Abstract
In the title compound, C16H17ClN2O4S, the dihedral angle between the phenyl and benzene rings is 19.4 (2)°. The crystal packing is stabilized by intermolecular N—H⋯O hydrogen bonds, as well as by intra- and intermolecular C—H⋯O hydrogen bonds.
Related literature
For general background, see Kemp (1991 ▶); Qui & Silverman (2000 ▶); Orlek & Stemp (1991 ▶), Han et al. (2007 ▶); Li et al. (2007 ▶). For bond-length data, see: Allen et al. (1987 ▶).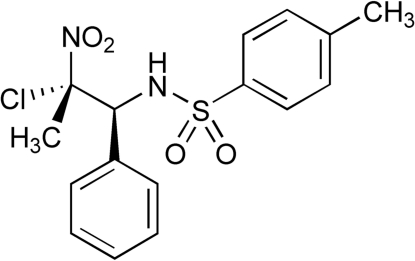
Experimental
Crystal data
C16H17ClN2O4S
M r = 368.83
Orthorhombic,

a = 7.8254 (8) Å
b = 19.610 (2) Å
c = 22.533 (3) Å
V = 3457.8 (7) Å3
Z = 8
Mo Kα radiation
μ = 0.36 mm−1
T = 291 (2) K
0.30 × 0.26 × 0.24 mm
Data collection
Bruker SMART APEX CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2000 ▶) T min = 0.901, T max = 0.921
17527 measured reflections
3396 independent reflections
2467 reflections with I > 2σ(I)
R int = 0.070
Refinement
R[F 2 > 2σ(F 2)] = 0.056
wR(F 2) = 0.125
S = 1.01
3396 reflections
222 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.28 e Å−3
Δρmin = −0.26 e Å−3
Data collection: SMART (Bruker, 2000 ▶); cell refinement: SAINT (Bruker, 2000 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Bruker, 2000 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL and PLATON (Spek, 2003 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536807067712/at2518sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536807067712/at2518Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C3—H3⋯O1 | 0.98 | 2.46 | 2.933 (4) | 109 |
| C3—H3⋯O3 | 0.98 | 2.42 | 2.779 (4) | 101 |
| C1—H1A⋯O2i | 0.96 | 2.52 | 3.369 (4) | 147 |
| C1—H1B⋯O1ii | 0.96 | 2.58 | 3.310 (4) | 133 |
| N2—H2A⋯O2i | 0.89 (2) | 2.32 (3) | 3.141 (3) | 153 |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Acknowledgments
This work was supported by the 863 High Technology Program (to YP). The research funds for YP from the Qing-Lan Program of Jiangsu Province and the Kua-Shi-Ji Program of the Education Ministry of China are also acknowledged.
supplementary crystallographic information
Comment
Since the vicinal haloamines are important building blocks in organic and medicinal chemistry, many attentions are attracted to the aminohalogenation reactions of functionalized alkenes (Kemp, 1991; Qui & Silverman, 2000). In recent years, many new aminohalogenation processes of several kinds of functionalized alkenes have been developed (Han et al., 2007), with the different nitrogen/halogen sources in the presence of metallic catalysts (Li et al., 2007). However, the aminohalogenation reactions of 2-nitro-propenyl benzene was not been well documented. Recently, we synthesized the title compound (I) from 2-nitro-propenyl benzene. As part of this study, we have undertaken the X-ray crystallographic analysis of (I) in order to elucidate the conformation and configuration of this product.
The bond lengths and angles in (I) are in good agree with expected values (Allen et al., 1987). The dihedral angle between the phenyl and benzene rings is 19.4 (2)°. The packing is stabilized by intermolecular N—H···O as well as intra and intermolecular C—H···O interactions in the crystal structure (Table 1).
