Abstract
In the title compound, {[Ho4(C12H6O4)6(H2O)5]·1.75H2O}n, which is isostructural with its Tb3+- and Eu3+-containing analogues, there are four crystallographically independent Ho3+ centres, each exhibiting a highly distorted HoO8 bicapped trigonal-prismatic coordination environment. Adjacent polyhedra are interconnected via the carboxylate groups and one μ2-bridging water molecule, forming one-dimensional chains propagating along [100]. The naphthalene-2,6-dicarboxylate ligands further interconnect these chains into a three-dimensional framework, which has zigzag channels housing the water molecules. Two naphthalene-2,6-dicarboxylate bridging ligands have their centroids located on crystallographic centres of inversion. One water O atom has a fixed site occupancy factor of 0.75.
Related literature
For isostructural materials, see: Min & Lee (2002 ▶); Zheng, Sun et al. (2004 ▶). For related structures, see: Zheng, Wang et al. (2004 ▶); Paz & Klinowski (2003 ▶); Almeida Paz & Klinowski (2008 ▶); Wang et al. (2002 ▶). For general background, see: Shi et al. (2008 ▶); Cunha-Silva et al. (2007 ▶). For bond-length data, see: Allen (2002 ▶).
Experimental
Crystal data
[Ho4(C12H6O4)6(H2O5)5]·1.75H2O
M r = 2066.34
Monoclinic,
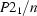
a = 17.0505 (4) Å
b = 15.1728 (4) Å
c = 24.9142 (6) Å
β = 106.126 (1)°
V = 6191.8 (3) Å3
Z = 4
Mo Kα radiation
μ = 5.16 mm−1
T = 180 (2) K
0.12 × 0.12 × 0.01 mm
Data collection
Nonius KappaCCD diffractometer
Absorption correction: multi-scan (SORTAV; Blessing, 1995 ▶) T min = 0.576, T max = 0.950
27842 measured reflections
10664 independent reflections
7421 reflections with I > 2σ(I)
R int = 0.060
Refinement
R[F 2 > 2σ(F 2)] = 0.062
wR(F 2) = 0.172
S = 1.04
10664 reflections
951 parameters
4 restraints
H-atom parameters constrained
Δρmax = 9.72 e Å−3
Δρmin = −2.00 e Å−3
Data collection: COLLECT (Nonius 1998 ▶); cell refinement: HKL SCALEPACK (Otwinowski & Minor 1997 ▶); data reduction: HKL DENZO (Otwinowski & Minor 1997 ▶) and SCALEPACK; program(s) used to solve structure: SIR92 (Altomare et al. 1994 ▶); program(s) used to refine structure: SHELXTL (Bruker, 2001 ▶); molecular graphics: DIAMOND (Brandenburg, 2006 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808000378/hb2668sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808000378/hb2668Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected bond lengths (Å).
| Ho1—O1 | 2.313 (8) |
| Ho1—O3i | 2.335 (7) |
| Ho1—O5 | 2.277 (8) |
| Ho1—O10ii | 2.314 (8) |
| Ho1—O15iii | 2.309 (8) |
| Ho1—O23iv | 2.847 (9) |
| Ho1—O24iv | 2.311 (8) |
| Ho1—O2W | 2.370 (8) |
| Ho2—O2 | 2.299 (8) |
| Ho2—O6 | 2.316 (8) |
| Ho2—O7 | 2.338 (8) |
| Ho2—O11 | 2.264 (8) |
| Ho2—O13 | 2.347 (8) |
| Ho2—O19ii | 2.338 (8) |
| Ho2—O20ii | 2.876 (8) |
| Ho2—O3W | 2.423 (9) |
| Ho3—O8 | 2.410 (8) |
| Ho3—O12 | 2.337 (8) |
| Ho3—O14 | 2.356 (8) |
| Ho3—O17 | 2.299 (7) |
| Ho3—O20ii | 2.299 (8) |
| Ho3—O21 | 2.300 (8) |
| Ho3—O4W | 2.469 (8) |
| Ho3—O5W | 2.640 (6) |
| Ho4—O4v | 2.360 (8) |
| Ho4—O9vi | 2.382 (7) |
| Ho4—O16vii | 2.342 (8) |
| Ho4—O18 | 2.337 (7) |
| Ho4—O22 | 2.276 (7) |
| Ho4—O23viii | 2.296 (8) |
| Ho4—O5W | 2.671 (6) |
| Ho4—O6W | 2.453 (10) |
Symmetry codes: (i) 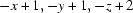 ; (ii)
; (ii) 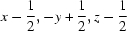 ; (iii)
; (iii) 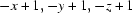 ; (iv)
; (iv) 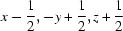 ; (v)
; (v) 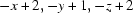 ; (vi)
; (vi) 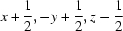 ; (vii)
; (vii) 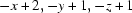 ; (viii)
; (viii) 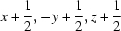 .
.
Acknowledgments
We are grateful to the Fundação para a Ciência e a Tecnologia (FCT, Portugal) for their general financial support under the POCI-PPCDT/QUI/58377/2004 research project supported by FEDER.
supplementary crystallographic information
Comment
Multi-dimensional (i.e., one-dimensional, two-dimensional or three-dimensional) networks, known as coordination polymers or metal-organic frameworks (MOFs), in which metallic centres are bridged via organic ligands, are of considerable interest. Even though structural diversity can be achieved by selecting different metallic centres (which implies a variation in the number and type of the coordination geometry), fascinating structural architectures are often produced by using uncommon bridging ligands. To reconcile the robustness and crystallinity of the synthesized networks, crystal engineers usually employ exo-carboxylate derivatives as the bridging ligands, usually associated with aromatic rings. It is therefore surprising that only a handful of papers reporting lanthanide centres coordinated to residues of naphthalene-2,6-dicarboxylic acid (H2NDC) have been published (Paz & Klinowski, 2008; Zheng, Sun et al., 2004; Zheng, Wang et al., 2004; Paz & Klinowski, 2003; Wang et al., 2002; Min & Lee, 2002), as confirmed by a search in the Cambridge Structural Database (CSD, Version 5.28 with three updates - August 2007; Allen, 2002).
Following our interest in the hydrothermal synthesis of MOFs, (e.g. Shi et al., 2008; Cunha-Silva et al., 2007), we report here the low temperature crystal structure of the title compound, (I), a three-dimensional MOF containing the naphthalene-2,6-dicarboxylate dianion (NDC2-) bound to Ho3+: [Ho4(NDC)6(H2O)5].1.75H2O. Despite being analogous the frameworks reported by Min & Lee (2002) (with Tb3+) and Zheng, Sun et al. (2004) (with Eu3+), this new crystal structure provides further insights into the self-assembly process. Thus crystals of a two-dimensional network, [Ho2(NDC)3(H2O)6], could also be isolated from the same synthetic batch (Paz & Klinowski, 2008). We infer that the ionic radius of the lanthanide employed determines whether a three-dimensional (for the lighter series of lanthanides - up to Dy3+) or a two-dimensional network (for lanthanides after and including Er3+) is obtained. Ho3+ always produces a mixture of the two materials, even though it is possible to vary the amount of each framework in the product by adjusting the composition of the synthesis mixture.
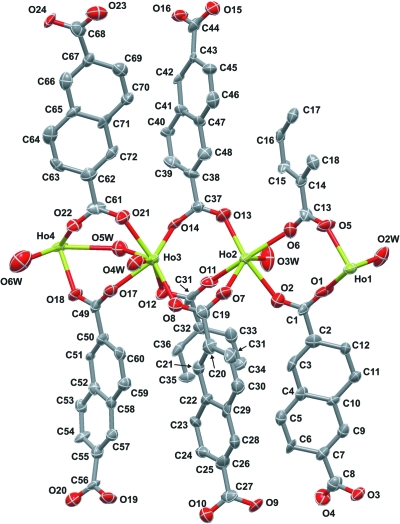
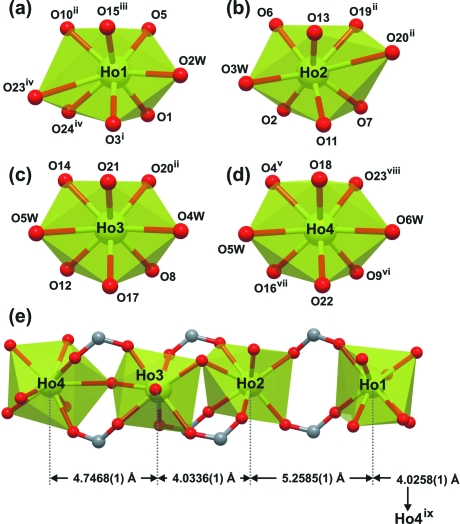
Compound (I) contains four crystallographically independent Ho3+ centres (Ho1 to Ho4) which are coordinated to a total of six NDC2- ligands (two of these have their centroids located at crystallographic inversion centres) and five water molecules. The coordination sphere of each metallic centre is composed by one unidentate water molecule, with the fifth water (O5W) bridging two neighbouring metallic centres (Ho3 and Ho4 - see Figure 1). Despite the large number of crystallographically independent moieties, the NDC2- moieties coordinate to the Ho3+ centres through only two distinct coordination fashions: a syn,syn-chelate coupled to a syn,syn-µ2-bridge (for the C56 and C68 carboxylate groups), and simple syn,syn-µ2-bridges (for all remaining carboxylate moieties). The {HoO8} coordination geometries for the Ho3+ centres remain strikingly similar, resembling highly distorted bicapped trigonal prisms (Figures 2a to 2 d), with the capping positions being either water molecules or the O-atoms involved in the syn,syn-µ2-bridges coupled to syn,syn-chelate mentioned above (O20 and O23 - Figure 2). Disregarding the Ho—O distances related to the O20, O23 and O5W atoms which occupy the capping positions of the coordination polyhedra, the remaining Ho—O distances are typical and well within the ranges registered for related materials (as revealed by a search in the CSD - 77 entries, range of 2.20–2.82 Å with a median of 2.34 Å): for Ho1 to Ho4, respectively, 2.277 (8)–2.370 (8) Å, 2.264 (8)–2.423 (9) Å, 2.299 (7)–2.469 (8) Å and 2.276 (7)–2.453 (10) Å (Table 1). We emphasize that even though the Ho—O distances associated with these capping positions are unusually long, they are still within the feasible range found in related materials. Moreover, we also note that the longest values of Ho—O for Ho1 to Ho4 found in the ranges given above are those with the coordinated water molecules. In fact, by restricting the search in the CSD to the geometrical parameters for coordinated water molecules to Ho3+ centres, the expected range is from 2.28 to 2.55 Å, which is in good agreement with the experimental data for the title compound.
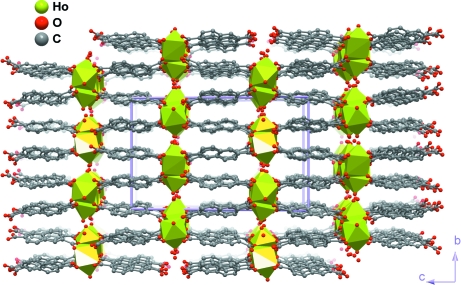
The connection between neighbouring {HoO8} polyhedra via the carboxylate groups and water molecules leads to the formation of a one-dimensional chain of metallic centres running along the [100] crystallographic direction (Figure 2 e). The Ho···Ho distances range from 4.0258 (1) to 5.2585 (1) Å. These chains are interconnected along the [001] direction via the NDC2- bridges forming a three-dimensional MOF (Figure 3). There is structural evidence that such connectivity creates small one-dimensional zigzag channels parallel to the a-axis, distributed in a typical brick-wall fashion in the bc plane containing the water molecules of crystallization O1W and O7W. Although the water H atoms could not be located in the present study, presumably O—H···O hydrogen bonds from the water molecules (both coordinated and uncoordinated) interconnect adjacent chains (not shown).
Experimental
To a solution of HoCl3.6H2O (1.062 g, 2.799 mmol) in distilled water (6.04 g), naphthalene-2,6-dicarboxylic acid (0.102 g, 0.472 mmol) and triethylamine (0.089 g, 0.880 mmol) were added and the mixture was stirred thoroughly for 5 minutes at ambient temperature. The suspension, with a molar composition of 5.93 Ho3+: 1.00 H2NDC: 1.86 TEA: 120 H2O, was transferred to a Parr teflon-lined stainless steel vessel (ca 21 cm3) and placed for 8 h at 418 K in a preheated oven. Before opening, the reaction vessel was allowed to cool slowly to ambient temperature at a rate of 10 K per hour over a period of 14 h. Colourless plates of (I) were manually selected from the product which also contains [Ho2(NDC)3(H2O)6] (Paz & Klinowski, 2008).
