes of increasing stringency (2× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]-0.1% [wt/vol] sodium dodecyl sulfate [SDS], 0.2× SSC- 0.1% SDS (wt/vol), and 0.1× SSC-0.1% SDS [wt/vol]), each for 15 min at 65°C and then dried at room temperature. PCV2 probe hybridization was detected after overnight exposure at −70°C by using BioMax film (Amersham) with intensifying screens.
Confocal microscopy analysis.
DCs were infected as described earlier and then cultured in eight-well culture slides (Becton Dickinson). At each analysis time point p.i., cells were washed twice with cold PBS (4°C) and then stained with anti-SWC3 plus anti-CD3 antibodies for 20 min on ice. Cells were washed twice in cold PBS, fixed in 4% (wt/vol) paraformaldehyde for 15 min, and washed again in PBS. Cells were permeabilized with 0.3% (wt/vol) saponin (S4521; Sigma), and washed with PBS containing 0.1% (wt/vol) saponin. Virus antigen was then stained with the anti-PCV2 (ORF2) antibody in PBS containing 0.1% (wt/vol) saponin for 20 min. After the cells were washed, the presence of reacted antibodies was detected by using Alexa fluorochrome-labeled anti-mouse secondary antibodies (Molecular Probes, Eugene, Oreg.). To view the actin microfilaments, the fixed and/or permeabilized cells were stained with phalloidin-Alexa 488 (Molecular Probes). The cells were analyzed by using a Leica TCS-SL spectral confocal microscope and Leica LCS software (Leica Microsystems AG, Glattbrugg, Switzerland), and the GIMP image analysis program (version 1.2.1; distributed under the terms of the GNU public license [http://www.gimp.org]) running on a Linux platform.
RESULTS
PCV2 interaction with DCs.
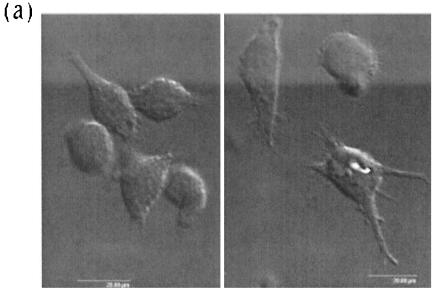
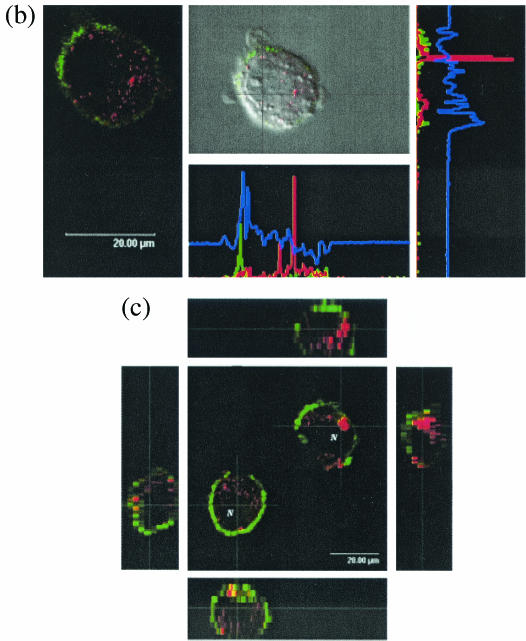
The generation of MoDCs and BMDCs yielded nonadherent cells with a typical DC-like morphology, present either as single cells or in clusters, as shown for MoDCs in Fig. 1a. Infection of these DCs with PCV2 at MOIs of 0.01, 0.2, 1, and 4 TCID50/cell or mock treatment for 24, 48, or 72 h did not influence this morphology (data not shown). The DCs were further identified by their surface staining with the anti-SWC3 panmyeloid marker antibody (green staining; Fig. 1b). With a monoclonal antibody to the PCV2 ORF2-encoded capsid protein, PCV2 antigen-positive DCs were detectable at each MOI and at all of the time points tested. An example is shown in Fig. 1b and c. Interestingly, PCV2 antigen-positive DCs could be detected as early as 4 h p.i. (data not shown). The infection did not alter the expression of the SWC3 marker, and the PCV2 antigen was only detected in the cytoplasm of infected DCs (Fig. 1b and c). The nucleus is clearly visible by Nomarski interference microscopy in Fig. 1b. No viral antigen was present.
FIG. 1.
Nomarski interference and confocal immunofluorescence photomicrographs showing the detection of PCV2 ORF2 capsid antigen in PCV2-infected MoDCs. (a) Typical nonadherent DCs, in cluster formation and as single cells, generated after treatment of monocytes with GM-CSF and IL-4 for 1 week. (b) MoDCs were infected with PCV2 at an MOI of 1 TCID50/cell for 2 h, washed to remove unadsorbed virus, and then stained at 24 h p.i. for surface expression of the panmyeloid marker SWC3 (green), followed by fixation and permeabilization and labeling for PCV2 antigen (red). A cross-section of the image was made by placing the cross-wire on the nucleus. This yielded the cross-section profiles shown (with the x axis below and the y axis to the right), with green representing the surface SWC3, red representing the PCV2, and blue representing the gray of the Nomarski interference image. (c) Sectional scanning of PCV2-infected MoDCs, which were labeled after fixation and/or permeabilization for microfilaments with phalloidin-Alexa 488 (green) and for PCV2 antigen (red). Analysis of the stacks, sections at 2-μm intervals, are shown to the left and bottom for the left-hand cells in the center image and to the right and top for the right-hand cell in the center image.
Comparison of fresh with fixed cells, as well as confocal scanning images of sequential sections, showed that the PCV2 antigen could not be seen on the cell surface (data not shown). Further analysis of the location of the detectable PCV2 is shown in Fig. 1c, with both x-y and x-z images obtained by scanning through the cell. This analysis was performed to confirm that nuclear antigen was not present in a plane different from that of the cytoplasmic antigen. The nucleus was again identified by Nomarski interference microscopy (marked “N” in this figure). These results confirmed that no viral antigen could be detected in the nucleus. Cross-section analysis of the images of infected DCs viewed as a single section stained for SWC3 (green) and PCV2 antigen (red) demonstrated the absence of PCV2 antigen in the nucleus (Fig. 1b). Scanning through infected cells at 2-μm intervals confirmed the cytoplasmic localization of the virus antigen (Fig. 1c). In this image, the cells were stained for microfilaments (green) and PCV2 antigen (red). The virus antigen was located mostly on the cytoplasmic side of the peripherally localized microfilaments. Occasionally, virus antigen inclusions could be seen associated with the microfilaments (the orange-yellow inclusions seen in the side-on views of the stacked scans in Fig. 1c).