Experimental
N-cChlorosuccinimide (400 mg, 3.0 mmol) was added into a solution of MnSO4 (30.2 mg, 0.20 mmol), 1-benzyl-2-nitro-propene (163 mg, 1 mmol), tolunesulfonamide (513 mg, 3 mmol) and 4 Å molecular sieves (500 mg) in CH2Cl2 (5.0 ml) with nitrogen atmosphere. The resulting mixture was stirred at room temperature for 48 h. Reaction was quenched with saturated aqueous Na2S2O3 solution. The solid precipitates were filtered off and washed with ethyl acetate (3 × 10 ml). The organic solution was concentrated and then purified via flash chromatography (ethyl acetate/ hexane, 1:4, v/v) provide the title compound (I) as white solid (276 mg) in yield of 75%. A colourless crystal of (I) for X-ray analysis was obtained by slow evaporation from ethyl acetate solution system.
Refinement
The H atom bonded to N was located in a difference map and refined with restraint of N—H = 0.89 (3) Å, and with Uiso(H) = 1.2Ueq(N). Other H atoms were geometrically placed and were treated as riding, with C—H distances of 0.93, 0.96 and 0.98 Å for aromatic, methyl and methine H atoms, respectively, and with Uiso(H) = 1.2Ueq(aromatic and methyne C) or 1.5Ueq(methyl C).
Figures
Fig. 1.
The molecular structure of (I), with atom labels and 30% probability displacement ellipsoids for non-H atoms.
Crystal data
| C16H17ClN2O4S | Dx = 1.417 Mg m−3 |
| Mr = 368.83 | Melting point: 423.2 K |
| Orthorhombic, Pbca | Mo Kα radiation λ = 0.71073 Å |
| Hall symbol: -P 2ac 2ab | Cell parameters from 7468 reflections |
| a = 7.8254 (8) Å | θ = 2.3–27.9º |
| b = 19.610 (2) Å | µ = 0.36 mm−1 |
| c = 22.533 (3) Å | T = 291 (2) K |
| V = 3457.8 (7) Å3 | Block, colourless |
| Z = 8 | 0.30 × 0.26 × 0.24 mm |
| F000 = 1536 |
Data collection
| Bruker SMART APEX CCD area-detector diffractometer | 3396 independent reflections |
| Radiation source: sealed tube | 2467 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.070 |
| T = 291(2) K | θmax = 26.0º |
| φ and ω scans | θmin = 2.1º |
| Absorption correction: multi-scan(SADABS; Bruker, 2000) | h = −9→8 |
| Tmin = 0.901, Tmax = 0.921 | k = −24→24 |
| 17527 measured reflections | l = −16→27 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.056 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.125 | w = 1/[σ2(Fo2) + (0.06P)2 + 0.58P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.01 | (Δ/σ)max < 0.001 |
| 3396 reflections | Δρmax = 0.28 e Å−3 |
| 222 parameters | Δρmin = −0.26 e Å−3 |