Refinement
The water molecules O1W, O5W and O7W were refined isotropically. Following structural evidence from unrestrained refinement cycles, the O7W water molecule was given a fixed occupancy of 75% in the final structural model.
It is important to stress that a considerable smeared-out electron density was found surrounding the water molecules O1W and O5W. Attempts to model this disorder (during the last stages of the overall structural refinement) over two (or more) partially occupied sites (for each water molecule) did not produce satisfactory models, with large shifts associated with these chemical moieties being observed. In order to achieve full convergence the positions of O1W and O5W were restrained to be equally distant from, respectively, Ho1 and Ho2, and Ho3 and Ho4 (one free variable for each pair of distances). The difficulties while modelling these two water molecules are attributed to the quality of the crystal used for data collection, which was a very small and thin colourless plate diffracting rather weakly at high angles [e.g., almost no reflections were observed for resolutions higher than 0.80 Å]. The highest difference peak is 0.78Å from O5W.
H atoms associated with all water molecules could not be located from difference Fourier maps, and attempts to place these atoms in calculated positions in order to maximize hydrogen bonding interactions did not lead to chemically reasonable structural models and they were omitted from the refinement. The H atoms bound to carbon were placed at idealized positions (C—H = 0.95 Å) and refined as riding with Uiso = 1.2Ueq(C).
Figures
Fig. 1.
Simplified representation of the asymmetric unit of (I) with displacement ellipsoids drawn at the 80% probability level. Water molecules O1W and O7W, and hydrogen atoms have been omitted for clarity.
Fig. 2.
Polyhedral representation of the {HoO8} coordination environments, which resemble highly distorted bicapped trigonal prisms, for: (a) Ho1, (b) Ho2, (c) Ho3 and (d) Ho4. (e) Interconnection of the individual {HoO8} polyhedra along the [100] crystallographic direction leading to the formation of one-dimensional chains. For selected bond lengths (in Å) see the dedicated Table in the main paper. Symmetry codes used to generate equivalent atoms: (i) -x + 1, -y + 1, -z + 2; (ii) x-1/2, -y + 1/2, z-1/2; (iii) -x + 1, -y + 1, -z + 1; (iv) x-1/2, -y + 1/2, z+1/2; (v) -x + 2, -y + 1, -z + 2; (vi) x+1/2, -y + 1/2, z-1/2; (vii) -x + 2, -y + 1, -z + 1; (viii) x+1/2, -y + 1/2, z+1/2; (ix) x-1, y, z.
Fig. 3.
Crystal packing of the title compound viewed in perspective along the [100] direction of the unit cell. Hydrogen atoms have been omitted for clarity.
Crystal data
| [Ho4(C12H6O4)6(H2O5)5]·1.75H2O | F(000) = 3982 |
| Mr = 2066.34 | Dx = 2.217 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2yn | Cell parameters from 70959 reflections |
| a = 17.0505 (4) Å | θ = 1.0–25.0° |
| b = 15.1728 (4) Å | µ = 5.16 mm−1 |
| c = 24.9142 (6) Å | T = 180 K |
| β = 106.126 (1)° | Plate, colourless |
| V = 6191.8 (3) Å3 | 0.12 × 0.12 × 0.01 mm |
| Z = 4 |
Data collection
| Nonius Kappa CCD diffractometer | 7421 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.060 |
| Thin slice ω and φ scans | θmax = 25.0°, θmin = 3.6° |
| Absorption correction: multi-scan (SORTAV; Blessing, 1995) | h = −20→20 |
| Tmin = 0.576, Tmax = 0.950 | k = −18→18 |
| 27842 measured reflections | l = −29→29 |
| 10664 independent reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.062 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.172 | H-atom parameters constrained |
| S = 1.04 | w = 1/[σ2(Fo2) + (0.0951P)2 + 49.5291P] where P = (Fo2 + 2Fc2)/3 |
| 10664 reflections | (Δ/σ)max = 0.001 |
| 951 parameters | Δρmax = 9.73 e Å−3 |
| 4 restraints | Δρmin = −2.01 e Å−3 |
Special details
| Experimental. See dedicated section in the main paper |
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Ho1 | 0.43036 (3) | 0.43328 (3) | 0.74931 (2) | 0.01641 (16) | |
| Ho2 | 0.73944 (3) | 0.44550 (4) | 0.75148 (2) | 0.01864 (16) | |
| Ho3 | 0.94596 (3) | 0.31502 (3) | 0.75250 (2) | 0.01782 (16) | |
| Ho4 | 1.22082 (3) | 0.30713 (4) | 0.74454 (2) | 0.02020 (16) | |
| O1W | 0.5958 (6) | 0.2081 (11) | 0.7441 (7) | 0.108 (5)* | |
| O2W | 0.4125 (5) | 0.5883 (5) | 0.7439 (4) | 0.028 (2) | |
| O3W | 0.7616 (5) | 0.6017 (6) | 0.7697 (4) | 0.039 (2) | |
| O4W | 0.9089 (5) | 0.1577 (5) | 0.7388 (3) | 0.0251 (18) | |
| O5W | 1.0839 (4) | 0.3895 (5) | 0.7479 (3) | 0.0263 (19)* | |
| O6W | 1.2552 (6) | 0.1500 (7) | 0.7565 (4) | 0.046 (3) | |
| O7W | 0.0661 (11) | 0.6098 (13) | 0.7506 (7) | 0.080 (5)* | 0.75 |
| O1 | 0.5535 (5) | 0.4870 (6) | 0.8042 (3) | 0.0273 (19) | |
| O2 | 0.6872 (5) | 0.4687 (6) | 0.8260 (4) | 0.030 (2) | |
| O3 | 0.6279 (5) | 0.5218 (5) | 1.1807 (3) | 0.0226 (18) | |
| O4 | 0.7584 (5) | 0.5611 (5) | 1.2030 (3) | 0.0265 (19) | |
| O5 | 0.4886 (5) | 0.4701 (6) | 0.6805 (3) | 0.0274 (19) | |
| O6 | 0.6189 (5) | 0.5100 (5) | 0.7000 (3) | 0.0251 (18) | |
| O7 | 0.7555 (5) | 0.3124 (5) | 0.8006 (3) | 0.0257 (19) | |
| O8 | 0.8854 (5) | 0.2691 (5) | 0.8246 (3) | 0.0224 (17) | |
| O9 | 0.7805 (5) | 0.2343 (6) | 1.1724 (3) | 0.0272 (19) | |
| O10 | 0.9123 (5) | 0.1926 (5) | 1.1948 (3) | 0.0271 (19) | |
| O11 | 0.8714 (5) | 0.4522 (5) | 0.8033 (3) | 0.0221 (18) | |
| O12 | 0.9998 (5) | 0.4138 (6) | 0.8254 (3) | 0.0263 (19) | |
| O13 | 0.7983 (5) | 0.4924 (6) | 0.6817 (3) | 0.0286 (19) | |
| O14 | 0.9277 (5) | 0.4469 (5) | 0.7005 (3) | 0.0255 (19) | |
| O15 | 0.7017 (5) | 0.5512 (6) | 0.3068 (3) | 0.029 (2) | |
| O16 | 0.8295 (5) | 0.5917 (6) | 0.3278 (3) | 0.0275 (19) | |
| O17 | 1.0540 (4) | 0.2363 (5) | 0.8072 (3) | 0.0193 (17) | |
| O18 | 1.1862 (5) | 0.2690 (6) | 0.8260 (3) | 0.0255 (19) | |
| O19 | 1.1803 (5) | 0.1574 (6) | 1.1822 (3) | 0.0244 (18) | |
| O20 | 1.3118 (5) | 0.1900 (5) | 1.2001 (3) | 0.0279 (19) | |
| O21 | 0.9784 (4) | 0.2705 (6) | 0.6730 (3) | 0.0237 (18) | |
| O22 | 1.1109 (4) | 0.2322 (5) | 0.6914 (3) | 0.0205 (17) | |
| O23 | 0.8546 (5) | 0.2013 (5) | 0.2970 (3) | 0.029 (2) | |
| O24 | 0.9861 (5) | 0.1763 (6) | 0.3129 (3) | 0.027 (2) | |
| C1 | 0.6227 (6) | 0.4803 (8) | 0.8388 (5) | 0.018 (2) | |
| C2 | 0.6267 (7) | 0.4936 (7) | 0.8990 (5) | 0.017 (2) | |
| C3 | 0.6942 (6) | 0.4698 (7) | 0.9413 (5) | 0.017 (2) | |
| H3 | 0.7390 | 0.4426 | 0.9321 | 0.020* | |
| C4 | 0.6985 (6) | 0.4851 (8) | 0.9988 (5) | 0.018 (2) | |
| C5 | 0.7657 (7) | 0.4613 (7) | 1.0441 (5) | 0.018 (2) | |
| H5 | 0.8115 | 0.4337 | 1.0367 | 0.021* | |
| C6 | 0.7666 (7) | 0.4770 (8) | 1.0988 (5) | 0.021 (3) | |
| H6 | 0.8137 | 0.4634 | 1.1284 | 0.025* | |
| C7 | 0.6966 (7) | 0.5137 (7) | 1.1106 (5) | 0.019 (2) | |
| C8 | 0.6950 (7) | 0.5322 (8) | 1.1689 (5) | 0.023 (3) | |
| C9 | 0.6303 (7) | 0.5372 (7) | 1.0675 (5) | 0.017 (2) | |
| H9 | 0.5837 | 0.5621 | 1.0756 | 0.021* | |
| C10 | 0.6301 (6) | 0.5250 (7) | 1.0112 (4) | 0.013 (2) | |
| C11 | 0.5608 (7) | 0.5494 (7) | 0.9665 (5) | 0.019 (2) | |
| H11 | 0.5152 | 0.5765 | 0.9746 | 0.023* | |