Association of infectious PCV2 with MoDCs and BMDCs.
The results shown in Fig. 1 demonstrated that PCV2 can interact with DCs and become internalized. This raised the question regarding the source and/or the fate of the detected viral antigen. One possibility was an accumulation of virus antigen endocytosed from the inoculum. The second possibility was that the observations were reflecting, at least in a part, active replication of the virus p.i. Consequently, viral replication was studied in a kinetic manner by using two approaches.
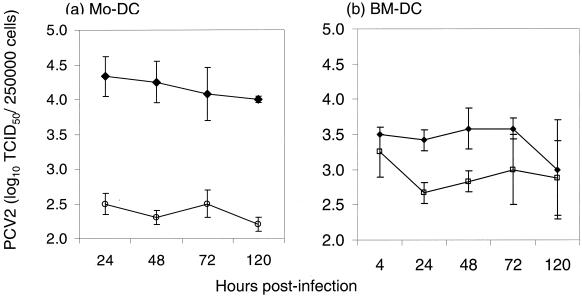
PCV2 replicates in the permissive PK-15A cells, producing infectious virus progeny. The presence of infectious PCV2 associated with the DCs was therefore analyzed by quantitative virus titration. For 5 days after infection of the DCs at an MOI of 1 TCID50/cell, the titers of infectious CAV and ECV associated with 250,000 DCs were determined by titration on the permissive PK-15A cell line (Fig. 2). Between 24 and 120 h p.i., these titers varied only slightly. Interestingly, there was almost 10 times more infectious PCV2 associated with the MoDCs compared to the BMDCs, even though both cultures received the same input of virus (105.4 TCID50/250,000 cells). This probably reflects the greater level of granulocytic cell contamination in the BMDC cultures (5 to 8%) compared to the MoDC cultures (no granulocytic cells) (9). The majority of PCV2 remained cell associated, particularly with the MoDCs, but both MoDCs and BMDCs released infectious PCV2 throughout the 5 days of observation. Regardless of the MOI used (0.02 and 4 TCID50/cell were also tested), no increase in infectious progeny was evident during the 5 days of infection (data not shown). PCV2-infected cells in lymphoid organs and tissues of infected pigs have also been described as macrophage- or DC-like cells and are only positive in the cytoplasm (29).
FIG. 2.
Replication kinetics of PCV2-infected MoDC and BMDCs. MoDCs (a) and BMDCs (b) were infected with PCV2 at an MOI of 1 TCID50/cell for 2 h, washed to remove unadsorbed virus, and then incubated for up to 5 days. ECV (open symbols) was harvested from the culture supernatant; CAV (solid symbols) was recovered from three cycles of freeze-thawing of 250,000 DCs at each of the time points shown. CAV and ECV were titrated on the permissive cell line PK15-A. Data are shown for three independent experiments performed in quadruplicate.
It was unclear how this virus was being released. Residual infectivity from the inoculum cannot totally explain these titers because the cultures were washed five times, leaving behind less than 102 TCID50/ml. It is possible that the virus had been internalized by the DCs and then exocytosed or that the extracellular titers were due to virus leaching from the cell surface. The exocytosis proposal certainly gains credence from the observation that PCV2 antigen was detectable primarily within the cell (on the cytosolic side of the plasma membrane) at later time points p.i. (see Fig. 1b and c and 7).
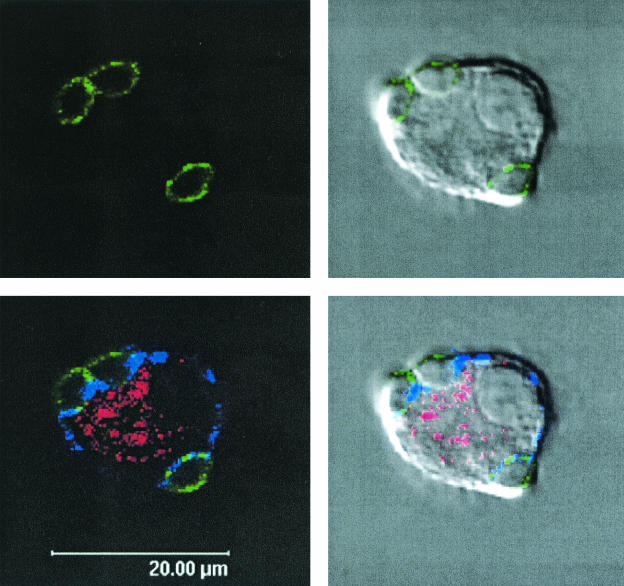
FIG. 7.
PCV2-infected MoDCs do not transmit virus to T lymphocytes. At 4 days after the initiation of coculture between PCV2-infected DCs and fresh autologous T lymphocytes, the cells were labeled with antibodies specific for the T lymphocyte marker CD3 (green), the PCV2 ORF2-encoded capsid protein (red), and the panmyeloid marker SWC3 (blue). Analysis was done with a Leica TCS-SL spectral confocal microscope and Leica LCS software, as well as the GIMP image analysis program (version 1.2.1). Images on the right show the fluorescent staining patterns, whereas images on the left show this staining pattern overplayed with the Nomarski interference images to show the position of the DC nucleus. Similar results were obtained by analyzing the cocultures at 2 days after initiation.
DNA RF analysis after PCV2 interaction with DCs.
Despite the absence of a detectable increase in virus titers associated with the DC, the prolonged presence of the infectious virus required further explanation. One possibility was a “steady-state” turnover of virus progeny production, yielding an equilibrium between virus replication and loss of infectivity. In order to test this hypothesis, MoDCs and BMDCs were infected at 1 TCID50/cell, and evidence for viral replicative intermediate production was sought at 24, 48, and 72 h p.i. During the productive PCV2 infection of permissive PK-15A cells, a typical pattern of dsDNA replicative intermediates with concomitant increase in progeny ssDNA can be identified (36). The presence of such dsDNA replicative intermediates is a clear indication of active virus replication.
