Abstract
The title compound, 2C17H13F3N3O+·SO4 2−·2H2O, crystallizes with four independent cations (A, B, C and D) in the asymmetric unit, which is composed of two groups of two cations, one anion and two water molecules (Z′ = 2). The dihedral angle between the mean planes of the 4-hydroxyphenyl and quinolinium groups is 8.9 (7)° in A, 30.1 (6)° in B, 28.8 (8)° in C and 12.8 (1)° in D. The crystal packing is stabilized by intermolecular O—H⋯O and N—H⋯O hydrogen bonding between H atoms from 4-hydroxyphenyl O atoms and the O atoms of nearby water molecules and sulfate anions, as well as H atoms from the N atom of the hydrazino group to O atoms of neighboring sulfate anions, linking the components into chains with the 4-hydroxyphenyl and quinolinium rings parallel to the (011) plane. There is also an extensive array of intermolecular hydrogen bonds between water molecules themselves and with sulfate O atoms, as well as hydrogen-bond interactions between H atoms from the hydrazino group and sulfate O atoms. In addition, intermolecular π–π stacking interactions occur between nearby 4-hydroxyphenyl and quinolinium groups, with distances between the centroids of interacting rings in the range 3.4140 (9)–3.9659 (9) Å.
Related literature
For related structures, see: Yathirajan et al. (2007 ▶); Fun et al. (1999 ▶); Wang et al. (1998 ▶); Sadık et al. (2004 ▶). For related literature, see: Roma et al. (2000 ▶); Chen et al. (2001 ▶); Maguire et al. (1994 ▶); Zhang et al. (2000 ▶); Kahwa et al. (1986 ▶); Santos et al. (2001 ▶); Saim et al. (2004 ▶); El-Masry et al. (2000 ▶); Pandey et al. (1999 ▶); Hodnett et al. (1970 ▶); Misra et al. (1981 ▶); Varma et al. (1986 ▶); Singh et al. (1988 ▶); Desai et al. (2001 ▶).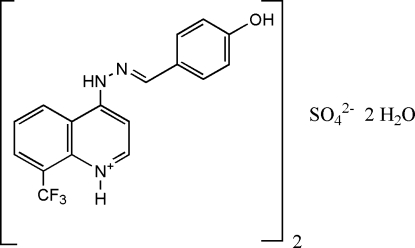
Experimental
Crystal data
2C17H13F3N3O+·SO4 2−·2H2O
M r = 796.70
Monoclinic,
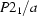
a = 13.4349 (1) Å
b = 23.8601 (1) Å
c = 21.7174 (1) Å
β = 96.8568 (4)°
V = 6911.89 (7) Å3
Z = 8
Cu Kα radiation
μ = 1.68 mm−1
T = 200 (2) K
0.55 × 0.37 × 0.19 mm
Data collection
Oxford Diffraction Gemini R CCD diffractometer
Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2007 ▶) T min = 0.441, T max = 0.727
45529 measured reflections
13742 independent reflections
11290 reflections with I > 2σ(I)
R int = 0.026
Refinement
R[F 2 > 2σ(F 2)] = 0.043
wR(F 2) = 0.127
S = 1.05
13742 reflections
1020 parameters
12 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.74 e Å−3
Δρmin = −0.41 e Å−3
Data collection: CrysAlisPro (Oxford Diffraction, 2007 ▶); cell refinement: CrysAlisPro; data reduction: CrysAlisPro; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808000561/nc2084sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808000561/nc2084Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O4W—H4W1⋯O11i | 0.835 (18) | 2.23 (3) | 2.986 (2) | 151 (5) |
| N2A—H2AB⋯O11i | 0.88 | 1.99 | 2.8592 (18) | 170 |
| C4A—H4AA⋯O11i | 0.95 | 2.28 | 3.199 (2) | 163 |
| O1A—H1A⋯O12ii | 0.84 | 1.89 | 2.6677 (18) | 154 |
| O1D—H1D⋯O13ii | 0.84 | 1.75 | 2.5771 (17) | 167 |
| O4W—H4W2⋯O14iii | 0.820 (19) | 2.06 (2) | 2.860 (2) | 167 (5) |
| C4D—H4DA⋯O14iii | 0.95 | 2.50 | 3.367 (2) | 152 |
| C11D—H11D⋯O14iii | 0.95 | 2.52 | 3.272 (2) | 136 |
| N1A—H1AA⋯O21i | 0.88 | 2.06 | 2.8636 (18) | 151 |