| Primary atom site location: structure-invariant direct methods | Extinction correction: none |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.7916 (4) | 0.10320 (17) | 0.61740 (13) | 0.0374 (7) | |
| H1A | 0.7709 | 0.1030 | 0.6594 | 0.056* | |
| H1B | 0.8606 | 0.1420 | 0.6072 | 0.056* | |
| H1C | 0.8503 | 0.0621 | 0.6064 | 0.056* | |
| C2 | 0.6185 (4) | 0.10723 (16) | 0.58376 (13) | 0.0333 (7) | |
| C3 | 0.5059 (4) | 0.16923 (14) | 0.59888 (13) | 0.0292 (6) | |
| H3 | 0.3995 | 0.1645 | 0.5763 | 0.035* | |
| C4 | 0.5853 (3) | 0.23727 (15) | 0.58138 (13) | 0.0293 (6) | |
| C5 | 0.5405 (4) | 0.26704 (17) | 0.52790 (13) | 0.0362 (7) | |
| H5 | 0.4651 | 0.2445 | 0.5028 | 0.043* | |
| C6 | 0.6055 (4) | 0.32954 (17) | 0.51103 (14) | 0.0415 (8) | |
| H6 | 0.5765 | 0.3484 | 0.4745 | 0.050* | |
| C7 | 0.7156 (4) | 0.36407 (18) | 0.54968 (15) | 0.0437 (8) | |
| H7 | 0.7570 | 0.4070 | 0.5396 | 0.052* | |
| C8 | 0.7623 (4) | 0.33466 (18) | 0.60226 (15) | 0.0444 (8) | |
| H8 | 0.8381 | 0.3572 | 0.6273 | 0.053* | |
| C9 | 0.6972 (4) | 0.27113 (17) | 0.61859 (14) | 0.0414 (8) | |
| H9 | 0.7290 | 0.2516 | 0.6545 | 0.050* | |
| C10 | 0.3185 (3) | 0.28495 (15) | 0.69070 (12) | 0.0314 (6) | |
| C11 | 0.2653 (4) | 0.32776 (18) | 0.64579 (14) | 0.0402 (7) | |
| H11 | 0.2085 | 0.3099 | 0.6131 | 0.048* | |
| C12 | 0.2959 (5) | 0.39679 (18) | 0.64904 (15) | 0.0465 (8) | |
| H12 | 0.2601 | 0.4252 | 0.6184 | 0.056* | |
| C13 | 0.3804 (4) | 0.42454 (18) | 0.69822 (14) | 0.0419 (8) | |
| C14 | 0.4350 (4) | 0.38054 (17) | 0.74268 (15) | 0.0423 (8) | |
| H14 | 0.4939 | 0.3980 | 0.7751 | 0.051* | |
| C15 | 0.4037 (4) | 0.31171 (16) | 0.73966 (13) | 0.0349 (7) | |
| H15 | 0.4392 | 0.2831 | 0.7702 | 0.042* | |
| C16 | 0.4064 (4) | 0.49975 (18) | 0.70326 (17) | 0.0491 (9) | |
| H16A | 0.3241 | 0.5184 | 0.7304 | 0.074* | |
| H16B | 0.3922 | 0.5204 | 0.6650 | 0.074* | |
| H16C | 0.5196 | 0.5087 | 0.7177 | 0.074* | |
| Cl1 | 0.65350 (9) | 0.10276 (4) | 0.50665 (3) | 0.03580 (19) | |
| N1 | 0.5083 (4) | 0.04372 (13) | 0.59707 (12) | 0.0407 (6) | |
| N2 | 0.4620 (3) | 0.16217 (13) | 0.66251 (11) | 0.0334 (6) | |
| H2A | 0.547 (4) | 0.1794 (17) | 0.6841 (15) | 0.040* | |
| O1 | 0.1557 (3) | 0.18367 (12) | 0.64347 (10) | 0.0439 (6) | |
| O2 | 0.2635 (3) | 0.17162 (12) | 0.74582 (9) | 0.0432 (6) | |
| O3 | 0.3667 (3) | 0.04155 (12) | 0.57591 (12) | 0.0547 (7) | |
| O4 | 0.5674 (3) | −0.00057 (13) | 0.62846 (12) | 0.0595 (7) | |