| C12 | 0.5597 (6) | 0.5342 (7) | 0.9130 (5) | 0.016 (2) | |
| H12 | 0.5131 | 0.5510 | 0.8839 | 0.019* | |
| C13 | 0.5503 (6) | 0.4905 (7) | 0.6661 (5) | 0.017 (2) | |
| C14 | 0.5434 (6) | 0.5002 (7) | 0.6056 (5) | 0.016 (2) | |
| C15 | 0.6068 (6) | 0.5430 (7) | 0.5885 (4) | 0.017 (2) | |
| H15 | 0.6541 | 0.5631 | 0.6158 | 0.020* | |
| C16 | 0.5998 (6) | 0.5554 (7) | 0.5329 (5) | 0.017 (2) | |
| H16 | 0.6416 | 0.5861 | 0.5222 | 0.020* | |
| C17 | 0.5312 (6) | 0.5233 (7) | 0.4910 (5) | 0.017 (2) | |
| C18 | 0.4772 (6) | 0.4677 (7) | 0.5661 (5) | 0.015 (2) | |
| H18 | 0.4357 | 0.4382 | 0.5778 | 0.018* | |
| C19 | 0.8176 (7) | 0.2826 (8) | 0.8349 (5) | 0.021 (3) | |
| C20 | 0.8111 (7) | 0.2621 (7) | 0.8932 (5) | 0.019 (2) | |
| C21 | 0.8757 (6) | 0.2863 (7) | 0.9394 (5) | 0.016 (2) | |
| H21 | 0.9242 | 0.3103 | 0.9337 | 0.019* | |
| C22 | 0.8690 (7) | 0.2752 (8) | 0.9943 (5) | 0.021 (3) | |
| C23 | 0.9341 (7) | 0.2967 (8) | 1.0427 (5) | 0.020 (2) | |
| H23 | 0.9828 | 0.3228 | 1.0387 | 0.024* | |
| C24 | 0.9259 (7) | 0.2794 (7) | 1.0947 (4) | 0.018 (2) | |
| H24 | 0.9704 | 0.2916 | 1.1264 | 0.021* | |
| C25 | 0.8527 (7) | 0.2439 (8) | 1.1029 (5) | 0.024 (3) | |
| C26 | 0.8468 (7) | 0.2230 (7) | 1.1597 (5) | 0.021 (3) | |
| C27 | 0.7881 (6) | 0.2240 (7) | 1.0570 (5) | 0.019 (2) | |
| H27 | 0.7388 | 0.2011 | 1.0621 | 0.023* | |
| C28 | 0.7956 (7) | 0.2380 (7) | 1.0023 (5) | 0.018 (2) | |
| C29 | 0.7310 (7) | 0.2155 (8) | 0.9532 (5) | 0.021 (3) | |
| H29 | 0.6820 | 0.1906 | 0.9577 | 0.026* | |
| C30 | 0.7375 (6) | 0.2283 (8) | 0.9012 (5) | 0.023 (3) | |
| H30 | 0.6929 | 0.2148 | 0.8698 | 0.027* | |
| C31 | 0.9407 (7) | 0.4500 (7) | 0.8369 (5) | 0.019 (2) | |
| C32 | 0.9522 (7) | 0.4816 (7) | 0.8958 (5) | 0.018 (2) | |
| C33 | 0.8895 (7) | 0.5255 (7) | 0.9109 (5) | 0.023 (3) | |
| H33 | 0.8419 | 0.5425 | 0.8827 | 0.028* | |
| C34 | 0.8955 (7) | 0.5444 (7) | 0.9651 (5) | 0.018 (2) | |
| H34 | 0.8516 | 0.5726 | 0.9747 | 0.021* | |
| C35 | 1.0323 (7) | 0.4782 (7) | 0.9923 (4) | 0.017 (2) | |
| C36 | 1.0245 (7) | 0.4606 (8) | 0.9367 (5) | 0.021 (3) | |
| H36 | 1.0684 | 0.4341 | 0.9261 | 0.025* | |
| C37 | 0.8618 (7) | 0.4769 (7) | 0.6682 (4) | 0.017 (2) | |
| C38 | 0.8630 (7) | 0.4925 (7) | 0.6092 (4) | 0.019 (2) | |
| C39 | 0.9333 (7) | 0.5273 (7) | 0.5949 (5) | 0.021 (3) | |
| H39 | 0.9816 | 0.5403 | 0.6236 | 0.026* | |
| C40 | 0.9309 (6) | 0.5418 (7) | 0.5409 (4) | 0.016 (2) | |
| H40 | 0.9775 | 0.5658 | 0.5325 | 0.019* | |
| C41 | 0.8605 (6) | 0.5219 (7) | 0.4964 (4) | 0.015 (2) | |
| C42 | 0.8534 (6) | 0.5405 (7) | 0.4398 (4) | 0.013 (2) | |
| H42 | 0.8984 | 0.5668 | 0.4303 | 0.015* | |
| C43 | 0.7835 (6) | 0.5220 (7) | 0.3975 (4) | 0.015 (2) | |
| C44 | 0.7714 (7) | 0.5548 (8) | 0.3403 (5) | 0.021 (3) | |
| C45 | 0.7181 (7) | 0.4779 (7) | 0.4120 (4) | 0.017 (2) | |
| H45 | 0.6710 | 0.4612 | 0.3832 | 0.021* | |
| C46 | 0.7218 (6) | 0.4594 (7) | 0.4660 (5) | 0.018 (2) | |
| H46 | 0.6772 | 0.4307 | 0.4745 | 0.021* | |
| C47 | 0.7923 (6) | 0.4830 (7) | 0.5103 (4) | 0.015 (2) | |
| C48 | 0.7949 (7) | 0.4712 (7) | 0.5676 (5) | 0.018 (2) | |
| H48 | 0.7485 | 0.4481 | 0.5768 | 0.021* | |
| C49 | 1.1229 (6) | 0.2538 (7) | 0.8407 (5) | 0.017 (2) | |
| C50 | 1.1273 (6) | 0.2519 (7) | 0.9011 (5) | 0.017 (2) | |
| C51 | 1.1979 (6) | 0.2820 (7) | 0.9410 (4) | 0.013 (2) | |
| H51 | 1.2405 | 0.3098 | 0.9294 | 0.016* | |
| C52 | 1.2048 (6) | 0.2704 (7) | 0.9985 (4) | 0.014 (2) | |
| C53 | 1.2777 (6) | 0.2962 (7) | 1.0406 (5) | 0.017 (2) | |
| H53 | 1.3196 | 0.3276 | 1.0303 | 0.020* | |
| C54 | 1.2864 (7) | 0.2755 (7) | 1.0951 (4) | 0.018 (2) | |
| H54 | 1.3353 | 0.2908 | 1.1226 | 0.022* | |
| C55 | 1.2227 (6) | 0.2308 (8) | 1.1113 (5) | 0.020 (2) | |
| C56 | 1.2393 (7) | 0.1930 (7) | 1.1688 (4) | 0.017 (2) | |
| C57 | 1.1509 (6) | 0.2115 (7) | 1.0724 (4) | 0.016 (2) | |
| H57 | 1.1080 | 0.1843 | 1.0838 | 0.019* | |
| C58 | 1.1396 (6) | 0.2314 (7) | 1.0155 (4) | 0.015 (2) | |
| C59 | 1.0675 (6) | 0.2059 (7) | 0.9728 (5) | 0.018 (2) | |
| H59 | 1.0224 | 0.1819 | 0.9832 | 0.021* | |
| C60 | 1.0627 (6) | 0.2154 (7) | 0.9185 (5) | 0.017 (2) | |
| H60 | 1.0147 | 0.1970 | 0.8912 | 0.020* | |
| C61 | 1.0416 (7) | 0.2537 (7) | 0.6579 (4) | 0.020 (3) | |
| C62 | 1.0362 (7) | 0.2519 (7) | 0.5969 (5) | 0.021 (3) | |
| C63 | 1.1013 (6) | 0.2124 (7) | 0.5797 (5) | 0.016 (2) | |
| H63 | 1.1487 | 0.1917 | 0.6067 | 0.019* | |
| C64 | 1.0947 (7) | 0.2049 (7) | 0.5240 (5) | 0.023 (3) | |
| H64 | 1.1384 | 0.1796 | 0.5125 | 0.027* | |
| C65 | 1.0234 (6) | 0.2345 (7) | 0.4826 (4) | 0.017 (2) | |
| C66 | 1.0120 (7) | 0.2199 (7) | 0.4252 (5) | 0.021 (3) | |
| H66 | 1.0551 | 0.1945 | 0.4131 | 0.025* | |
| C67 | 0.9396 (7) | 0.2414 (7) | 0.3858 (5) | 0.018 (2) | |
| C68 | 0.9257 (7) | 0.2056 (8) | 0.3278 (5) | 0.023 (3) | |
| C69 | 0.8791 (7) | 0.2877 (7) | 0.4044 (5) | 0.021 (2) | |
| H69 | 0.8306 | 0.3070 | 0.3778 | 0.025* | |
| C70 | 0.8896 (7) | 0.3041 (7) | 0.4580 (5) | 0.019 (2) | |
| H70 | 0.8482 | 0.3351 | 0.4690 | 0.023* | |
| C71 | 0.9601 (6) | 0.2772 (7) | 0.4996 (4) | 0.015 (2) | |
| C72 | 0.9682 (6) | 0.2855 (7) | 0.5576 (5) | 0.016 (2) | |
| H72 | 0.9266 | 0.3143 | 0.5698 | 0.019* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Ho1 | 0.0159 (3) | 0.0228 (3) | 0.0110 (3) | −0.0031 (2) | 0.00474 (19) | −0.0011 (2) |
| Ho2 | 0.0156 (3) | 0.0290 (3) | 0.0126 (3) | 0.0039 (2) | 0.0060 (2) | −0.0003 (2) |
| Ho3 | 0.0158 (3) | 0.0273 (3) | 0.0115 (3) | 0.0032 (2) | 0.0057 (2) | −0.0002 (2) |
| Ho4 | 0.0173 (3) | 0.0331 (3) | 0.0116 (3) | −0.0074 (2) | 0.0063 (2) | −0.0006 (2) |
| O2W | 0.029 (5) | 0.023 (4) | 0.043 (5) | −0.006 (4) | 0.026 (4) | −0.002 (4) |
| O3W | 0.026 (5) | 0.026 (5) | 0.065 (7) | 0.000 (4) | 0.012 (4) | −0.011 (5) |
| O4W | 0.026 (5) | 0.026 (4) | 0.026 (5) | −0.004 (4) | 0.012 (4) | −0.003 (4) |
| O6W | 0.050 (6) | 0.044 (6) | 0.052 (7) | −0.012 (5) | 0.030 (5) | −0.006 (5) |
| O1 | 0.027 (5) | 0.044 (5) | 0.015 (4) | −0.011 (4) | 0.013 (4) | −0.003 (4) |
| O2 | 0.024 (5) | 0.045 (5) | 0.024 (5) | 0.008 (4) | 0.009 (4) | −0.001 (4) |
| O3 | 0.022 (4) | 0.031 (5) | 0.018 (4) | 0.000 (3) | 0.011 (3) | −0.004 (3) |
| O4 | 0.021 (4) | 0.031 (5) | 0.026 (5) | −0.003 (4) | 0.003 (4) | −0.006 (4) |
| O5 | 0.023 (4) | 0.046 (5) | 0.016 (4) | −0.008 (4) | 0.011 (3) | 0.003 (4) |
| O6 | 0.021 (4) | 0.030 (5) | 0.027 (5) | 0.007 (4) | 0.009 (4) | 0.004 (4) |
| O7 | 0.027 (5) | 0.035 (5) | 0.018 (4) | 0.002 (4) | 0.010 (4) | 0.005 (4) |
| O8 | 0.021 (4) | 0.027 (4) | 0.019 (4) | 0.001 (3) | 0.005 (3) | −0.002 (3) |
| O9 | 0.028 (5) | 0.041 (5) | 0.020 (4) | 0.009 (4) | 0.018 (4) | 0.004 (4) |
| O10 | 0.020 (4) | 0.037 (5) | 0.024 (5) | 0.006 (4) | 0.005 (4) | 0.006 (4) |
| O11 | 0.022 (4) | 0.031 (5) | 0.012 (4) | −0.009 (4) | 0.002 (3) | −0.003 (3) |
| O12 | 0.019 (4) | 0.040 (5) | 0.022 (4) | −0.002 (4) | 0.009 (3) | −0.010 (4) |
| O13 | 0.027 (5) | 0.039 (5) | 0.024 (5) | 0.006 (4) | 0.014 (4) | 0.007 (4) |
| O14 | 0.027 (5) | 0.039 (5) | 0.016 (4) | 0.006 (4) | 0.016 (4) | 0.002 (4) |