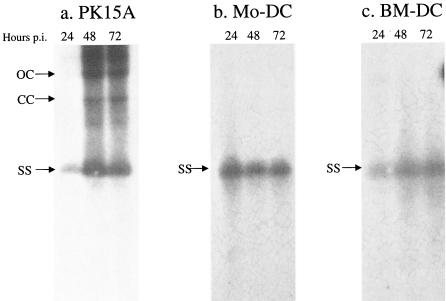
Total DNA was extracted from the infected DCs, and Southern blots were performed to identify the occurrence of viral dsDNA intermediates indicative of active virus replication (Fig. 3). For comparison, infection of the permissive PK-15A cells was used as a positive control (Fig. 3a). As expected for PCV2 replication in the latter, three PCV2 DNA forms were identifiable: closed circular (CC), open circular (OC), and single stranded (SS). In contrast, infected MoDCs (Fig. 3b) and infected BMDCs (Fig. 3c) revealed no evidence for either the CC or the OC viral RFs p.i. The ssDNA persisting throughout the course of the experiment represents the uptake of the initial input virus inoculum.
FIG. 3.
Detection of viral replicative intermediates (OC and CC forms of viral DNA) in PCV2-infected MoDCs and BMDCs. The permissive PK-15A cells (a), MoDCs (b), and BMDCs (c) were infected with PCV2 at MOIs of 1 TCID50/cell for 2 h, washed to remove unadsorbed virus, and then analyzed over a 3-day period. Total DNA was extracted from 400,000 DCs at each time point shown, processed for Southern blotting, and hybridized by using a PCV2-specific whole genomic radiolabeled probe. The positions of OC, CC, and SS forms of the PCV2 DNA normally found during the replication cycle are shown by arrows. These results are representative of three different experiments.
This inability to detect viral replicative intermediates, along with the results from the measurements of infectious virus titers, indicates an absence of PCV2 replication in infected MoDCs and BMDCs. These results are intriguing, given the initial efficient interaction of DCs with PCV2. Of particular significance is the observation that infectious PCV2 remained associated within the cells for at least 5 days and retained infectivity after release.
Viability of DCs after PCV2 infection.
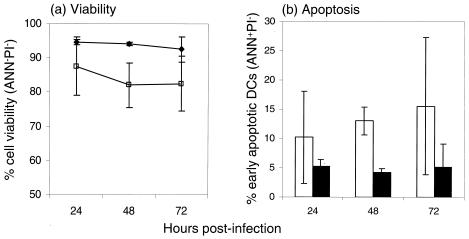
Kinetic studies on DC viability were performed to determine whether the inability to support PCV2 replication was due to DC death or apoptosis. MoDCs and BMDCs were either mock treated or PCV2 infected at an MOI of 1 TCID50/cell. Double labeling with Annexin V and PI was performed at 24, 48, and 72 h p.i. (see Fig. 4a for an MoDC example). The viability of mock-treated DCs (unfilled symbols in Fig. 4) dropped to 87% at 24 h p.i. and was ∼82% at 72 h p.i., although the variations between the samples were high. In contrast, the viability was clearly higher in PCV2-infected DCs, remaining always at >90% (solid symbols in Fig. 4).
FIG. 4.
Cell viability and apoptosis analysis of PCV2-infected MoDCs. PCV2-infected DC (solid symbols) and mock-treated DCs (open symbols) were infected with PCV2 at MOIs of 1 TCID50/cell for 2 h and washed to remove unadsorbed virus, and cells collected during a 3-day period for analysis. Discrimination was determined by dual-parameter analysis of annexin V-FITC (ANN) staining and PI uptake by using flow cytometry. The percentage of viable DCs was evaluated by gating on the ANN−/PI− cells, and early apoptotic cells were identified as ANN+/PI−. The data shown are representative of three independent experiments.
When apoptotic cells (annexin V+/PI−) were analyzed, the PCV2 infection of DCs again clearly did not induce apoptosis. In fact, the infected DCs could contain fewer apoptotic cells than the mock-treated DCs, especially when monitored at 48 h p.i. This was not always observable in replicate experiments at all time points, hence the size of the error bars (Fig. 4b). Taken together with the findings of the viability analysis, it is clear that PCV2 infection does not induce cell death in the DCs.
PCV2 influence on DC surface phenotype.
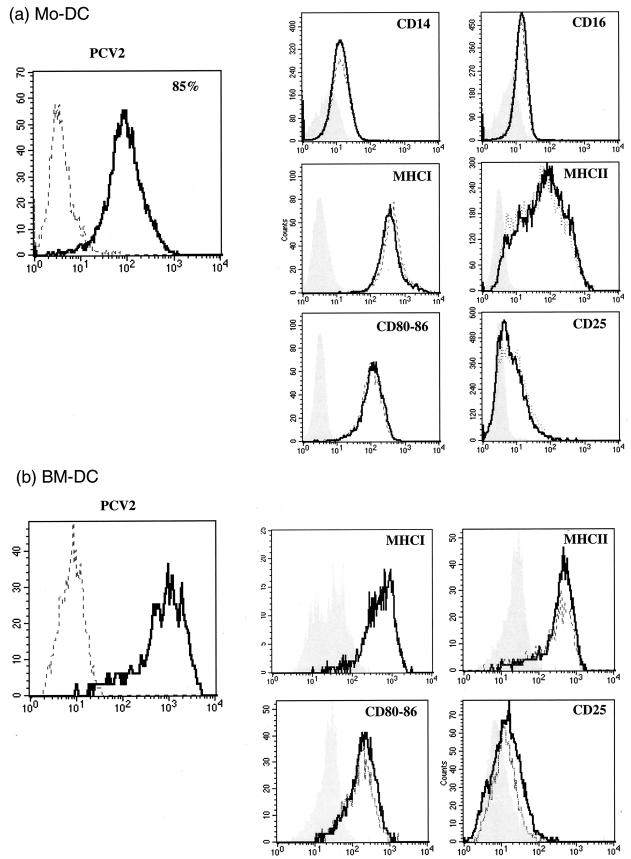
The continued presence of infectious PCV2 associated with the DCs, in the absence of detectable virus replication, may have resulted in modulation of cell activity. One important feature, insofar as DCs are concerned, is the expression of MHC-I and MHC-II and costimulatory molecules, as well as the receptors used during immune response development. DC were therefore mock treated or PCV2 infected at 1 TCID50/cell and then analyzed at 24, 48, and 72 h p.i. for their expression of MHC-I and -II, CD80/86, CD14, CD16, and CD25. As observed in the other experiments, the percentage of PCV2 antigen-positive DCs reached a maximum at 24 h p.i. (Fig. 5). Irrespective of the time after infection, the expression of the various markers did not change due to the PCV2 infection (Fig. 5 shows the results obtained at 24 h p.i. as an example). Expression of the panmyeloid marker SWC3 was also not modulated by the virus infection (data not shown). Furthermore, the interaction of PCV2 with the DCs did not modify the inducible cytokine profiles. Neither coculture with T lymphocytes nor infection at higher or lower MOIs modified these results (data not shown).