| S1 | 0.28433 (9) | 0.19677 (4) | 0.68662 (3) | 0.03081 (19) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0406 (16) | 0.0483 (18) | 0.0233 (14) | 0.0236 (14) | −0.0020 (12) | −0.0033 (14) |
| C2 | 0.0365 (16) | 0.0373 (16) | 0.0261 (14) | 0.0020 (13) | 0.0056 (12) | −0.0015 (13) |
| C3 | 0.0296 (15) | 0.0309 (14) | 0.0270 (14) | −0.0002 (12) | 0.0055 (11) | −0.0002 (12) |
| C4 | 0.0267 (14) | 0.0322 (15) | 0.0290 (15) | −0.0027 (11) | 0.0033 (12) | −0.0024 (12) |
| C5 | 0.0344 (15) | 0.0448 (17) | 0.0295 (15) | −0.0005 (14) | −0.0042 (12) | 0.0001 (13) |
| C6 | 0.056 (2) | 0.0446 (19) | 0.0242 (16) | 0.0022 (15) | 0.0026 (14) | 0.0134 (14) |
| C7 | 0.0534 (19) | 0.0383 (18) | 0.0393 (19) | −0.0083 (15) | 0.0079 (15) | 0.0100 (15) |
| C8 | 0.0513 (19) | 0.0471 (19) | 0.0349 (18) | −0.0214 (16) | 0.0037 (15) | 0.0031 (15) |
| C9 | 0.0496 (18) | 0.0467 (19) | 0.0279 (15) | −0.0154 (15) | −0.0060 (14) | 0.0084 (14) |
| C10 | 0.0265 (14) | 0.0402 (17) | 0.0275 (15) | 0.0008 (12) | 0.0057 (11) | 0.0011 (12) |
| C11 | 0.0452 (17) | 0.0461 (19) | 0.0293 (16) | 0.0124 (14) | −0.0042 (14) | 0.0062 (14) |
| C12 | 0.059 (2) | 0.0433 (19) | 0.0368 (18) | 0.0120 (16) | −0.0016 (16) | 0.0137 (16) |
| C13 | 0.0474 (19) | 0.0432 (18) | 0.0350 (17) | −0.0008 (14) | 0.0099 (14) | 0.0068 (15) |
| C14 | 0.0435 (18) | 0.0471 (19) | 0.0363 (17) | −0.0077 (14) | −0.0042 (14) | −0.0025 (15) |
| C15 | 0.0373 (15) | 0.0392 (16) | 0.0281 (15) | −0.0009 (13) | −0.0026 (12) | 0.0021 (13) |
| C16 | 0.0394 (17) | 0.050 (2) | 0.058 (2) | −0.0181 (15) | 0.0056 (17) | 0.0087 (18) |
| Cl1 | 0.0403 (4) | 0.0396 (4) | 0.0275 (4) | 0.0017 (3) | 0.0128 (3) | −0.0108 (3) |
| N1 | 0.0507 (17) | 0.0366 (15) | 0.0347 (15) | −0.0014 (13) | 0.0048 (13) | −0.0007 (12) |
| N2 | 0.0326 (13) | 0.0325 (14) | 0.0351 (15) | 0.0000 (10) | 0.0028 (11) | −0.0005 (11) |
| O1 | 0.0323 (10) | 0.0607 (15) | 0.0387 (12) | −0.0070 (10) | −0.0006 (10) | −0.0059 (11) |
| O2 | 0.0565 (14) | 0.0451 (13) | 0.0281 (11) | −0.0041 (10) | 0.0182 (11) | 0.0042 (10) |
| O3 | 0.0564 (15) | 0.0482 (14) | 0.0597 (17) | −0.0259 (12) | 0.0007 (13) | −0.0081 (12) |
| O4 | 0.0644 (16) | 0.0474 (15) | 0.0667 (18) | 0.0107 (12) | 0.0152 (14) | 0.0303 (14) |
| S1 | 0.0299 (3) | 0.0364 (4) | 0.0261 (4) | −0.0028 (3) | 0.0059 (3) | 0.0004 (3) |
Geometric parameters (Å, °)
| C1—C2 | 1.555 (4) | C10—C11 | 1.379 (4) |
| C1—H1A | 0.9600 | C10—C15 | 1.392 (4) |
| C1—H1B | 0.9600 | C10—S1 | 1.752 (3) |
| C1—H1C | 0.9600 | C11—C12 | 1.377 (5) |
| C2—C3 | 1.539 (4) | C11—H11 | 0.9300 |