| O3W—H3W2⋯O22i | 0.841 (18) | 2.039 (19) | 2.864 (2) | 167 (4) |
| N2B—H2BB⋯O22iv | 0.88 | 1.93 | 2.7909 (18) | 166 |
| C4B—H4BA⋯O22iv | 0.95 | 2.37 | 3.294 (2) | 165 |
| C11B—H11B⋯O22iv | 0.95 | 2.55 | 3.319 (2) | 138 |
| O3W—H3W1⋯O23iii | 0.824 (18) | 1.952 (19) | 2.776 (2) | 179 (5) |
| O1W—H1W1⋯O24iii | 0.810 (17) | 1.933 (17) | 2.725 (2) | 165 (3) |
| C8A—H8AA⋯O24i | 0.95 | 2.39 | 3.134 (2) | 135 |
| C7C—H7CA⋯O1Av | 0.95 | 2.46 | 3.0647 (19) | 122 |
| C8C—H8CA⋯O1Av | 0.95 | 2.47 | 3.078 (2) | 122 |
| O2W—H2W1⋯O1Dvi | 0.821 (17) | 2.114 (18) | 2.933 (2) | 175 (4) |
| C8B—H8BA⋯O1Dv | 0.95 | 2.34 | 3.234 (2) | 157 |
| O1C—H1C⋯O3Wv | 0.84 | 1.82 | 2.651 (2) | 168 |
| O2W—H2W2⋯O4Wi | 0.828 (17) | 1.931 (17) | 2.759 (3) | 177 (3) |
Symmetry codes: (i) 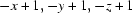 ; (ii)
; (ii) 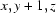 ; (iii)
; (iii) 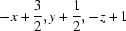 ; (iv)
; (iv) 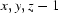 ; (v)
; (v) 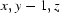 ; (vi)
; (vi) 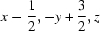 .
.
Acknowledgments
KS thanks the Department of Chemistry, Mangalore University, for use of their research facilities. RJB acknowledges the NSF MRI program (grant No. CHE-0619278) for funds to purchase the X-ray diffractometer.
supplementary crystallographic information
Comment
It is well known that the quinoline ring system is an important structural unit widely existing in alkaloids, therapeutics and synthetic analogues with interesting biological activities (Roma et al. 2000 and Chen et al. 2001). A large variety of quinoline derivatives have been used as antimalarial, anti-inflammatory agents, antiasthmatic, antibacterial, antihypertensive and tyrokinase PDGF-RTK inhibiting agents (Maguire et al. 1994). Furthermore, poly-substituted quinolines have been found to undergo hierarchical self-assembly into a variety of nano- and mesostructures with enhanced electronic and photonic functions (Zhang et al. 2000). The synthesis and structure of Schiff bases have attracted much attention in biology and chemistry (Kahwa et al. 1986). One of the aims of investigating the structural chemistry of Schiff bases is to develop protein and enzyme mimics (Santos et al. 2001). Structural information is useful in investigating the coordination properties of Schiff bases functioning as ligands (Saim et al. 2004). Some Schiff base derivatives were reported to possess antimicrobial, anti-inflammatory and central nervous system activities. Moreover, Schiff bases are also known to have biological activities such as antimicrobial (El-Masry et al.. 2000 & Pandey et al. 1999), antifungal (Singh et al. 1988 & Varma et al. 1986), antitumor (Hodnett et al. 1970; Misra et al. 1981 & Desai et al. 2001), and as herbicides. The crystal structures of Schiff base compounds, viz, bis{4-[(2-hydroxybenzylidine)hydrazino]-8-(trifluoromethyl)quinolinium} sulfate tetrahydrate, (Yathirajan et al. 2007), p-hydroxybenzaldehyde nicotinoylhydrazone monohydrate (Fun et al. 1999), 2-(2-hydroxybenzylidene)-1-(2-picoloyl)hydrazine hemihydrate (Wang et al. 1998), 5-bromo-2-hydroxybenzaldehyde (4-phenyl-1,3-thiazol-2-yl)hydrazone (Sadık et al. 2004) have been reported. A new Schiff base, C34H30F6N6O8S, was synthesized and its crystal structure is reported.