FIG.5.
Expression of cell surface markers on PCV2-infected MoDCs (a) and BMDCs (b). The DCs were mock treated or PCV2 infected at 1 TCID50/cell for 2 h, washed to remove unadsorbed virus, and tested by flow cytometry after 24 h for the expression of the cell surface markers indicated. To the left of each set of marker analyses is the profile of PCV2 antigen expression within the cells. Shaded histograms, conjugate control; dashed-line histograms, mock-treated DCs; heavy-line histograms, PCV2-infected DCs. The results shown are representative of three independent experiments.
PCV2 titers and cell viability after interaction of infected MoDCs with syngeneic T lymphocytes.
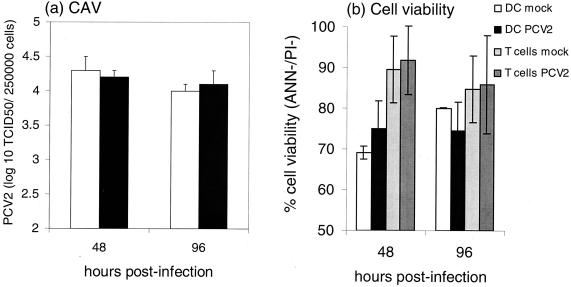
The results shown in Fig. 2 and 3 suggest that PCV2 replication is not initiated or is impaired in DCs. Consequently, cocultures of PCV2-infected MoDCs with syngeneic T lymphocytes were used to determine whether PCV2 replication could be activated in the DCs. In addition, transmission of infectious virus to T cells was also analyzed, along with viability and apoptosis in both the DCs and T lymphocytes. After infection of MoDCs with PCV2 at an MOI of 1 TCID50/cell for 2 or 24 h and then removal of the unadsorbed virus, freshly sorted syngeneic CD6+ T lymphocytes or SWC3− PBMC were added to give DC/T-cell ratios of 1:10, 1:100, and 1:1,000. Coculture of the lymphocytes with the infected DCs did not induce PCV2 replication. The same titers of infectious virus were recovered, regardless of whether the DCs were cocultured with lymphocytes or not (Fig. 6a). Furthermore, the coculture did not augment cell death in either the DCs or the T cells (Fig. 6b).
FIG. 6.
Coculture of PCV2-infected MoDCs with syngeneic lymphocytes does not induce PCV2 replication. DCs were infected with PCV2 at 1 TCID50/cell as described in the previous figures prior to the coculture with freshly sorted syngeneic CD6+ T lymphocytes or SWC3− PBMC at a 1:10 DC/T-cell ratio. The figure shows the results obtained with SWC3− PBMC, but studies with CD6 T cells yielded similar results. At 2 and 4 days after initiation of the coculture, the CAV was recovered from the cells by three cycles of freeze-thawing, followed by clarification at 3,000 × g for 30 min. (a) The resulting CAV was titrated on PK-15A cells. (b) Cell viability (ANN−/PI−) was also measured as described for Fig. 4. The results shown are representative of three independent experiments.
At 2 and 4 days after initiation of the coculture, the cells were labeled with antibodies specific for the T lymphocyte marker CD3, the panmyeloid marker SWC3, and the PCV2 ORF2-encoded capsid protein. Both confocal microscopy and flow cytometry identified the T lymphocytes as CD3+ (Fig. 7, green), and the DC as SWC3+ (Fig. 7, blue). Only the SWC3+ cells were PCV2+. This was noted even when the T cells were in direct contact with the DCs, as shown in the example given in Fig. 7.
Similar results were obtained when the T lymphocytes were activated. For this purpose, SEB was used as a cross-linker of the MHC-II on the DCs with the T-cell receptor on the lymphocytes, or ConA was used as a polyclonal lymphocyte activator. Lymphocyte activation was monitored by flow cytometry, thus confirming the presence of lymphoblasts, and also by [3H]thymidine incorporation, demonstrating high counts per minute (data not shown). Again, DC-T cell contact was observed, but no transmission of the PCV2 from the DCs to the syngeneic lymphocytes could be demonstrated (data not shown).
PCV2 infection of DC precursors (MoDCs or BMHCs).
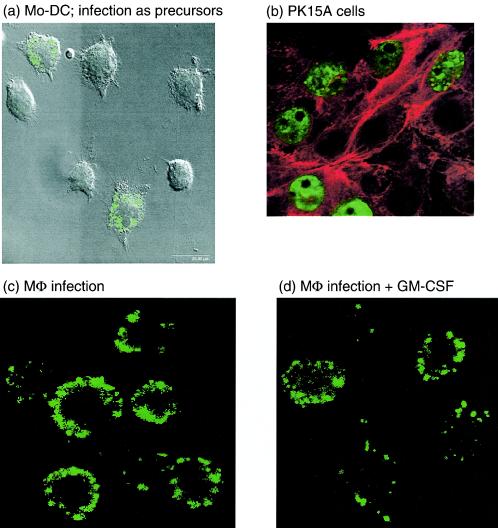
Monocytes and BMHCs infected with PCV2 before induction of DC differentiation by the addition of GM-CSF-IL-4 or GM-CSF alone for 6 days could still develop into cells typical of DCs (Fig. 8a). PCV2 antigen was detected in these cells by using the same monoclonal antibody to the PCV2 ORF2-encoded capsid protein used for staining the predifferentiated DCs after interaction with PCV2 (shown in Fig. 1 and 7). When analyzed after their differentiation into DCs, between 50 and 70% cells were found to be carrying PCV2 antigen, relating to the MOI used (Fig. 8a shows a typical example obtained with MoDCs [BMDCs yielded similar results]). Only cytoplasmic staining was observable, a finding contrasting clearly with the staining pattern obtained in the infected PK-15A cells, in which the virus does replicate productively (Fig. 8b). Furthermore, if GM-CSF was applied to preformed DCs subsequent to their interaction with PCV2, no apparent influence of the cytokine was observable (data not shown). Thus, the presence of GM-CSF subsequent to the PCV2 infection did not modify the interaction of the virus with DCs. Similar results were obtained with infected macrophages, with or without GM-CSF stimulation (Fig. 8c and d) or phorbol myristate acetate stimulation (data not shown).