| C2—N1 | 1.544 (4) | C12—C13 | 1.400 (5) |
| C2—Cl1 | 1.761 (3) | C12—H12 | 0.9300 |
| C3—N2 | 1.481 (4) | C13—C14 | 1.390 (5) |
| C3—C4 | 1.524 (4) | C13—C16 | 1.493 (5) |
| C3—H3 | 0.9800 | C14—C15 | 1.373 (4) |
| C4—C9 | 1.382 (4) | C14—H14 | 0.9300 |
| C4—C5 | 1.384 (4) | C15—H15 | 0.9300 |
| C5—C6 | 1.380 (4) | C16—H16A | 0.9600 |
| C5—H5 | 0.9300 | C16—H16B | 0.9600 |
| C6—C7 | 1.400 (5) | C16—H16C | 0.9600 |
| C6—H6 | 0.9300 | N1—O3 | 1.207 (3) |
| C7—C8 | 1.367 (5) | N1—O4 | 1.212 (3) |
| C7—H7 | 0.9300 | N2—S1 | 1.639 (3) |
| C8—C9 | 1.395 (4) | N2—H2A | 0.89 (3) |
| C8—H8 | 0.9300 | O1—S1 | 1.423 (2) |
| C9—H9 | 0.9300 | O2—S1 | 1.432 (2) |
| C2—C1—H1A | 109.5 | C11—C10—C15 | 119.8 (3) |
| C2—C1—H1B | 109.5 | C11—C10—S1 | 121.1 (2) |
| H1A—C1—H1B | 109.5 | C15—C10—S1 | 119.1 (2) |
| C2—C1—H1C | 109.5 | C12—C11—C10 | 120.5 (3) |
| H1A—C1—H1C | 109.5 | C12—C11—H11 | 119.8 |
| H1B—C1—H1C | 109.5 | C10—C11—H11 | 119.8 |
| C3—C2—N1 | 105.9 (2) | C11—C12—C13 | 120.4 (3) |
| C3—C2—C1 | 115.5 (2) | C11—C12—H12 | 119.8 |
| N1—C2—C1 | 110.5 (2) | C13—C12—H12 | 119.8 |
| C3—C2—Cl1 | 110.3 (2) | C14—C13—C12 | 118.3 (3) |
| N1—C2—Cl1 | 103.78 (19) | C14—C13—C16 | 121.1 (3) |
| C1—C2—Cl1 | 110.1 (2) | C12—C13—C16 | 120.6 (3) |
| N2—C3—C4 | 115.3 (2) | C15—C14—C13 | 121.3 (3) |
| N2—C3—C2 | 105.9 (2) | C15—C14—H14 | 119.3 |
| C4—C3—C2 | 113.6 (2) | C13—C14—H14 | 119.3 |
| N2—C3—H3 | 107.2 | C14—C15—C10 | 119.7 (3) |
| C4—C3—H3 | 107.2 | C14—C15—H15 | 120.2 |
| C2—C3—H3 | 107.2 | C10—C15—H15 | 120.2 |
| C9—C4—C5 | 119.1 (3) | C13—C16—H16A | 109.5 |
| C9—C4—C3 | 121.5 (3) | C13—C16—H16B | 109.5 |
| C5—C4—C3 | 119.4 (3) | H16A—C16—H16B | 109.5 |
| C6—C5—C4 | 121.4 (3) | C13—C16—H16C | 109.5 |
| C6—C5—H5 | 119.3 | H16A—C16—H16C | 109.5 |
| C4—C5—H5 | 119.3 | H16B—C16—H16C | 109.5 |
| C5—C6—C7 | 119.0 (3) | O3—N1—O4 | 123.8 (3) |
| C5—C6—H6 | 120.5 | O3—N1—C2 | 117.7 (3) |
| C7—C6—H6 | 120.5 | O4—N1—C2 | 118.6 (3) |
| C8—C7—C6 | 120.0 (3) | C3—N2—S1 | 118.6 (2) |
| C8—C7—H7 | 120.0 | C3—N2—H2A | 109 (2) |
| C6—C7—H7 | 120.0 | S1—N2—H2A | 107 (2) |
| C7—C8—C9 | 120.5 (3) | O1—S1—O2 | 119.59 (14) |
| C7—C8—H8 | 119.8 | O1—S1—N2 | 107.37 (14) |
| C9—C8—H8 | 119.8 | O2—S1—N2 | 105.25 (14) |
| C4—C9—C8 | 120.0 (3) | O1—S1—C10 | 108.79 (14) |
| C4—C9—H9 | 120.0 | O2—S1—C10 | 107.97 (14) |
| C8—C9—H9 | 120.0 | N2—S1—C10 | 107.24 (13) |
| N1—C2—C3—N2 | 59.6 (3) | C12—C13—C14—C15 | −1.7 (5) |
| C1—C2—C3—N2 | −63.2 (3) | C16—C13—C14—C15 | 176.7 (3) |