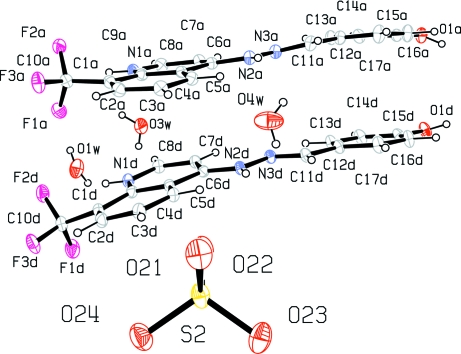
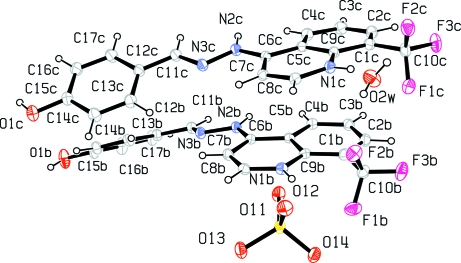
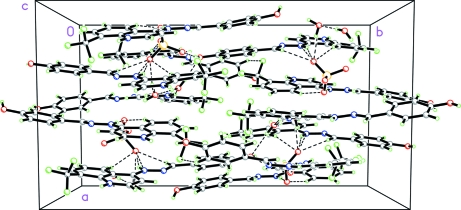
The title compound, C34H30F6N6O8S, crystallizes with four independent cations (A, B, C and D) in the asymmetric unit which is composed of two groups of two cations, one anion and two water molecules [Z'= 2], respectively (Figs. 1–2). The dihedral angle between the mean planes of the 4-hydroxyphenyl and quinolinium groups is 8.9 (7)° in A, 30.1 (6)° in B, 28.8 (8)° in C and 12.8 (1)° in D. Crystal packing is stabilized by intermolecular O—H···O and N—H···O hydrogen bonding between H atoms from 4-hydroxyphenyl oxygen atoms and oxygen atoms from nearby water and sulfate molecules as well as H atoms from the nitrogen atom of the hydrazino group to oxygen atoms from neighboring sulfate molecules linking the molecules into chains with the 4-hydroxyphenyl and quinolimium rings parallel to the [011] plane of the unit cell (Fig. 3). There is also an extensive array of intermolecular hydrogen bonds between water molecules themselves and with sulfate oxygen atoms,in the unit cell as well as hydrogen bond interactions between hydrogen atoms from the hydrazino group and sulfate oxygen atoms. Also, intermolecular π-π stacking interactions occur between adjacent 4-hydroxyphenyl and quinolinium rings as follows: [Cg1, Cg2, Cg3, Cg5, Cg6, Cg9, Cg10, Cg13, Cg14 & Cg15 = center of gravity of (N1A, C5A–C9A), (C1A–C5A, C9A), (C12A–C17A), (N1B, C5B–C9B), (C1B–C5B, C9B), (N1C, C5C–C9C), (C1C–C5C, C9C), (N1C, C5C–C9C), (C1D–C5D, C9D), (C12D–C17D); Cg1···Cg13 = 3.7262 (9) & 3.4140 (9)ix Å; Cg2···Cg13 = 3.5888 (9)ix Å; Cg2···Cg14 = 3.9659 (9)ix Å; Cg3···Cg15 = 3.9401 (10)ix Å; Cg5···Cg9 = 3.5848 (8)ix & 3.6314 (9)x Å; Cg6···Cg9 = 3.8223 (9)ix & 3.5486 (9)x Å; Cg6···Cg10 = 3.6649 (9)xi Å; ix = x, y, z; x = 1/2 - x, 1/2 - y, z; xi = -1/2 + x, 1/2 - y, z].
Experimental
A mixture of 4-hydrazino-8-(trifluoromethyl)quinoline (1.135 g, 0.005 mol) and 4-hydroxybenzaldehyde (0.61 g, 0.005 mol) in 15 ml of absolute alcohol containing 2 drops of sulfuric acid was refluxed for about 3 h. On cooling, the solid separated, was filtered and recrystallized from DMF (m.p.: Above 523 K).