| O15 | 0.013 (4) | 0.053 (6) | 0.018 (4) | 0.002 (4) | 0.000 (3) | −0.002 (4) |
| O16 | 0.022 (4) | 0.047 (5) | 0.015 (4) | 0.001 (4) | 0.007 (3) | 0.004 (4) |
| O17 | 0.024 (4) | 0.020 (4) | 0.017 (4) | 0.002 (3) | 0.012 (3) | 0.004 (3) |
| O18 | 0.018 (4) | 0.044 (5) | 0.018 (4) | 0.002 (4) | 0.011 (3) | 0.006 (4) |
| O19 | 0.021 (4) | 0.036 (5) | 0.018 (4) | −0.002 (4) | 0.007 (3) | 0.011 (4) |
| O20 | 0.030 (5) | 0.034 (5) | 0.022 (5) | 0.003 (4) | 0.011 (4) | −0.002 (4) |
| O21 | 0.015 (4) | 0.038 (5) | 0.019 (4) | −0.002 (4) | 0.005 (3) | −0.005 (4) |
| O22 | 0.013 (4) | 0.033 (5) | 0.014 (4) | −0.007 (3) | 0.001 (3) | −0.005 (3) |
| O23 | 0.031 (5) | 0.036 (5) | 0.019 (4) | −0.010 (4) | 0.006 (4) | 0.001 (4) |
| O24 | 0.021 (4) | 0.046 (5) | 0.018 (4) | 0.009 (4) | 0.010 (3) | −0.009 (4) |
| C1 | 0.010 (5) | 0.033 (7) | 0.014 (6) | −0.003 (5) | 0.005 (4) | −0.007 (5) |
| C2 | 0.019 (6) | 0.015 (5) | 0.019 (6) | −0.007 (5) | 0.008 (5) | 0.003 (5) |
| C3 | 0.016 (6) | 0.016 (6) | 0.024 (6) | 0.000 (4) | 0.013 (5) | −0.006 (5) |
| C4 | 0.010 (5) | 0.027 (6) | 0.019 (6) | −0.004 (5) | 0.007 (4) | 0.002 (5) |
| C5 | 0.015 (6) | 0.017 (6) | 0.024 (7) | 0.001 (4) | 0.009 (5) | 0.000 (5) |
| C6 | 0.014 (6) | 0.033 (7) | 0.021 (6) | 0.004 (5) | 0.014 (5) | 0.004 (5) |
| C7 | 0.021 (6) | 0.022 (6) | 0.019 (6) | −0.001 (5) | 0.012 (5) | −0.002 (5) |
| C8 | 0.024 (7) | 0.023 (6) | 0.027 (7) | −0.003 (5) | 0.014 (5) | 0.002 (5) |
| C9 | 0.018 (6) | 0.016 (6) | 0.021 (6) | 0.001 (4) | 0.011 (5) | 0.000 (5) |
| C10 | 0.018 (6) | 0.013 (5) | 0.010 (5) | −0.002 (4) | 0.008 (4) | 0.000 (4) |
| C11 | 0.021 (6) | 0.023 (6) | 0.017 (6) | 0.005 (5) | 0.011 (5) | 0.003 (5) |
| C12 | 0.015 (6) | 0.019 (6) | 0.015 (6) | 0.005 (4) | 0.006 (4) | 0.002 (4) |
| C13 | 0.010 (5) | 0.021 (6) | 0.017 (6) | −0.003 (5) | −0.001 (4) | −0.005 (5) |
| C14 | 0.012 (5) | 0.020 (6) | 0.018 (6) | 0.000 (4) | 0.006 (4) | −0.003 (5) |
| C15 | 0.013 (5) | 0.023 (6) | 0.013 (6) | −0.002 (5) | 0.003 (4) | −0.012 (5) |
| C16 | 0.009 (5) | 0.022 (6) | 0.022 (6) | 0.002 (4) | 0.008 (4) | 0.001 (5) |
| C17 | 0.014 (5) | 0.017 (6) | 0.022 (6) | −0.001 (4) | 0.008 (5) | −0.006 (5) |
| C18 | 0.013 (5) | 0.009 (5) | 0.021 (6) | −0.002 (4) | 0.002 (4) | −0.002 (4) |
| C19 | 0.016 (6) | 0.026 (6) | 0.019 (6) | −0.002 (5) | 0.002 (5) | −0.001 (5) |
| C20 | 0.023 (6) | 0.011 (5) | 0.022 (6) | 0.000 (5) | 0.006 (5) | −0.004 (5) |
| C21 | 0.014 (5) | 0.013 (5) | 0.021 (6) | 0.001 (4) | 0.006 (4) | 0.001 (4) |
| C22 | 0.019 (6) | 0.032 (7) | 0.015 (6) | 0.008 (5) | 0.009 (5) | 0.003 (5) |
| C23 | 0.017 (6) | 0.029 (6) | 0.013 (6) | 0.010 (5) | 0.004 (4) | 0.006 (5) |
| C24 | 0.025 (6) | 0.016 (6) | 0.012 (6) | 0.003 (5) | 0.004 (4) | 0.002 (4) |
| C25 | 0.028 (7) | 0.023 (6) | 0.028 (7) | 0.007 (5) | 0.019 (5) | 0.011 (5) |
| C26 | 0.036 (7) | 0.014 (6) | 0.015 (6) | 0.003 (5) | 0.010 (5) | −0.004 (5) |
| C27 | 0.014 (6) | 0.025 (6) | 0.019 (6) | 0.008 (5) | 0.005 (4) | 0.002 (5) |
| C28 | 0.021 (6) | 0.011 (5) | 0.027 (7) | 0.008 (4) | 0.015 (5) | 0.001 (5) |
| C29 | 0.017 (6) | 0.024 (6) | 0.025 (7) | 0.003 (5) | 0.007 (5) | 0.002 (5) |
| C30 | 0.010 (5) | 0.034 (7) | 0.022 (6) | −0.001 (5) | 0.001 (4) | −0.005 (5) |
| C31 | 0.022 (6) | 0.020 (6) | 0.023 (6) | 0.000 (5) | 0.019 (5) | 0.001 (5) |
| C32 | 0.017 (6) | 0.019 (6) | 0.019 (6) | −0.001 (5) | 0.006 (5) | 0.006 (5) |
| C33 | 0.018 (6) | 0.022 (6) | 0.031 (7) | 0.005 (5) | 0.011 (5) | 0.009 (5) |
| C34 | 0.018 (6) | 0.020 (6) | 0.016 (6) | −0.002 (5) | 0.006 (5) | −0.004 (5) |
| C35 | 0.021 (6) | 0.018 (6) | 0.013 (6) | −0.006 (5) | 0.006 (5) | 0.002 (4) |
| C36 | 0.019 (6) | 0.028 (6) | 0.021 (6) | −0.003 (5) | 0.014 (5) | −0.008 (5) |
| C37 | 0.021 (6) | 0.017 (6) | 0.014 (6) | 0.002 (5) | 0.007 (5) | 0.001 (4) |
| C38 | 0.024 (6) | 0.020 (6) | 0.011 (6) | 0.001 (5) | 0.001 (5) | 0.004 (4) |
| C39 | 0.019 (6) | 0.025 (6) | 0.016 (6) | −0.003 (5) | −0.001 (5) | 0.000 (5) |
| C40 | 0.015 (6) | 0.023 (6) | 0.012 (6) | −0.003 (5) | 0.006 (4) | 0.004 (4) |
| C41 | 0.017 (6) | 0.014 (5) | 0.015 (6) | 0.000 (4) | 0.005 (4) | 0.005 (4) |
| C42 | 0.011 (5) | 0.019 (5) | 0.011 (6) | −0.002 (4) | 0.008 (4) | −0.002 (4) |
| C43 | 0.012 (5) | 0.022 (6) | 0.010 (5) | 0.011 (4) | 0.004 (4) | −0.001 (4) |
| C44 | 0.019 (6) | 0.026 (6) | 0.024 (7) | −0.001 (5) | 0.014 (5) | −0.002 (5) |
| C45 | 0.020 (6) | 0.020 (6) | 0.012 (6) | 0.001 (5) | 0.005 (4) | 0.002 (4) |
| C46 | 0.011 (5) | 0.016 (6) | 0.027 (7) | −0.005 (4) | 0.007 (5) | 0.001 (5) |
| C47 | 0.014 (5) | 0.018 (6) | 0.015 (6) | 0.003 (4) | 0.006 (4) | 0.008 (4) |
| C48 | 0.017 (6) | 0.018 (6) | 0.017 (6) | −0.005 (5) | 0.004 (5) | −0.005 (5) |
| C49 | 0.014 (6) | 0.021 (6) | 0.019 (6) | 0.002 (5) | 0.009 (5) | −0.002 (5) |
| C50 | 0.014 (5) | 0.024 (6) | 0.016 (6) | 0.000 (5) | 0.008 (4) | 0.002 (5) |
| C51 | 0.013 (5) | 0.020 (6) | 0.010 (5) | 0.006 (4) | 0.007 (4) | 0.005 (4) |
| C52 | 0.012 (5) | 0.013 (5) | 0.017 (6) | −0.001 (4) | 0.003 (4) | −0.002 (4) |
| C53 | 0.013 (5) | 0.016 (6) | 0.022 (6) | 0.000 (4) | 0.005 (4) | 0.003 (5) |
| C54 | 0.021 (6) | 0.024 (6) | 0.012 (6) | −0.007 (5) | 0.010 (4) | −0.006 (5) |
| C55 | 0.015 (6) | 0.026 (6) | 0.020 (6) | 0.002 (5) | 0.006 (5) | −0.001 (5) |
| C56 | 0.017 (6) | 0.026 (6) | 0.011 (5) | −0.003 (5) | 0.010 (4) | −0.002 (4) |
| C57 | 0.013 (5) | 0.023 (6) | 0.013 (6) | 0.000 (5) | 0.005 (4) | 0.000 (5) |
| C58 | 0.015 (5) | 0.016 (5) | 0.017 (6) | 0.005 (4) | 0.006 (4) | 0.002 (4) |
| C59 | 0.015 (6) | 0.021 (6) | 0.018 (6) | −0.002 (5) | 0.006 (4) | 0.001 (5) |
| C60 | 0.008 (5) | 0.023 (6) | 0.019 (6) | 0.000 (4) | 0.003 (4) | 0.005 (5) |
| C61 | 0.019 (6) | 0.025 (6) | 0.014 (6) | −0.015 (5) | 0.001 (5) | −0.006 (5) |
| C62 | 0.023 (6) | 0.021 (6) | 0.020 (6) | −0.005 (5) | 0.008 (5) | −0.001 (5) |
| C63 | 0.012 (5) | 0.014 (5) | 0.019 (6) | −0.002 (4) | 0.001 (4) | 0.009 (4) |
| C64 | 0.020 (6) | 0.018 (6) | 0.035 (7) | −0.004 (5) | 0.015 (5) | 0.003 (5) |
| C65 | 0.017 (6) | 0.024 (6) | 0.014 (6) | −0.007 (5) | 0.010 (4) | −0.007 (5) |
| C66 | 0.022 (6) | 0.013 (6) | 0.030 (7) | 0.000 (5) | 0.009 (5) | −0.002 (5) |
| C67 | 0.019 (6) | 0.018 (6) | 0.020 (6) | 0.001 (5) | 0.012 (5) | −0.001 (5) |
| C68 | 0.016 (6) | 0.028 (6) | 0.023 (6) | −0.003 (5) | 0.003 (5) | 0.001 (5) |
| C69 | 0.018 (6) | 0.022 (6) | 0.021 (6) | −0.002 (5) | 0.004 (5) | 0.004 (5) |
| C70 | 0.019 (6) | 0.023 (6) | 0.017 (6) | 0.003 (5) | 0.008 (5) | −0.003 (5) |
| C71 | 0.013 (5) | 0.018 (6) | 0.019 (6) | −0.009 (4) | 0.013 (4) | −0.005 (5) |
| C72 | 0.014 (5) | 0.019 (6) | 0.019 (6) | −0.005 (4) | 0.011 (4) | −0.006 (5) |
Geometric parameters (Å, °)
| Ho1—O1 | 2.313 (8) | C17—C18iii | 1.395 (16) |
| Ho1—O3i | 2.335 (7) | C17—C17iii | 1.45 (2) |
| Ho1—O5 | 2.277 (8) | C18—C17iii | 1.395 (16) |
| Ho1—O10ii | 2.314 (8) | C18—H18 | 0.9500 |
| Ho1—O15iii | 2.309 (8) | C19—C20 | 1.519 (16) |
| Ho1—O23iv | 2.847 (9) | C20—C21 | 1.402 (15) |
| Ho1—O24iv | 2.311 (8) | C20—C30 | 1.420 (16) |
| Ho1—O2W | 2.370 (8) | C21—C22 | 1.415 (15) |