| Cl1—C2—C3—N2 | 171.28 (19) | C13—C14—C15—C10 | 1.2 (5) |
| N1—C2—C3—C4 | −172.9 (2) | C11—C10—C15—C14 | −0.4 (4) |
| C1—C2—C3—C4 | 64.4 (3) | S1—C10—C15—C14 | 178.4 (2) |
| Cl1—C2—C3—C4 | −61.2 (3) | C3—C2—N1—O3 | 50.9 (3) |
| N2—C3—C4—C9 | 36.7 (4) | C1—C2—N1—O3 | 176.7 (3) |
| C2—C3—C4—C9 | −85.7 (3) | Cl1—C2—N1—O3 | −65.3 (3) |
| N2—C3—C4—C5 | −141.2 (3) | C3—C2—N1—O4 | −128.4 (3) |
| C2—C3—C4—C5 | 96.3 (3) | C1—C2—N1—O4 | −2.5 (4) |
| C9—C4—C5—C6 | 0.2 (5) | Cl1—C2—N1—O4 | 115.5 (3) |
| C3—C4—C5—C6 | 178.1 (3) | C4—C3—N2—S1 | 80.2 (3) |
| C4—C5—C6—C7 | −1.6 (5) | C2—C3—N2—S1 | −153.3 (2) |
| C5—C6—C7—C8 | 2.4 (5) | C3—N2—S1—O1 | 41.9 (3) |
| C6—C7—C8—C9 | −1.8 (5) | C3—N2—S1—O2 | 170.3 (2) |
| C5—C4—C9—C8 | 0.5 (5) | C3—N2—S1—C10 | −74.9 (2) |
| C3—C4—C9—C8 | −177.4 (3) | C11—C10—S1—O1 | −18.0 (3) |
| C7—C8—C9—C4 | 0.4 (5) | C15—C10—S1—O1 | 163.2 (2) |
| C15—C10—C11—C12 | 0.0 (5) | C11—C10—S1—O2 | −149.2 (2) |
| S1—C10—C11—C12 | −178.8 (3) | C15—C10—S1—O2 | 32.0 (3) |
| C10—C11—C12—C13 | −0.4 (5) | C11—C10—S1—N2 | 97.8 (3) |
| C11—C12—C13—C14 | 1.2 (5) | C15—C10—S1—N2 | −80.9 (2) |
| C11—C12—C13—C16 | −177.1 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C3—H3···O1 | 0.98 | 2.46 | 2.933 (4) | 109 |
| C3—H3···O3 | 0.98 | 2.42 | 2.779 (4) | 101 |
| C1—H1A···O2i | 0.96 | 2.52 | 3.369 (4) | 147 |
| C1—H1B···O1ii | 0.96 | 2.58 | 3.310 (4) | 133 |
| N2—H2A···O2i | 0.89 (2) | 2.32 (3) | 3.141 (3) | 153 |
Symmetry codes: (i) x+1/2, y, −z+3/2; (ii) x+1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: AT2518).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Bruker (2000). SMART (Version 5.625), SAINT (Version 6.01), SHELXTL (Version 6.10) and SADABS (Version 2.03). Bruker AXS Inc., Madison, Wisconsin, USA.
- Han, J., Zhi, S., Wang, L., Pan, Y. & Li, G. (2007). Eur. J. Org. Chem. pp. 1332–1337.
- Kemp, J. E. G. (1991). Comprehensive Organic Synthesis, Vol. 3, edited by B. M. Trost & I. Fleming, pp. 469–513. Oxford: Pergamon Press.
- Li, G., Saibabu, K. S. R. S. & Timmons, C. (2007). Eur. J. Org. Chem. pp. 2745–2758.
- Orlek, B. S. & Stemp, G. (1991). Tetrahedron Lett.32, 4045–4048.
- Qui, J. & Silverman, R. B. (2000). J. Med. Chem.43, 706–720. [DOI] [PubMed]
- Spek, A. L. (2003). J. Appl. Cryst.36, 7–13.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536807067712/at2518sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536807067712/at2518Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