FIG. 8.
(a) Detection of the PCV2 ORF2 capsid antigen in DC after infection of precursors prior to differentiation. Monocytes were infected with PCV2 at MOIs of 1 TCID50/cell for 4 h, washed five times to remove unadsorbed virus, and then cultured with GM-CSF and IL-4 for 1 week to generate DCs. The MoDCs thus generated presented a typical veiled morphology, shown with the Nomarski interference, and PCV2 antigen (green) was found to be localized within the cytoplasm of the cells, demonstrated by using confocal immunofluorescence. (b) PK15a cells (used as a positive control) are shown at 48 h p.i., with PCV2 antigen staining clearly within the nucleus. (c) Pulmonary (lung lavage) macrophages were infected with PCV2 in a manner similar to that used for the DC precursors shown in panel a. Again, the PCV2 antigen (green) localized in the cytoplasm. (d) Treatment of the macrophages in panel c with GM-CSF did not alter the pattern of PCV2 antigen localization.
DISCUSSION
DCs represent a unique and essential leukocyte population with a particularly high ability to initiate and modulate the immune response and control lymphocyte homeostasis (20). The presence of DC networks associated with the skin, mucosal surfaces, and blood predispose them to encounter viruses early postexposure. As sensors of danger, DCs capture and process antigens and migrate to lymph nodes and lymphoid tissues, where they can activate naive T cells or recall antigen-specific memory T cells (5, 20). However, pathogens have developed various strategies to alter different steps in the immune response, in particular the function of DCs, leading to viral escape from attack by immune defenses and the absence of protective immunity (6, 23, 27). In this context, nothing is known about the causative agent of the disease PMWS: the ssDNA virus PCV2. PMWS is characterized immunopathologically as showing enlarged lymph nodes (2, 12) with lymphocyte depletion and histiocyte infiltration in the lymphoid organs (43, 45). Recent studies have demonstrated that PCV2 infection of pigs induces a B-cell and T-cell lymphopenia and a general collapse of the immune system (37). Comparison of these latter observations to those for classical swine fever virus infection (28) suggests that PCV2 is also a monocytotropic virus.
The present results demonstrated that both MoDCs and BMDCs are susceptible to PCV2 infection. Virus antigen was clearly identifiable in these cells, but only within the cytoplasm. Such characteristics contrast with those of a productive replication by PCV2 in the susceptible PK-15A cell line, wherein both nuclear and cytoplasmic antigen is detectable. This lack of PCV2 antigen in the nucleus of DC is not an in vitro artifact. Viral antigen detected in vivo is also associated primarily with the cytoplasm rather than the nucleus (42, 29) of targets referred to as “DC-like” (42).
The interaction of PCV2 with DCs did not relate to what was expected from a productive replication of the virus, if the characteristics of infection in PK-15A cells can be applied. It is unclear whether this is an abortive infection in the DCs or is reflecting the capacity of DCs to endocytose potentially dangerous material. There was no evidence for an increase in virus progeny, nor was there evidence for the production of viral replicative intermediates, as seen after infection of the permissive PK-15A cells. Occasionally, there was an apparent increase in the abundance of the ssDNA form with time p.i. (data not shown). However, this was observed in only a minority of the replicate experiments performed. More importantly, neither the OC nor the CC replicative intermediates were observed in any of the DC experimental replicates. PCV2 replicates via a rolling-circle method of DNA replication (36), whereby a significant amplification of virion ssDNA molecules can be accomplished from only a few replicative intermediate molecules. If virion ssDNA were being produced in the DCs from replicative intermediate dsDNA molecules too low in copy number for detection, it was not a consistent feature of PCV2 interaction with DCs.
This presence of both virus antigen and ssDNA in the DCs would be expected after endocytosis by the DCs rather than an “active” infection by the virus. The different levels of antigen signal obtained would reflect the differing rates of endocytosis by DCs, which are known to exist (48). Blocking DC endocytosis with cytochalasin D at 4 h after interaction with PCV2 prohibited an increase in the ssDNA signal (data not shown). Treatment of the DC prior to infection also reduced the signal for virus antigen associated with the cells (data not shown). This would imply that the detectable viral antigen and DNA in the DCs was being endocytosed and may reflect more the response of the DCs to the presence of the virus rather than infection in the classical sense.
The various processes of endocytosis affected by DCs result in the ultimate degradation of the internalized material. It was therefore surprising that internalized PCV2 was not degraded as expected. Not only the virus antigen but also virus infectivity remained DC associated for at least 5 days. This did not reflect an inability of the in vitro-derived DCs to support virus replication. Such in vitro-derived DCs are perfectly capable of supporting the productive replication of a known monocytotropic virus (classical swine fever virus) with a clear increase in virus progeny both extracellularly and cell-associated (C. P. Carrasco et al., unpublished data). The in vitro-derived DCs were also endocytically active, as observed through their ability to internalize and degrade both bovine serum albumin and ovalbumin (A. Summerfield et al., unpublished data; K. C. McCullough and H. Gerber, unpublished results). Furthermore, PCV2 interaction with blood DCs (identifiable as SWC3+ CD14− [Summerfield et al., unpublished]) yielded images identical to those presented here.