| Ho2—O2 | 2.299 (8) | C21—H21 | 0.9500 |
| Ho2—O6 | 2.316 (8) | C22—C23 | 1.431 (16) |
| Ho2—O7 | 2.338 (8) | C22—C28 | 1.437 (16) |
| Ho2—O11 | 2.264 (8) | C23—C24 | 1.365 (15) |
| Ho2—O13 | 2.347 (8) | C23—H23 | 0.9500 |
| Ho2—O19ii | 2.338 (8) | C24—C25 | 1.425 (16) |
| Ho2—O20ii | 2.876 (8) | C24—H24 | 0.9500 |
| Ho2—O3W | 2.423 (9) | C25—C27 | 1.383 (16) |
| Ho3—O8 | 2.410 (8) | C25—C26 | 1.482 (16) |
| Ho3—O12 | 2.337 (8) | C27—C28 | 1.420 (16) |
| Ho3—O14 | 2.356 (8) | C27—H27 | 0.9500 |
| Ho3—O17 | 2.299 (7) | C28—C29 | 1.441 (16) |
| Ho3—O20ii | 2.299 (8) | C29—C30 | 1.346 (16) |
| Ho3—O21 | 2.300 (8) | C29—H29 | 0.9500 |
| Ho3—O4W | 2.469 (8) | C30—H30 | 0.9500 |
| Ho3—O5W | 2.640 (6) | C31—C32 | 1.503 (16) |
| Ho4—O4v | 2.360 (8) | C32—C33 | 1.397 (16) |
| Ho4—O9vi | 2.382 (7) | C32—C36 | 1.400 (16) |
| Ho4—O16vii | 2.342 (8) | C33—C34 | 1.357 (16) |
| Ho4—O18 | 2.337 (7) | C33—H33 | 0.9500 |
| Ho4—O22 | 2.276 (7) | C34—C35v | 1.426 (15) |
| Ho4—O23viii | 2.296 (8) | C34—H34 | 0.9500 |
| Ho4—O5W | 2.671 (6) | C35—C36 | 1.380 (16) |
| Ho4—O6W | 2.453 (10) | C35—C35v | 1.43 (2) |
| O1—C1 | 1.258 (13) | C35—C34v | 1.426 (15) |
| O2—C1 | 1.239 (13) | C36—H36 | 0.9500 |
| O3—C8 | 1.269 (14) | C37—C38 | 1.494 (15) |
| O3—Ho1i | 2.335 (7) | C38—C48 | 1.364 (15) |
| O4—C8 | 1.254 (14) | C38—C39 | 1.443 (16) |
| O4—Ho4v | 2.360 (8) | C39—C40 | 1.352 (16) |
| O5—C13 | 1.240 (13) | C39—H39 | 0.9500 |
| O6—C13 | 1.272 (13) | C40—C41 | 1.421 (15) |
| O7—C19 | 1.246 (14) | C40—H40 | 0.9500 |
| O8—C19 | 1.269 (14) | C41—C42 | 1.410 (15) |
| O9—C26 | 1.268 (14) | C41—C47 | 1.429 (15) |
| O9—Ho4iv | 2.382 (7) | C42—C43 | 1.383 (15) |
| O10—C26 | 1.297 (14) | C42—H42 | 0.9500 |
| O10—Ho1viii | 2.314 (8) | C43—C45 | 1.429 (15) |
| O11—C31 | 1.245 (14) | C43—C44 | 1.470 (16) |
| O12—C31 | 1.249 (13) | C44—Ho4vii | 3.005 (12) |
| O13—C37 | 1.241 (13) | C45—C46 | 1.359 (16) |
| O14—C37 | 1.271 (13) | C45—H45 | 0.9500 |
| O15—C44 | 1.249 (14) | C46—C47 | 1.433 (15) |
| O15—Ho1iii | 2.309 (7) | C46—H46 | 0.9500 |
| O16—C44 | 1.250 (13) | C47—C48 | 1.428 (15) |
| O16—Ho4vii | 2.342 (8) | C48—H48 | 0.9500 |
| O17—C49 | 1.266 (13) | C49—C50 | 1.486 (15) |
| O18—C49 | 1.255 (13) | C50—C60 | 1.404 (14) |
| O19—C56 | 1.266 (13) | C50—C51 | 1.408 (15) |
| O19—Ho2viii | 2.338 (8) | C51—C52 | 1.417 (14) |
| O20—C56 | 1.266 (14) | C51—H51 | 0.9500 |
| O20—Ho3viii | 2.299 (8) | C52—C58 | 1.424 (15) |
| O20—Ho2viii | 2.876 (8) | C52—C53 | 1.439 (15) |
| O21—C61 | 1.263 (13) | C53—C54 | 1.361 (15) |
| O22—C61 | 1.285 (13) | C53—H53 | 0.9500 |
| O23—C68 | 1.243 (14) | C54—C55 | 1.431 (15) |
| O23—Ho4ii | 2.296 (8) | C54—H54 | 0.9500 |
| O23—Ho1vi | 2.847 (9) | C55—C57 | 1.366 (15) |
| O24—C68 | 1.267 (14) | C55—C56 | 1.495 (15) |
| O24—Ho1vi | 2.311 (8) | C56—Ho2viii | 2.942 (11) |
| C1—C2 | 1.496 (15) | C57—C58 | 1.409 (15) |
| C2—C3 | 1.375 (16) | C57—H57 | 0.9500 |
| C2—C12 | 1.422 (15) | C58—C59 | 1.438 (15) |
| C3—C4 | 1.433 (16) | C59—C60 | 1.340 (15) |
| C3—H3 | 0.9500 | C59—H59 | 0.9500 |
| C4—C5 | 1.414 (16) | C60—H60 | 0.9500 |
| C4—C10 | 1.422 (15) | C61—C62 | 1.496 (16) |
| C5—C6 | 1.379 (16) | C62—C72 | 1.390 (16) |
| C5—H5 | 0.9500 | C62—C63 | 1.428 (15) |
| C6—C7 | 1.419 (15) | C63—C64 | 1.366 (16) |
| C6—H6 | 0.9500 | C63—H63 | 0.9500 |
| C7—C9 | 1.373 (16) | C64—C65 | 1.431 (16) |
| C7—C8 | 1.485 (16) | C64—H64 | 0.9500 |
| C9—C10 | 1.415 (15) | C65—C66 | 1.406 (16) |
| C9—H9 | 0.9500 | C65—C71 | 1.421 (14) |
| C10—C11 | 1.428 (15) | C66—C67 | 1.386 (16) |
| C11—C12 | 1.349 (15) | C66—H66 | 0.9500 |
| C11—H11 | 0.9500 | C67—C69 | 1.428 (15) |
| C12—H12 | 0.9500 | C67—C68 | 1.500 (16) |
| C13—C14 | 1.487 (15) | C68—Ho1vi | 2.890 (12) |
| C14—C18 | 1.367 (15) | C69—C70 | 1.322 (16) |
| C14—C15 | 1.423 (15) | C69—H69 | 0.9500 |
| C15—C16 | 1.371 (16) | C70—C71 | 1.412 (15) |
| C15—H15 | 0.9500 | C70—H70 | 0.9500 |
| C16—C17 | 1.421 (15) | C71—C72 | 1.419 (15) |
| C16—H16 | 0.9500 | C72—H72 | 0.9500 |
| O5—Ho1—O15iii | 94.3 (3) | C9—C10—C11 | 120.8 (10) |
| O5—Ho1—O24iv | 120.7 (3) | C4—C10—C11 | 119.5 (10) |
| O15iii—Ho1—O24iv | 129.4 (3) | C12—C11—C10 | 120.5 (10) |
| O5—Ho1—O1 | 81.0 (3) | C12—C11—H11 | 119.8 |
| O15iii—Ho1—O1 | 153.5 (3) | C10—C11—H11 | 119.8 |
| O24iv—Ho1—O1 | 73.5 (3) | C11—C12—C2 | 121.6 (10) |
| O5—Ho1—O10ii | 77.0 (3) | C11—C12—H12 | 119.2 |
| O15iii—Ho1—O10ii | 77.3 (3) | C2—C12—H12 | 119.2 |
| O24iv—Ho1—O10ii | 76.7 (3) | O5—C13—O6 | 124.3 (10) |
| O1—Ho1—O10ii | 126.1 (3) | O5—C13—C14 | 118.9 (9) |
| O5—Ho1—O3i | 148.8 (3) | O6—C13—C14 | 116.6 (9) |
| O15iii—Ho1—O3i | 82.9 (3) | C18—C14—C15 | 119.5 (10) |
| O24iv—Ho1—O3i | 83.0 (3) | C18—C14—C13 | 120.7 (10) |
| O1—Ho1—O3i | 87.7 (3) | C15—C14—C13 | 119.7 (9) |
| O10ii—Ho1—O3i | 131.6 (3) | C16—C15—C14 | 120.3 (10) |
| O5—Ho1—O2W | 77.9 (3) | C16—C15—H15 | 119.8 |
| O15iii—Ho1—O2W | 77.1 (3) | C14—C15—H15 | 119.8 |
| O24iv—Ho1—O2W | 140.8 (3) | C15—C16—C17 | 121.2 (10) |
| O1—Ho1—O2W | 76.4 (3) | C15—C16—H16 | 119.4 |
| O10ii—Ho1—O2W | 142.3 (3) | C17—C16—H16 | 119.4 |
| O3i—Ho1—O2W | 71.2 (3) | C18iii—C17—C16 | 123.1 (10) |
| O5—Ho1—O23iv | 146.5 (3) | C18iii—C17—C17iii | 119.2 (12) |
| O15iii—Ho1—O23iv | 81.2 (3) | C16—C17—C17iii | 117.7 (13) |
| O24iv—Ho1—O23iv | 49.1 (2) | C14—C18—C17iii | 121.9 (10) |
| O1—Ho1—O23iv | 116.7 (3) | C14—C18—H18 | 119.0 |
| O10ii—Ho1—O23iv | 69.6 (3) | C17iii—C18—H18 | 119.0 |
| O3i—Ho1—O23iv | 64.0 (3) | O7—C19—O8 | 124.6 (11) |
| O2W—Ho1—O23iv | 132.0 (2) | O7—C19—C20 | 117.2 (10) |
| O5—Ho1—C68iv | 142.0 (3) | O8—C19—C20 | 118.2 (10) |
| O15iii—Ho1—C68iv | 106.2 (3) | C21—C20—C30 | 120.2 (10) |
| O24iv—Ho1—C68iv | 25.2 (3) | C21—C20—C19 | 118.8 (10) |
| O1—Ho1—C68iv | 92.7 (3) | C30—C20—C19 | 120.6 (10) |
| O10ii—Ho1—C68iv | 76.8 (3) | C20—C21—C22 | 120.5 (10) |
| O3i—Ho1—C68iv | 67.1 (3) | C20—C21—H21 | 119.8 |
| O2W—Ho1—C68iv | 137.3 (3) | C22—C21—H21 | 119.8 |
| O23iv—Ho1—C68iv | 25.0 (3) | C21—C22—C23 | 122.5 (10) |
| O11—Ho2—O2 | 94.6 (3) | C21—C22—C28 | 119.2 (10) |
| O11—Ho2—O6 | 152.3 (3) | C23—C22—C28 | 118.3 (10) |
| O2—Ho2—O6 | 84.2 (3) | C24—C23—C22 | 119.7 (11) |
| O11—Ho2—O19ii | 127.4 (3) | C24—C23—H23 | 120.1 |
| O2—Ho2—O19ii | 120.9 (3) | C22—C23—H23 | 120.1 |
| O6—Ho2—O19ii | 74.8 (3) | C23—C24—C25 | 122.2 (11) |
| O11—Ho2—O7 | 77.7 (3) | C23—C24—H24 | 118.9 |
| O2—Ho2—O7 | 74.0 (3) | C25—C24—H24 | 118.9 |
| O6—Ho2—O7 | 127.7 (3) | C27—C25—C24 | 119.5 (10) |
| O19ii—Ho2—O7 | 76.9 (3) | C27—C25—C26 | 119.5 (10) |
| O11—Ho2—O13 | 81.2 (3) | C24—C25—C26 | 120.9 (11) |
| O2—Ho2—O13 | 153.4 (3) | O9—C26—O10 | 122.8 (10) |
| O6—Ho2—O13 | 87.6 (3) | O9—C26—C25 | 120.8 (10) |
| O19ii—Ho2—O13 | 80.9 (3) | O10—C26—C25 | 116.4 (10) |
| O7—Ho2—O13 | 129.6 (3) | C25—C27—C28 | 119.8 (10) |
| O11—Ho2—O3W | 76.8 (3) | C25—C27—H27 | 120.1 |
| O2—Ho2—O3W | 76.9 (3) | C28—C27—H27 | 120.1 |
| O6—Ho2—O3W | 76.0 (3) | C27—C28—C22 | 120.4 (10) |
| O19ii—Ho2—O3W | 143.6 (3) | C27—C28—C29 | 121.8 (10) |
| O7—Ho2—O3W | 139.2 (3) | C22—C28—C29 | 117.8 (10) |
| O13—Ho2—O3W | 76.5 (3) | C30—C29—C28 | 122.3 (11) |