Taken together, these results suggest that PCV2 replication would not necessarily occur in DCs in vivo, but the infected cells could carry and release infectious virus as a source of infection for other cells. Considering that the detectable viral antigen was intracellular, this release of infectious virus would have required exocytosis of the internalized material. An alternative explanation would be the “leaching” of infectious virus that had adsorbed to and remained on the cell surface. This leaching may indeed have occurred at early time points after infection, when surface-associated antigen could be found (data not shown), but seems unlikely as time progressed. At the later time points, it was consistently noted that the majority if not all detectable viral antigen was within the cell (on the cytosolic side of the plasma membrane; see Fig. 1b and c and Fig. 7). In vivo observations have also shown that infectious PCV2 must be released from cells, due to its presence in the feces, blood, and tissues (31). Although exocytosis would appear to be a feasible proposition, the exact mechanism or mechanisms of PCV2 release from DCs in vitro and in vivo remain to be elucidated. It must also be taken into consideration that with the “released” virus may reflect a low-level release with prolonged survival of the virus extracellularly.
Studies with human immunodeficiency virus and measles virus have shown that a low productivity of infection with virus-infected DCs (14, 41) can be enhanced through contact with T lymphocytes (23). PCV2 clearly contrasts with such viruses because cocultures of PCV2-infected DCs with syngeneic lymphocytes did not result in virus transmission to the lymphocytes, nor in the induction of virus replication. Furthermore, no cell death in DCs or lymphocytes was discernible. Activation of the lymphocytes did not change this image. Consequently, the collapse of the lymphocytic compartment in animals that subsequently develop PMWS (37) is not a direct effect of interaction with the virus. Indeed, PCV2-infected animals that do not develop the disease appear to be immunologically normal in that they can mount an anti-PCV2 immune response (31). Furthermore, gnotobiotic animals infected with PCV2 alone develop healthy germinal centers but no disease (29, 30). This would imply that the severity of PMWS is dependent on the involvement of other factors, including infectious agents and the status of the immune system (30, 31). Nevertheless, the critical factor is the PCV2 interaction with the animal immune system, being the essential element in the development of the disease (30, 31).
In conclusion, although PCV2 interacts efficiently with DCs, there is no evidence for virus replication in these cells. Infectious virus remains associated with the DCs for several days without any modulation of the cell surface molecules, induction of cell death, or transmission to syngeneic T lymphocytes. Unlike viruses known to infect and replicate in DCs (27), the interaction of PCV2 with DCs is more typical of DC endocytosis of potentially dangerous material rather than virus infection of the cells. Thus, the DCs may inadvertently act as a safe haven for PCV2: DCs do serve as reservoirs for other viruses (4), such as human immunodeficiency virus (27, 33). PCV2-infected animals can carry infectious virus associated with various organs for at least 125 days after the initial PCV2 contact (7). Such persistence and shedding of infectious PCV2 implies that even if an animal recovers from infection, the immune response cannot complete virus clearance. Consequently, DCs could serve as a vehicle for PCV2 trafficking rather than as a target cell for its replication. In this sense, PCV2 is somewhat unique in that it does not appear to modulate the DC, neither in terms of activation nor to prevent detection of the PCV2 presence. However, despite the apparent innocuity of PCV2 for the immune system, the virus is responsible for the development of severe clinical disease, implying that subsequent additional events must account for this pathology. Studies to identify the events that lead to the PCV2-induced lymphopenia characteristic of PMWS are under way.
FIG. 1—Continued.
Acknowledgments
This study was supported by the Swiss Federal Office for Education and Science (number 99.0588) through an EU Framework 5 project (number QLK2-CT-1999-00445).
We thank Valérie Tache for assistance with the VSV bioassays and Heidi Gerber for assistance with the confocal microscopy and for producing the monoclonal antibodies. We also thank the animal handlers for taking care of the blood donor pigs and for routine bleeding.
REFERENCES
- 1.Allan, G. M., S. Kennedy, F. McNeilly, J. C. Foster, J. A. Ellis, S. J. Krakowka, B. M. Meehan, and B. M. Adair. 1999. Experimental reproduction of severe wasting disease by coinfection of pigs with porcine circovirus and porcine parvovirus. J. Comp. Pathol. 121:1-11. [DOI] [PubMed] [Google Scholar]
- 2.Allan, G. M., F. McNeilly, S. Kennedy, B. Daft, E. G. Clarke, J. A. Ellis, D. M. Haines, B. M. Meehan, and B. M. Adair. 1998. Isolation of porcine circovirus-like viruses from pigs with a wasting disease in the USA and Europe. J. Vet. Diagn. Investig. 10:3-10. [DOI] [PubMed] [Google Scholar]
- 3.Andrews, D. M., C. E. Andoniou, F. Granucci, P. Ricciardi-Castagnoli, and M. A. Degli-Esposti. 2001. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat. Immunol. 2:1077-1084. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 6.Bhardwaj, N. 1997. Interactions of viruses with dendritic cells: a double-edged sword. J. Exp. Med. 186:795-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolin, S. R., W. C. Stoffregen, G. P. Nayar, and A. L. Hamel. 2001. Postweaning multisystemic wasting syndrome induced after experimental inoculation of cesarean-derived, colostrum-deprived piglets with type 2 porcine circovirus. J. Vet. Diagn. Investig. 13:185-194. [DOI] [PubMed] [Google Scholar]
- 8.Botchan, M., W. Topp, and J. Sambrook. 1976. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell 9:269-287. [DOI] [PubMed] [Google Scholar]
- 9.Carrasco, C. P., R. C. Rigden, R. Schaffner, H. Gerber, V. Neuhaus, S. Inumaru, H. Takamatsu, G. Bertoni, K. C. McCullough, and A. Summerfield. 2001. Porcine dendritic cells generated in vitro: morphological, phenotypic and functional properties. Immunology 104:175-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark E. G. 1996. Pathology of the post-weaning multisystemic syndrome of pigs. Proc. West. Can. Assoc. Swine Pract. 1996:22-25.