| O11—Ho2—O20ii | 78.7 (3) | C30—C29—H29 | 118.9 |
| O2—Ho2—O20ii | 141.2 (3) | C28—C29—H29 | 118.9 |
| O6—Ho2—O20ii | 118.7 (3) | C29—C30—C20 | 120.0 (10) |
| O19ii—Ho2—O20ii | 49.0 (2) | C29—C30—H30 | 120.0 |
| O7—Ho2—O20ii | 67.2 (3) | C20—C30—H30 | 120.0 |
| O13—Ho2—O20ii | 64.0 (3) | O11—C31—O12 | 122.1 (11) |
| O3W—Ho2—O20ii | 136.0 (3) | O11—C31—C32 | 119.4 (9) |
| O11—Ho2—C56ii | 103.8 (3) | O12—C31—C32 | 118.0 (10) |
| O2—Ho2—C56ii | 138.5 (3) | O11—C31—Ho3 | 75.5 (6) |
| O6—Ho2—C56ii | 94.9 (3) | O12—C31—Ho3 | 49.1 (6) |
| O19ii—Ho2—C56ii | 24.5 (3) | C32—C31—Ho3 | 153.4 (7) |
| O7—Ho2—C56ii | 74.1 (3) | C33—C32—C36 | 119.8 (11) |
| O13—Ho2—C56ii | 67.4 (3) | C33—C32—C31 | 120.8 (10) |
| O3W—Ho2—C56ii | 143.1 (3) | C36—C32—C31 | 119.2 (10) |
| O20ii—Ho2—C56ii | 25.1 (3) | C34—C33—C32 | 121.1 (11) |
| O20ii—Ho3—O17 | 145.8 (3) | C34—C33—H33 | 119.5 |
| O20ii—Ho3—O21 | 86.6 (3) | C32—C33—H33 | 119.5 |
| O17—Ho3—O21 | 90.7 (3) | C33—C34—C35v | 120.1 (10) |
| O20ii—Ho3—O12 | 125.3 (3) | C33—C34—H34 | 119.9 |
| O17—Ho3—O12 | 78.2 (3) | C35v—C34—H34 | 119.9 |
| O21—Ho3—O12 | 136.1 (3) | C36—C35—C35v | 119.6 (13) |
| O20ii—Ho3—O14 | 75.9 (3) | C36—C35—C34v | 121.6 (10) |
| O17—Ho3—O14 | 136.8 (3) | C35v—C35—C34v | 118.8 (12) |
| O21—Ho3—O14 | 78.6 (3) | C35—C36—C32 | 120.4 (10) |
| O12—Ho3—O14 | 81.1 (3) | C35—C36—H36 | 119.8 |
| O20ii—Ho3—O8 | 81.3 (3) | C32—C36—H36 | 119.8 |
| O17—Ho3—O8 | 81.2 (3) | O13—C37—O14 | 125.4 (10) |
| O21—Ho3—O8 | 144.2 (3) | O13—C37—C38 | 119.2 (10) |
| O12—Ho3—O8 | 76.3 (3) | O14—C37—C38 | 115.4 (9) |
| O14—Ho3—O8 | 129.5 (3) | C48—C38—C39 | 119.3 (10) |
| O20ii—Ho3—O4W | 73.3 (3) | C48—C38—C37 | 118.0 (10) |
| O17—Ho3—O4W | 73.2 (3) | C39—C38—C37 | 122.7 (10) |
| O21—Ho3—O4W | 73.2 (3) | C40—C39—C38 | 120.5 (10) |
| O12—Ho3—O4W | 139.3 (3) | C40—C39—H39 | 119.7 |
| O14—Ho3—O4W | 139.1 (3) | C38—C39—H39 | 119.7 |
| O8—Ho3—O4W | 71.1 (3) | C39—C40—C41 | 121.8 (10) |
| O20ii—Ho3—O5W | 138.4 (3) | C39—C40—H40 | 119.1 |
| O17—Ho3—O5W | 70.8 (2) | C41—C40—H40 | 119.1 |
| O21—Ho3—O5W | 70.1 (3) | C42—C41—C40 | 123.8 (10) |
| O12—Ho3—O5W | 66.2 (3) | C42—C41—C47 | 118.4 (10) |
| O14—Ho3—O5W | 66.2 (3) | C40—C41—C47 | 117.8 (10) |
| O8—Ho3—O5W | 136.6 (2) | C43—C42—C41 | 122.5 (9) |
| O4W—Ho3—O5W | 127.3 (2) | C43—C42—H42 | 118.8 |
| O20ii—Ho3—C31 | 101.9 (3) | C41—C42—H42 | 118.8 |
| O17—Ho3—C31 | 96.7 (3) | C42—C43—C45 | 118.1 (10) |
| O21—Ho3—C31 | 151.5 (3) | C42—C43—C44 | 121.9 (10) |
| O12—Ho3—C31 | 23.8 (3) | C45—C43—C44 | 119.6 (9) |
| O14—Ho3—C31 | 77.1 (3) | O15—C44—O16 | 121.7 (11) |
| O8—Ho3—C31 | 64.3 (3) | O15—C44—C43 | 119.0 (10) |
| O4W—Ho3—C31 | 135.3 (3) | O16—C44—C43 | 119.0 (10) |
| O5W—Ho3—C31 | 86.4 (3) | O15—C44—Ho4vii | 77.3 (7) |
| O22—Ho4—O23viii | 146.1 (3) | O16—C44—Ho4vii | 47.1 (6) |
| O22—Ho4—O18 | 90.9 (3) | C43—C44—Ho4vii | 153.7 (8) |
| O23viii—Ho4—O18 | 86.7 (3) | C46—C45—C43 | 121.4 (10) |
| O22—Ho4—O16vii | 79.3 (3) | C46—C45—H45 | 119.3 |
| O23viii—Ho4—O16vii | 124.6 (3) | C43—C45—H45 | 119.3 |
| O18—Ho4—O16vii | 134.8 (3) | C45—C46—C47 | 120.6 (10) |
| O22—Ho4—O4v | 135.9 (3) | C45—C46—H46 | 119.7 |
| O23viii—Ho4—O4v | 76.1 (3) | C47—C46—H46 | 119.7 |
| O18—Ho4—O4v | 76.4 (3) | C48—C47—C41 | 119.5 (10) |
| O16vii—Ho4—O4v | 80.7 (3) | C48—C47—C46 | 121.6 (10) |
| O22—Ho4—O9vi | 83.4 (3) | C41—C47—C46 | 118.8 (10) |
| O23viii—Ho4—O9vi | 81.4 (3) | C38—C48—C47 | 120.9 (10) |
| O18—Ho4—O9vi | 148.5 (3) | C38—C48—H48 | 119.5 |
| O16vii—Ho4—O9vi | 74.6 (3) | C47—C48—H48 | 119.5 |
| O4v—Ho4—O9vi | 127.6 (3) | O18—C49—O17 | 124.2 (10) |
| O22—Ho4—O6W | 73.5 (3) | O18—C49—C50 | 119.6 (10) |
| O23viii—Ho4—O6W | 73.2 (3) | O17—C49—C50 | 116.1 (9) |
| O18—Ho4—O6W | 76.3 (3) | C60—C50—C51 | 120.0 (10) |
| O16vii—Ho4—O6W | 139.0 (3) | C60—C50—C49 | 119.8 (10) |
| O4v—Ho4—O6W | 139.6 (3) | C51—C50—C49 | 120.1 (9) |
| O9vi—Ho4—O6W | 72.4 (3) | C50—C51—C52 | 119.3 (9) |
| O22—Ho4—O5W | 70.5 (3) | C50—C51—H51 | 120.4 |
| O23viii—Ho4—O5W | 138.4 (3) | C52—C51—H51 | 120.4 |
| O18—Ho4—O5W | 69.8 (3) | C51—C52—C58 | 120.0 (9) |
| O16vii—Ho4—O5W | 65.3 (3) | C51—C52—C53 | 121.0 (9) |
| O4v—Ho4—O5W | 65.4 (3) | C58—C52—C53 | 119.0 (10) |
| O9vi—Ho4—O5W | 135.1 (3) | C54—C53—C52 | 119.7 (10) |
| O6W—Ho4—O5W | 129.2 (3) | C54—C53—H53 | 120.1 |
| O22—Ho4—C44vii | 97.5 (3) | C52—C53—H53 | 120.1 |
| O23viii—Ho4—C44vii | 102.0 (3) | C53—C54—C55 | 120.7 (10) |
| O18—Ho4—C44vii | 148.7 (3) | C53—C54—H54 | 119.6 |
| O16vii—Ho4—C44vii | 23.0 (3) | C55—C54—H54 | 119.6 |
| O4v—Ho4—C44vii | 76.7 (3) | C57—C55—C54 | 120.1 (10) |
| O9vi—Ho4—C44vii | 62.7 (3) | C57—C55—C56 | 119.4 (10) |
| O6W—Ho4—C44vii | 135.0 (3) | C54—C55—C56 | 119.8 (10) |
| O5W—Ho4—C44vii | 84.6 (3) | O19—C56—O20 | 122.3 (10) |
| Ho3—O5W—Ho4 | 126.7 (3) | O19—C56—C55 | 117.6 (10) |
| C1—O1—Ho1 | 154.4 (8) | O20—C56—C55 | 119.7 (9) |
| C1—O2—Ho2 | 143.2 (8) | O19—C56—Ho2viii | 50.0 (5) |
| C8—O3—Ho1i | 137.4 (8) | O20—C56—Ho2viii | 74.5 (6) |
| C8—O4—Ho4v | 129.9 (8) | C55—C56—Ho2viii | 155.2 (8) |
| C13—O5—Ho1 | 149.4 (7) | C55—C57—C58 | 120.9 (10) |
| C13—O6—Ho2 | 141.3 (7) | C55—C57—H57 | 119.5 |
| C19—O7—Ho2 | 128.7 (8) | C58—C57—H57 | 119.5 |
| C19—O8—Ho3 | 135.0 (7) | C57—C58—C52 | 119.2 (10) |
| C26—O9—Ho4iv | 138.6 (7) | C57—C58—C59 | 122.5 (10) |
| C26—O10—Ho1viii | 129.6 (7) | C52—C58—C59 | 118.0 (10) |
| C31—O11—Ho2 | 171.8 (7) | C60—C59—C58 | 121.2 (10) |
| C31—O12—Ho3 | 107.0 (7) | C60—C59—H59 | 119.4 |
| C37—O13—Ho2 | 136.7 (7) | C58—C59—H59 | 119.4 |
| C37—O14—Ho3 | 127.7 (7) | C59—C60—C50 | 121.4 (10) |
| C44—O15—Ho1iii | 170.7 (8) | C59—C60—H60 | 119.3 |
| C44—O16—Ho4vii | 109.8 (7) | C50—C60—H60 | 119.3 |
| C49—O17—Ho3 | 136.5 (7) | O21—C61—O22 | 124.3 (10) |
| C49—O18—Ho4 | 138.1 (7) | O21—C61—C62 | 119.3 (10) |
| C56—O19—Ho2viii | 105.6 (7) | O22—C61—C62 | 116.2 (10) |
| C56—O20—Ho3viii | 176.9 (7) | C72—C62—C63 | 120.5 (10) |
| C56—O20—Ho2viii | 80.4 (6) | C72—C62—C61 | 120.8 (10) |
| Ho3viii—O20—Ho2viii | 101.8 (3) | C63—C62—C61 | 118.6 (10) |
| C61—O21—Ho3 | 138.2 (7) | C64—C63—C62 | 119.3 (10) |
| C61—O22—Ho4 | 135.3 (7) | C64—C63—H63 | 120.4 |
| C68—O23—Ho4ii | 176.8 (8) | C62—C63—H63 | 120.4 |
| C68—O23—Ho1vi | 79.4 (7) | C63—C64—C65 | 121.3 (10) |
| Ho4ii—O23—Ho1vi | 102.5 (3) | C63—C64—H64 | 119.3 |
| C68—O24—Ho1vi | 103.9 (7) | C65—C64—H64 | 119.3 |
| O2—C1—O1 | 124.4 (10) | C66—C65—C71 | 118.3 (10) |
| O2—C1—C2 | 118.8 (10) | C66—C65—C64 | 122.2 (10) |
| O1—C1—C2 | 116.7 (9) | C71—C65—C64 | 119.4 (10) |
| C3—C2—C12 | 119.0 (10) | C67—C66—C65 | 121.9 (10) |
| C3—C2—C1 | 121.8 (10) | C67—C66—H66 | 119.1 |
| C12—C2—C1 | 119.2 (10) | C65—C66—H66 | 119.1 |
| C2—C3—C4 | 121.5 (10) | C66—C67—C69 | 117.9 (10) |
| C2—C3—H3 | 119.2 | C66—C67—C68 | 117.9 (10) |
| C4—C3—H3 | 119.2 | C69—C67—C68 | 123.8 (10) |
| C5—C4—C10 | 117.8 (10) | O23—C68—O24 | 122.3 (11) |
| C5—C4—C3 | 124.3 (10) | O23—C68—C67 | 118.6 (10) |
| C10—C4—C3 | 118.0 (10) | O24—C68—C67 | 119.1 (10) |
| C6—C5—C4 | 122.0 (10) | O23—C68—Ho1vi | 75.6 (7) |
| C6—C5—H5 | 119.0 | O24—C68—Ho1vi | 50.9 (6) |
| C4—C5—H5 | 119.0 | C67—C68—Ho1vi | 152.8 (8) |
| C5—C6—C7 | 119.6 (11) | C70—C69—C67 | 120.8 (10) |
| C5—C6—H6 | 120.2 | C70—C69—H69 | 119.6 |
| C7—C6—H6 | 120.2 | C67—C69—H69 | 119.6 |
| C9—C7—C6 | 119.8 (10) | C69—C70—C71 | 122.5 (10) |
| C9—C7—C8 | 118.7 (10) | C69—C70—H70 | 118.8 |
| C6—C7—C8 | 121.4 (10) | C71—C70—H70 | 118.8 |
| O4—C8—O3 | 123.2 (11) | C70—C71—C72 | 122.9 (10) |
| O4—C8—C7 | 118.9 (10) | C70—C71—C65 | 118.3 (10) |
| O3—C8—C7 | 117.8 (10) | C72—C71—C65 | 118.6 (10) |
| C7—C9—C10 | 121.1 (10) | C62—C72—C71 | 120.7 (10) |
| C7—C9—H9 | 119.5 | C62—C72—H72 | 119.7 |
| C10—C9—H9 | 119.5 | C71—C72—H72 | 119.7 |
| C9—C10—C4 | 119.7 (10) | ||
| O20ii—Ho3—O5W—Ho4 | −119.2 (4) | C20—C21—C22—C28 | 0.6 (16) |
| O17—Ho3—O5W—Ho4 | 38.6 (3) | C21—C22—C23—C24 | −176.0 (10) |
| O21—Ho3—O5W—Ho4 | −59.5 (4) | C28—C22—C23—C24 | 1.7 (16) |
| O12—Ho3—O5W—Ho4 | 123.8 (4) | C22—C23—C24—C25 | −2.8 (17) |
| O14—Ho3—O5W—Ho4 | −145.6 (4) | C23—C24—C25—C27 | 1.5 (17) |
| O8—Ho3—O5W—Ho4 | 91.3 (5) | C23—C24—C25—C26 | 177.8 (10) |
| O4W—Ho3—O5W—Ho4 | −11.0 (5) | Ho4iv—O9—C26—O10 | −14.1 (19) |
| C31—Ho3—O5W—Ho4 | 136.9 (4) | Ho4iv—O9—C26—C25 | 165.6 (8) |
| O22—Ho4—O5W—Ho3 | 38.8 (4) | Ho1viii—O10—C26—O9 | 52.1 (15) |
| O23viii—Ho4—O5W—Ho3 | −119.3 (4) | Ho1viii—O10—C26—C25 | −127.6 (9) |
| O18—Ho4—O5W—Ho3 | −59.7 (4) | C27—C25—C26—O9 | −39.3 (16) |
| O16vii—Ho4—O5W—Ho3 | 125.6 (5) | C24—C25—C26—O9 | 144.3 (11) |
| O4v—Ho4—O5W—Ho3 | −143.5 (5) | C27—C25—C26—O10 | 140.3 (11) |
| O9vi—Ho4—O5W—Ho3 | 96.9 (5) | C24—C25—C26—O10 | −36.0 (15) |
| O6W—Ho4—O5W—Ho3 | −8.5 (6) | C24—C25—C27—C28 | 0.9 (17) |
| C44vii—Ho4—O5W—Ho3 | 138.7 (4) | C26—C25—C27—C28 | −175.5 (10) |
| O5—Ho1—O1—C1 | −111.2 (17) | C25—C27—C28—C22 | −1.9 (16) |
| O15iii—Ho1—O1—C1 | 167.1 (15) | C25—C27—C28—C29 | 177.8 (11) |
| O24iv—Ho1—O1—C1 | 14.6 (16) | C21—C22—C28—C27 | 178.4 (10) |
| O10ii—Ho1—O1—C1 | −44.6 (18) | C23—C22—C28—C27 | 0.6 (16) |
| O3i—Ho1—O1—C1 | 97.9 (17) | C21—C22—C28—C29 | −1.3 (16) |
| O2W—Ho1—O1—C1 | 169.2 (17) | C23—C22—C28—C29 | −179.2 (10) |
| O23iv—Ho1—O1—C1 | 38.7 (17) | C27—C28—C29—C30 | −179.9 (11) |
| C68iv—Ho1—O1—C1 | 31.0 (17) | C22—C28—C29—C30 | −0.2 (17) |
| O11—Ho2—O2—C1 | 174.7 (14) | C28—C29—C30—C20 | 2.4 (18) |
| O6—Ho2—O2—C1 | 22.5 (14) | C21—C20—C30—C29 | −3.2 (17) |
| O19ii—Ho2—O2—C1 | −46.0 (15) | C19—C20—C30—C29 | −176.0 (10) |
| O7—Ho2—O2—C1 | −109.5 (14) | Ho3—O12—C31—O11 | −20.8 (13) |
| O13—Ho2—O2—C1 | 95.3 (15) | Ho3—O12—C31—C32 | 151.5 (8) |
| O3W—Ho2—O2—C1 | 99.4 (14) | O20ii—Ho3—C31—O11 | −7.7 (7) |
| O20ii—Ho2—O2—C1 | −107.6 (13) | O17—Ho3—C31—O11 | −158.8 (6) |
| C56ii—Ho2—O2—C1 | −68.4 (15) | O21—Ho3—C31—O11 | 97.2 (8) |
| O15iii—Ho1—O5—C13 | 168.4 (16) | O12—Ho3—C31—O11 | 161.9 (11) |
| O24iv—Ho1—O5—C13 | −50.0 (16) | O14—Ho3—C31—O11 | 64.7 (6) |
| O1—Ho1—O5—C13 | 14.7 (15) | O8—Ho3—C31—O11 | −81.9 (6) |
| O10ii—Ho1—O5—C13 | −115.7 (16) | O4W—Ho3—C31—O11 | −85.9 (7) |
| O3i—Ho1—O5—C13 | 84.9 (17) | O5W—Ho3—C31—O11 | 131.0 (6) |
| O2W—Ho1—O5—C13 | 92.6 (16) | O20ii—Ho3—C31—O12 | −169.6 (7) |
| O23iv—Ho1—O5—C13 | −111.1 (15) | O17—Ho3—C31—O12 | 39.3 (8) |
| C68iv—Ho1—O5—C13 | −68.3 (17) | O21—Ho3—C31—O12 | −64.7 (10) |
| O11—Ho2—O6—C13 | 166.5 (10) | O14—Ho3—C31—O12 | −97.2 (7) |
| O2—Ho2—O6—C13 | −104.7 (12) | O8—Ho3—C31—O12 | 116.2 (8) |
| O19ii—Ho2—O6—C13 | 19.5 (11) | O4W—Ho3—C31—O12 | 112.2 (7) |
| O7—Ho2—O6—C13 | −40.0 (13) | O5W—Ho3—C31—O12 | −30.9 (7) |
| O13—Ho2—O6—C13 | 100.7 (12) | O20ii—Ho3—C31—C32 | 120.3 (16) |
| O3W—Ho2—O6—C13 | 177.4 (12) | O17—Ho3—C31—C32 | −30.8 (17) |
| O20ii—Ho2—O6—C13 | 42.3 (12) | O21—Ho3—C31—C32 | −134.8 (15) |
| C56ii—Ho2—O6—C13 | 33.7 (12) | O12—Ho3—C31—C32 | −70.1 (17) |
| O11—Ho2—O7—C19 | −0.8 (9) | O14—Ho3—C31—C32 | −167.3 (17) |
| O2—Ho2—O7—C19 | −99.2 (10) | O8—Ho3—C31—C32 | 46.1 (16) |
| O6—Ho2—O7—C19 | −168.5 (9) | O4W—Ho3—C31—C32 | 42.1 (18) |
| O19ii—Ho2—O7—C19 | 132.8 (10) | O5W—Ho3—C31—C32 | −100.9 (16) |
| O13—Ho2—O7—C19 | 66.7 (10) | O11—C31—C32—C33 | −9.9 (16) |
| O3W—Ho2—O7—C19 | −53.0 (11) | O12—C31—C32—C33 | 177.6 (10) |
| O20ii—Ho2—O7—C19 | 82.0 (9) | Ho3—C31—C32—C33 | −128.7 (14) |
| C56ii—Ho2—O7—C19 | 107.6 (10) | O11—C31—C32—C36 | 165.2 (10) |
| O20ii—Ho3—O8—C19 | −32.2 (10) | O12—C31—C32—C36 | −7.3 (16) |
| O17—Ho3—O8—C19 | 177.4 (11) | Ho3—C31—C32—C36 | 46 (2) |
| O21—Ho3—O8—C19 | −103.7 (11) | C36—C32—C33—C34 | −3.4 (17) |
| O12—Ho3—O8—C19 | 97.5 (10) | C31—C32—C33—C34 | 171.6 (10) |
| O14—Ho3—O8—C19 | 31.5 (11) | C32—C33—C34—C35v | 2.0 (17) |
| O4W—Ho3—O8—C19 | −107.4 (11) | C35v—C35—C36—C32 | −3.0 (19) |
| O5W—Ho3—O8—C19 | 127.9 (10) | C34v—C35—C36—C32 | 178.6 (10) |
| C31—Ho3—O8—C19 | 75.6 (10) | C33—C32—C36—C35 | 3.9 (17) |
| O20ii—Ho3—O12—C31 | 12.5 (9) | C31—C32—C36—C35 | −171.2 (10) |
| O17—Ho3—O12—C31 | −140.0 (8) | Ho2—O13—C37—O14 | −22.7 (19) |
| O21—Ho3—O12—C31 | 141.4 (7) | Ho2—O13—C37—C38 | 156.1 (8) |
| O14—Ho3—O12—C31 | 78.2 (7) | Ho3—O14—C37—O13 | 57.0 (15) |
| O8—Ho3—O12—C31 | −56.3 (7) | Ho3—O14—C37—C38 | −121.8 (9) |
| O4W—Ho3—O12—C31 | −93.9 (8) | O13—C37—C38—C48 | −38.8 (15) |
| O5W—Ho3—O12—C31 | 146.0 (8) | O14—C37—C38—C48 | 140.0 (11) |
| O11—Ho2—O13—C37 | 38.7 (11) | O13—C37—C38—C39 | 142.3 (11) |
| O2—Ho2—O13—C37 | 121.3 (11) | O14—C37—C38—C39 | −38.9 (15) |
| O6—Ho2—O13—C37 | −166.7 (11) | C48—C38—C39—C40 | 2.3 (17) |
| O19ii—Ho2—O13—C37 | −91.7 (11) | C37—C38—C39—C40 | −178.8 (10) |
| O7—Ho2—O13—C37 | −27.2 (13) | C38—C39—C40—C41 | −1.2 (17) |
| O3W—Ho2—O13—C37 | 117.1 (12) | C39—C40—C41—C42 | 176.2 (11) |
| O20ii—Ho2—O13—C37 | −43.0 (11) | C39—C40—C41—C47 | −2.1 (16) |
| C56ii—Ho2—O13—C37 | −70.4 (11) | C40—C41—C42—C43 | −178.7 (10) |
| O20ii—Ho3—O14—C37 | 5.3 (8) | C47—C41—C42—C43 | −0.4 (16) |
| O17—Ho3—O14—C37 | 173.3 (8) | C41—C42—C43—C45 | −3.5 (15) |
| O21—Ho3—O14—C37 | 94.6 (9) | C41—C42—C43—C44 | 169.5 (10) |
| O12—Ho3—O14—C37 | −124.6 (9) | Ho4vii—O16—C44—O15 | 22.1 (14) |
| O8—Ho3—O14—C37 | −60.6 (10) | Ho4vii—O16—C44—C43 | −152.1 (8) |
| O4W—Ho3—O14—C37 | 47.6 (10) | C42—C43—C44—O15 | −166.0 (10) |
| O5W—Ho3—O14—C37 | 167.6 (9) | C45—C43—C44—O15 | 7.0 (16) |
| C31—Ho3—O14—C37 | −100.6 (9) | C42—C43—C44—O16 | 8.4 (16) |
| O20ii—Ho3—O17—C49 | −166.4 (9) | C45—C43—C44—O16 | −178.7 (10) |
| O21—Ho3—O17—C49 | 108.6 (10) | C42—C43—C44—Ho4vii | −42 (2) |
| O12—Ho3—O17—C49 | −28.6 (10) | C45—C43—C44—Ho4vii | 130.7 (14) |
| O14—Ho3—O17—C49 | 34.6 (11) | C42—C43—C45—C46 | 4.1 (16) |
| O8—Ho3—O17—C49 | −106.3 (10) | C44—C43—C45—C46 | −169.1 (10) |
| O4W—Ho3—O17—C49 | −179.2 (10) | C43—C45—C46—C47 | −0.7 (16) |
| O5W—Ho3—O17—C49 | 40.1 (9) | C42—C41—C47—C48 | −174.1 (9) |
| C31—Ho3—O17—C49 | −43.7 (10) | C40—C41—C47—C48 | 4.3 (15) |
| O22—Ho4—O18—C49 | −31.4 (12) | C42—C41—C47—C46 | 3.8 (15) |
| O23viii—Ho4—O18—C49 | −177.7 (12) | C40—C41—C47—C46 | −177.8 (10) |
| O16vii—Ho4—O18—C49 | 44.2 (13) | C45—C46—C47—C48 | 174.6 (10) |
| O4v—Ho4—O18—C49 | 105.9 (12) | C45—C46—C47—C41 | −3.3 (16) |
| O9vi—Ho4—O18—C49 | −110.2 (11) | C39—C38—C48—C47 | −0.1 (17) |