- 11.Darwich, L., J. Segales, M. Domingo, and E. Mateu. 2002. Changes in CD4+, CD8+, CD4+ CD8+, and immunoglobulin M-positive peripheral blood mononuclear cells of postweaning multisystemic wasting syndrome-affected pigs and age-matched uninfected wasted and healthy pigs correlate with lesions and porcine circovirus type 2 load in lymphoid tissues. Clin. Diagn. Lab. Immunol. 9:236-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis, J., L. Hassard, E. Clark, J. Harding, G. Allan, P. Willson, J. Strokappe, K. Martin, F. McNeilly, B. Meehan, D. Todd, and D. Haines. 1998. Isolation of circovirus from lesions of pigs with postweaning multisystemic wasting syndrome. Can. Vet. J. 39:44-51. [PMC free article] [PubMed] [Google Scholar]
- 13.Engelmayer, J., M. Larsson, M. Subklewe, A. Chahroudi, W. I. Cox, R. M. Steinman, and N. Bhardwaj. 1999. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J. Immunol. 163:6762-6768. [PubMed] [Google Scholar]
- 14.Fugier-Vivier, I., C. Servet-Delprat, P. Rivailler, M. C. Rissoan, Y. J. Liu, and C. Rabourdin-Combe. 1997. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J. Exp. Med. 186:813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilpin, D., K. McCullough, B. Meehan, F. McNeilly, I. McNair, L. Stevenson, J. Foster, J. Ellis, S. Krakowka, B. Adair, and G. Allan. 2003. In vitro studies on the infection and replication of porcine circovirus type 2 antigen in cells of the porcine immune system. Vet. Immunol. Immunopathol. 94:149-161. [DOI] [PubMed]
- 16.Grosjean, I., C. Caux, C. Bella, I. Berger, F. Wild, J. Banchereau, and D. Kaiserlian. 1997. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J. Exp. Med. 186:801-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halloran, P. J., S. E. Sweeney, C. M. Strohmeier, and Y. B. Kim. 1994. Molecular cloning and identification of the porcine cytolytic trigger molecule G7 as a Fcγ RIIIα (CD16) homologue. J. Immunol. 153:2631-2641. [PubMed] [Google Scholar]
- 18.Hamel, A. L., L. L. Lin, and G. P. Nayar. 1998. Nucleotide sequence of porcine circovirus associated with postweaning multisystemic wasting syndrome in pigs. J. Virol. 72:5262-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammerberg, C., and G. G. Schurig. 1986. Characterization of monoclonal antibodies directed against swine leukocytes. Vet. Immunol. Immunopathol. 11:107-121. [DOI] [PubMed] [Google Scholar]
- 20.Hart, D. N. 1997. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood 90:3245-3287. [PubMed] [Google Scholar]
- 21.Ho, L. J., J. J. Wang, M. F. Shaio, C. L. Kao, D. M. Chang, S. W. Han, and J. H. Lai. 2001. Infection of human dendritic cells by dengue virus causes cell maturation and cytokine production. J. Immunol. 166:1499-1506. [DOI] [PubMed] [Google Scholar]
- 22.Inumaru, S., T. Kokuho, S. Denham, M. S. Denyer, E. Momotani, S. Kitamura, A. Corteyn, S. Brookes, R. M. Parkhouse, and H. Takamatsu. 1998. Expression of biologically active recombinant porcine GM-CSF by baculovirus gene expression system. Immunol. Cell Biol. 76:195-201. [DOI] [PubMed] [Google Scholar]
- 23.Kaiserlian, D., and B. Dubois. 2001. Dendritic cells and viral immunity: friends or foes? Semin. Immunol. 13:303-310. [DOI] [PubMed] [Google Scholar]
- 24.Kim, J., C. Choi, and C. Chae. 2003. Pathogenesis of postweaning multisystemic wasting syndrome reproduced by co-infection with Korean isolates of porcine circovirus 2 and porcine parvovirus. J. Comp. Pathol. 128:52-59. [DOI] [PubMed] [Google Scholar]
- 25.Kiupel, M., G. W. Stevenson, J. Choi, K. S. Latimer, C. L. Kanitz, and S. K. Mittal. 2001. Viral replication and lesions in BALB/c mice experimentally inoculated with porcine circovirus isolated from a pig with postweaning multisystemic wasting disease. Vet. Pathol. 38:74-82. [DOI] [PubMed] [Google Scholar]
- 26.Kiupel, M., G. W. Stevenson, S. K. Mittal, E. G. Clark, and D. M. Haines. 1998. Circovirus-like viral associated disease in weaned pigs in Indiana. Vet. Pathol. 35:303-307. [DOI] [PubMed] [Google Scholar]
- 27.Klagge, I. M., and S. Schneider-Schaulies. 1999. Virus interactions with dendritic cells. J. Gen. Virol. 80(Pt. 4):823-833. [DOI] [PubMed] [Google Scholar]
- 28.Knoetig, S. M., A. Summerfield, M. Spagnuolo-Weaver, and K. C. McCullough. 1999. Immunopathogenesis of classical swine fever: role of monocytic cells. Immunology 97:359-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krakowka, S., J. A. Ellis, F. McNeilly, D. Gilpin, B. Meehan, K. McCullough, and G. Allan. 2002. Immunologic features of porcine circovirus type 2 infection. Viral Immunol. 15:567-582. [DOI] [PubMed] [Google Scholar]
- 30.Krakowka, S., J. A. Ellis, B. Meehan, S. Kennedy, F. McNeilly, and G. Allan. 2000. Viral wasting syndrome of swine: experimental reproduction of postweaning multisystemic wasting syndrome in gnotobiotic swine by coinfection with porcine circovirus 2 and porcine parvovirus. Vet. Pathol. 37:254-263. [DOI] [PubMed] [Google Scholar]
- 31.Ladekjaer-Mikkelsen, A. S., J. Nielsen, T. Stadejek, T. Storgaard, S. Krakowka, J. Ellis, F. McNeilly, G. Allan, and A. Botner. 2002. Reproduction of postweaning multisystemic wasting syndrome (PMWS) in immunostimulated and nonimmunostimulated 3-week-old piglets experimentally infected with porcine circovirus type 2 (PCV2). Vet. Microbiol. 89:97-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magar, R., R. Larochelle, S. Thibault, and L. Lamontagne. 2000. Experimental transmission of porcine circovirus type 2 (PCV2) in weaned pigs: a sequential study. J. Comp. Pathol. 123:258-269. [DOI] [PubMed] [Google Scholar]
- 33.Masurier, C., B. Salomon, N. Guettari, C. Pioche, F. Lachapelle, M. Guigon, and D. Klatzmann. 1998. Dendritic cells route human immunodeficiency virus to lymph nodes after vaginal or intravenous administration to mice. J. Virol. 72:7822-7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCullough, K. C., S. Basta, S. Knotig, H. Gerber, R. Schaffner, Y. B. Kim, A. Saalmuller, and A. Summerfield. 1999. Intermediate stages in monocyte-macrophage differentiation modulate phenotype and susceptibility to virus infection. Immunology 98:203-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNeilly, F., I. McNair, D. P. Mackie, B. M. Meehan, S. Kennedy, D. Moffett, J. Ellis, S. Krakowka, and G. M. Allan. 2001. Production, characterisation and applications of monoclonal antibodies to porcine circovirus 2. Arch. Virol. 146:909-922. [DOI] [PubMed] [Google Scholar]
- 36.Meehan, B. M., F. McNeilly, D. Todd, S. Kennedy, V. A. Jewhurst, J. A. Ellis, L. E. Hassard, E. G. Clark, D. M. Haines, and G. M. Allan. 1998. Characterization of novel circovirus DNAs associated with wasting syndromes in pigs. J. Gen. Virol. 79(Pt. 9):2171-2179. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen, J., I. Vincent, A. Bøtner, A.-S. Ladekjær-Mikkelsen, G. Allan, A. Summerfield, and K. McCullough. 2003. Association of lymphopenia with porcine circovirus type 2 induced postweaning multisystemic wasting syndrome (PMWS). Vet. Immunol. Immunopathol. 92:97-111. [DOI] [PubMed] [Google Scholar]
- 38.Nishizawa, T., H. Okamoto, K. Konishi, H. Yoshizawa, Y. Miyakawa, and M. Mayumi. 1997. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem. Biophys. Res. Commun. 241:92-97. [DOI] [PubMed] [Google Scholar]
- 39.Pauly, T., E. Weiland, W. Hirt, C. Dreyer-Bux, S. Maurer, A. Summerfield, and A. Saalmuller. 1996. Differentiation between MHC-restricted and non-MHC-restricted porcine cytolytic T lymphocytes. Immunology 88:238-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pescovitz, M. D., J. K. Lunney, and D. H. Sachs. 1984. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J. Immunol. 133:368-375. [PubMed] [Google Scholar]
- 41.Pope, M., M. G. Betjes, N. Romani, H. Hirmand, P. U. Cameron, L. Hoffman, S. Gezelter, G. Schuler, and R. M. Steinman. 1994. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell 78:389-398. [DOI] [PubMed] [Google Scholar]
- 42.Rosell, C., J. Segales, J. Plana-Duran, M. Balasch, G. M. Rodriguez-Arrioja, S. Kennedy, G. M. Allan, F. McNeilly, K. S. Latimer, and M. Domingo. 1999. Pathological, immunohistochemical, and in-situ hybridization studies of natural cases of postweaning multisystemic wasting syndrome (PMWS) in pigs. J. Comp. Pathol. 120:59-78. [DOI] [PubMed] [Google Scholar]
- 43.Rovira, A., M. Balasch, J. Segales, L. Garcia, J. Plana-Duran, C. Rosell, H. Ellerbrok, A. Mankertz, and M. Domingo. 2002. Experimental inoculation of conventional pigs with porcine reproductive and respiratory syndrome virus and porcine circovirus 2. J. Virol. 76:3232-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saalmuller, A. 1996. Characterization of swine leukocyte differentiation antigens. Immunol. Today. 17:352-354. [DOI] [PubMed] [Google Scholar]
- 45.Segales, J., F. Alonso, C. Rosell, J. Pastor, F. Chianini, E. Campos, L. Lopez-Fuertes, J. Quintana, G. Rodriguez-Arrioja, M. Calsamiglia, J. Pujols, J. Dominguez, and M. Domingo. 2001. Changes in peripheral blood leukocyte populations in pigs with natural postweaning multisystemic wasting syndrome (PMWS). Vet. Immunol. Immunopathol. 81:37-44. [DOI] [PubMed] [Google Scholar]
- 46.Segales, J., M. Sitjar, M. Domingo, S. Dee, M. Del Pozo, R. Noval, C. Sacristan, H. A. De Las, A. Ferro, and K. S. Latimer. 1997. First report of post-weaning multisystemic wasting syndrome in pigs in Spain. Vet. Rec. 141:600-601. [PubMed] [Google Scholar]
- 47.Shibahara, T., K. Sato, Y. Ishikawa, and K. Kadota. 2000. Porcine circovirus induces B lymphocyte depletion in pigs with wasting disease syndrome. J. Vet. Med. Sci. 62:1125-1131. [DOI] [PubMed] [Google Scholar]
- 48.Steinman, R. M., and J. Swanson. 1995. The endocytic activity of dendritic cells. J. Exp. Med. 182:283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Summerfield, A., and K. C. McCullough. 1997. Porcine bone marrow myeloid cells: phenotype and adhesion molecule expression. J. Leukoc. Biol. 62:176-185. [DOI] [PubMed] [Google Scholar]
- 50.Thacker, E., A. Summerfield, K. McCullough, A. Ezquerra, J. Dominguez, F. Alonso, J. Lunney, J. Sinkora, and K. Haverson. 2001. Summary of workshop findings for porcine myelomonocytic markers. Vet. Immunol. Immunopathol. 80:93-109. [DOI] [PubMed] [Google Scholar]
- 51.Vaughan, A. N., P. Malde, N. J. Rogers, I. M. Jackson, R. I. Lechler, and A. Dorling. 2000. Porcine CTLA4-Ig lacks a MYPPPY motif, binds inefficiently to human B7 and specifically suppresses human CD4+ T-cell responses costimulated by pig but not human B7. J. Immunol. 165:3175-3181. [DOI] [PubMed] [Google Scholar]
- 52.Von Niederhausern, B., G. Bertoni, C. Hertig, H. Pfister, E. Peterhans, and U. Pauli. 1993. Cloning and expression in mammalian cells of porcine tumor necrosis factor alpha: examination of biological properties. Vet. Immunol. Immunopathol. 38:57-74. [DOI] [PubMed] [Google Scholar]