| O6W—Ho4—O18—C49 | −104.2 (12) | C37—C38—C48—C47 | −179.0 (10) |
| O5W—Ho4—O18—C49 | 37.4 (11) | C41—C47—C48—C38 | −3.3 (16) |
| C44vii—Ho4—O18—C49 | 74.5 (13) | C46—C47—C48—C38 | 178.9 (11) |
| O20ii—Ho3—O21—C61 | 179.4 (12) | Ho4—O18—C49—O17 | 17.4 (18) |
| O17—Ho3—O21—C61 | −34.7 (11) | Ho4—O18—C49—C50 | −165.5 (8) |
| O12—Ho3—O21—C61 | 38.9 (13) | Ho3—O17—C49—O18 | −83.7 (14) |
| O14—Ho3—O21—C61 | 103.1 (12) | Ho3—O17—C49—C50 | 99.1 (11) |
| O8—Ho3—O21—C61 | −110.6 (11) | O18—C49—C50—C60 | −165.1 (10) |
| O4W—Ho3—O21—C61 | −107.0 (12) | O17—C49—C50—C60 | 12.2 (15) |
| O5W—Ho3—O21—C61 | 34.5 (11) | O18—C49—C50—C51 | 11.2 (16) |
| C31—Ho3—O21—C61 | 70.7 (14) | O17—C49—C50—C51 | −171.4 (10) |
| O23viii—Ho4—O22—C61 | −167.4 (9) | C60—C50—C51—C52 | 3.5 (16) |
| O18—Ho4—O22—C61 | 107.3 (10) | C49—C50—C51—C52 | −172.8 (9) |
| O16vii—Ho4—O22—C61 | −28.3 (10) | C50—C51—C52—C58 | −2.0 (15) |
| O4v—Ho4—O22—C61 | 36.1 (11) | C50—C51—C52—C53 | 176.9 (10) |
| O9vi—Ho4—O22—C61 | −103.8 (10) | C51—C52—C53—C54 | −172.7 (10) |
| O6W—Ho4—O22—C61 | −177.3 (10) | C58—C52—C53—C54 | 6.2 (16) |
| O5W—Ho4—O22—C61 | 39.1 (10) | C52—C53—C54—C55 | −2.1 (16) |
| C44vii—Ho4—O22—C61 | −42.5 (10) | C53—C54—C55—C57 | −2.5 (17) |
| Ho2—O2—C1—O1 | −4(2) | C53—C54—C55—C56 | 168.0 (10) |
| Ho2—O2—C1—C2 | −179.4 (8) | Ho2viii—O19—C56—O20 | −19.2 (12) |
| Ho1—O1—C1—O2 | 92 (2) | Ho2viii—O19—C56—C55 | 153.9 (8) |
| Ho1—O1—C1—C2 | −92.3 (18) | Ho2viii—O20—C56—O19 | 15.1 (10) |
| O2—C1—C2—C3 | −18.2 (17) | Ho2viii—O20—C56—C55 | −157.9 (10) |
| O1—C1—C2—C3 | 165.8 (11) | C57—C55—C56—O19 | −12.4 (16) |
| O2—C1—C2—C12 | 160.1 (11) | C54—C55—C56—O19 | 177.0 (10) |
| O1—C1—C2—C12 | −15.9 (15) | C57—C55—C56—O20 | 161.0 (10) |
| C12—C2—C3—C4 | −0.2 (16) | C54—C55—C56—O20 | −9.6 (16) |
| C1—C2—C3—C4 | 178.1 (10) | C57—C55—C56—Ho2viii | 41 (2) |
| C2—C3—C4—C5 | 179.2 (11) | C54—C55—C56—Ho2viii | −129.7 (15) |
| C2—C3—C4—C10 | 0.8 (16) | C54—C55—C57—C58 | 2.9 (17) |
| C10—C4—C5—C6 | −1.1 (16) | C56—C55—C57—C58 | −167.6 (10) |
| C3—C4—C5—C6 | −179.5 (11) | C55—C57—C58—C52 | 1.3 (16) |
| C4—C5—C6—C7 | 3.6 (17) | C55—C57—C58—C59 | 175.0 (11) |
| C5—C6—C7—C9 | −3.2 (17) | C51—C52—C58—C57 | 173.1 (9) |
| C5—C6—C7—C8 | −179.6 (10) | C53—C52—C58—C57 | −5.8 (15) |
| Ho4v—O4—C8—O3 | −55.0 (16) | C51—C52—C58—C59 | −0.9 (15) |
| Ho4v—O4—C8—C7 | 121.4 (10) | C53—C52—C58—C59 | −179.9 (9) |
| Ho1i—O3—C8—O4 | 14.9 (19) | C57—C58—C59—C60 | −171.3 (10) |
| Ho1i—O3—C8—C7 | −161.5 (8) | C52—C58—C59—C60 | 2.6 (16) |
| C9—C7—C8—O4 | −138.4 (12) | C58—C59—C60—C50 | −1.2 (17) |
| C6—C7—C8—O4 | 38.0 (16) | C51—C50—C60—C59 | −1.9 (17) |
| C9—C7—C8—O3 | 38.2 (16) | C49—C50—C60—C59 | 174.4 (10) |
| C6—C7—C8—O3 | −145.4 (11) | Ho3—O21—C61—O22 | 22.6 (18) |
| C6—C7—C9—C10 | 0.4 (17) | Ho3—O21—C61—C62 | −161.9 (8) |
| C8—C7—C9—C10 | 176.9 (10) | Ho4—O22—C61—O21 | −85.9 (14) |
| C7—C9—C10—C4 | 2.1 (16) | Ho4—O22—C61—C62 | 98.5 (11) |
| C7—C9—C10—C11 | 179.8 (10) | O21—C61—C62—C72 | 13.2 (16) |
| C5—C4—C10—C9 | −1.7 (16) | O22—C61—C62—C72 | −171.0 (10) |
| C3—C4—C10—C9 | 176.8 (9) | O21—C61—C62—C63 | −165.0 (10) |
| C5—C4—C10—C11 | −179.5 (10) | O22—C61—C62—C63 | 10.9 (15) |
| C3—C4—C10—C11 | −0.9 (16) | C72—C62—C63—C64 | −2.6 (16) |
| C9—C10—C11—C12 | −177.2 (10) | C61—C62—C63—C64 | 175.6 (10) |
| C4—C10—C11—C12 | 0.5 (16) | C62—C63—C64—C65 | −1.0 (16) |
| C10—C11—C12—C2 | 0.1 (16) | C63—C64—C65—C66 | −173.8 (10) |
| C3—C2—C12—C11 | −0.3 (16) | C63—C64—C65—C71 | 3.8 (16) |
| C1—C2—C12—C11 | −178.6 (10) | C71—C65—C66—C67 | −3.6 (16) |
| Ho1—O5—C13—O6 | −10 (2) | C64—C65—C66—C67 | 173.9 (10) |
| Ho1—O5—C13—C14 | 175.0 (10) | C65—C66—C67—C69 | 6.3 (16) |
| Ho2—O6—C13—O5 | 88.6 (15) | C65—C66—C67—C68 | −166.6 (10) |
| Ho2—O6—C13—C14 | −96.4 (13) | Ho1vi—O23—C68—O24 | 21.2 (11) |
| O5—C13—C14—C18 | −15.3 (16) | Ho1vi—O23—C68—C67 | −154.9 (10) |
| O6—C13—C14—C18 | 169.3 (10) | Ho1vi—O24—C68—O23 | −26.8 (13) |
| O5—C13—C14—C15 | 165.3 (10) | C66—C67—C68—O23 | 159.0 (11) |
| O6—C13—C14—C15 | −10.0 (15) | C69—C67—C68—O23 | −13.5 (17) |
| C18—C14—C15—C16 | 3.1 (16) | C66—C67—C68—O24 | −17.2 (16) |
| C13—C14—C15—C16 | −177.5 (10) | C69—C67—C68—O24 | 170.3 (11) |
| C14—C15—C16—C17 | −2.4 (16) | C66—C67—C68—Ho1vi | 43 (2) |
| C15—C16—C17—C18iii | −178.1 (10) | C69—C67—C68—Ho1vi | −129.5 (15) |
| C15—C16—C17—C17iii | −0.1 (18) | C66—C67—C69—C70 | −4.5 (16) |
| C15—C14—C18—C17iii | −1.3 (16) | C68—C67—C69—C70 | 168.0 (11) |
| C13—C14—C18—C17iii | 179.3 (10) | C67—C69—C70—C71 | −0.1 (17) |
| Ho2—O7—C19—O8 | −57.9 (16) | C69—C70—C71—C72 | −172.6 (11) |
| Ho2—O7—C19—C20 | 121.5 (9) | C69—C70—C71—C65 | 2.9 (16) |
| Ho3—O8—C19—O7 | 15.9 (18) | C66—C65—C71—C70 | −1.0 (15) |
| Ho3—O8—C19—C20 | −163.5 (7) | C64—C65—C71—C70 | −178.7 (10) |
| O7—C19—C20—C21 | −137.2 (11) | C66—C65—C71—C72 | 174.7 (10) |
| O8—C19—C20—C21 | 42.2 (15) | C64—C65—C71—C72 | −3.0 (15) |
| O7—C19—C20—C30 | 35.8 (16) | C63—C62—C72—C71 | 3.3 (16) |
| O8—C19—C20—C30 | −144.8 (11) | C61—C62—C72—C71 | −174.8 (9) |
| C30—C20—C21—C22 | 1.7 (16) | C70—C71—C72—C62 | 175.0 (10) |
| C19—C20—C21—C22 | 174.6 (10) | C65—C71—C72—C62 | −0.5 (15) |
| C20—C21—C22—C23 | 178.3 (11) |
Symmetry codes: (i) −x+1, −y+1, −z+2; (ii) x−1/2, −y+1/2, z−1/2; (iii) −x+1, −y+1, −z+1; (iv) x−1/2, −y+1/2, z+1/2; (v) −x+2, −y+1, −z+2; (vi) x+1/2, −y+1/2, z−1/2; (vii) −x+2, −y+1, −z+1; (viii) x+1/2, −y+1/2, z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HB2668).
References
- Allen, F. H. (2002). Acta Cryst. B58, 380–388. [DOI] [PubMed]
- Almeida Paz, F. A. & Klinowski, J. (2008). Acta Cryst. E64, m140–m141. [DOI] [PMC free article] [PubMed]
- Altomare, A., Cascarano, G., Giacovazzo, C., Guagliardi, A., Burla, M. C., Polidori, G. & Camalli, M. (1994). J. Appl. Cryst.27, 435.
- Blessing, R. H. (1995). Acta Cryst. A51, 33–38. [DOI] [PubMed]
- Brandenburg, K. (2006). DIAMOND Version 3.1e. Crystal Impact GbR, Bonn, Germany.
- Bruker (2001). SHELXTL. Version 6.12. Bruker AXS, Inc. Madison, Wisconsin, USA.
- Cunha-Silva, L., Shi, F.-N., Klinowski, J., Trindade, T., Rocha, J. & Paz, F. A. A. (2007). Acta Cryst. E63, m372–m375.
- Min, D. & Lee, S. W. (2002). Bull. Korean Chem. Soc.23, 948–952.
- Nonius (1998). COLLECT Nonius BV, Delft, The Netherlands.
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
- Paz, F. A. A. & Klinowski, J. (2003). Chem. Commun. pp. 1484–1485.
- Shi, F.-N., Cunha-Silva, L., Sá Ferreira, R. A., Mafra, L., Trindade, T., Carlos, L. D., Paz, F. A. A. & Rocha, J. (2008). J. Am. Chem. Soc.130, 150–167. [DOI] [PubMed]
- Wang, Z., Jin, C.-M., Shao, T., Li, Y.-Z., Zhang, K.-L., Zhang, H.-T. & You, X.-Z. (2002). Inorg. Chem. Commun.5, 642–648.
- Zheng, X., Sun, C., Lu, S., Liao, F., Gao, S. & Jin, L. (2004). Eur. J. Inorg. Chem. pp. 3262–3268.
- Zheng, X.-J., Wang, Z.-M., Gao, S., Liao, F.-H., Yan, C.-H. & Jin, L.-P. (2004). Eur. J. Inorg. Chem. pp. 2968–2973.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808000378/hb2668sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808000378/hb2668Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report