Refinement
The hydroxyl hydrogen atoms (H1A, H1B, H1C, H1D) and the amine hydrogen atoms (H1AA, H2AB, H1BA, H2BB, H1CA, H2CB, H1DA, H2DB) were located in a difference Fourier map and refined using the riding model with O—H = 0.84 Å, N—H = 0.88 Å. All other H atoms in cations A, B, C & D were placed in their calculated positions and then refined using the riding model with C—H = 0.95 Å, and with Uiso(H) = 1.18–1.21Ueq(C, N, O). O–H bonds and H–H distances in each of the water molecules were refined with restraints at 0.82 Å and 1.297 Å, respectively.
Figures
Fig. 1.
Molecular structure of molecules A & D, a sulfate ion and 3 water molecules in a partial description of the asymmetric unit for the title compound, showing atom labeling and 30% probability displacement ellipsoids.
Fig. 2.
Molecular structure of molecules B & C, a sulfate ion and 1 water molecule in a partial description of the asymmetric unit for the title compound, showing atom labeling and 30% probability displacement ellipsoids.
Fig. 3.
Packing diagram of the title compound, viewed down the c axis. Dashed lines indicate intermolecular O—H···O and N—H···O hydrogen bonds.
Crystal data
| 2C17H13F3N3O+·S1O42–·2H2O | F000 = 3280 |
| Mr = 796.70 | Dx = 1.531 Mg m−3 |
| Monoclinic, P21/a | Cu Kα radiation λ = 1.54184 Å |
| Hall symbol: -P 2yab | Cell parameters from 28034 reflections |
| a = 13.4349 (1) Å | θ = 4.1–73.8º |
| b = 23.8601 (1) Å | µ = 1.68 mm−1 |
| c = 21.7174 (1) Å | T = 200 (2) K |
| β = 96.8568 (4)º | Thick plate, yellow |
| V = 6911.89 (7) Å3 | 0.55 × 0.37 × 0.19 mm |
| Z = 8 |
Data collection
| Oxford Diffraction Gemini R CCD diffractometer | 13742 independent reflections |
| Radiation source: fine-focus sealed tube | 11290 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.026 |
| Detector resolution: 10.5081 pixels mm-1 | θmax = 74.1º |
| T = 200(2) K | θmin = 4.1º |
| φ and ω scans | h = −16→15 |
| Absorption correction: multi-scan(CrysAlis RED; Oxford Diffraction, 2007) | k = −29→29 |
| Tmin = 0.441, Tmax = 0.727 | l = −27→27 |
| 45529 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.043 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.127 | w = 1/[σ2(Fo2) + (0.0961P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 1.05 | (Δ/σ)max = 0.001 |
| 13742 reflections | Δρmax = 0.74 e Å−3 |
| 1020 parameters | Δρmin = −0.41 e Å−3 |
| 12 restraints | Extinction correction: none |
| Primary atom site location: structure-invariant direct methods |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.66689 (3) | 0.187616 (15) | 0.499522 (16) | 0.02836 (10) | |
| S2 | 0.75736 (3) | 0.264569 (16) | 0.980196 (16) | 0.03112 (10) | |
| F1A | 0.38287 (9) | 0.60885 (6) | 0.16001 (6) | 0.0492 (3) | |
| F2A | 0.23142 (8) | 0.62688 (5) | 0.17582 (6) | 0.0456 (3) | |
| F3A | 0.30734 (11) | 0.55656 (5) | 0.22072 (6) | 0.0549 (3) | |
| F1B | 0.77439 (9) | 0.30958 (6) | 0.39611 (5) | 0.0505 (3) | |
| F2B | 0.61525 (9) | 0.32051 (5) | 0.38017 (5) | 0.0454 (3) | |
| F3B | 0.71268 (11) | 0.39064 (5) | 0.37049 (6) | 0.0528 (3) | |
| F1C | 0.52317 (9) | 0.42538 (5) | 0.29453 (6) | 0.0497 (3) | |
| F2C | 0.36467 (9) | 0.43361 (5) | 0.27143 (7) | 0.0504 (3) | |
| F3C | 0.46481 (11) | 0.48564 (5) | 0.22683 (7) | 0.0548 (3) | |
| F1D | 0.72434 (11) | 0.57600 (6) | 0.15532 (6) | 0.0581 (3) | |
| F2D | 0.56809 (9) | 0.55653 (5) | 0.14721 (5) | 0.0463 (3) | |
| F3D | 0.67439 (11) | 0.49953 (5) | 0.19391 (6) | 0.0552 (3) | |
| O1A | 0.48294 (10) | 1.15354 (5) | 0.36284 (6) | 0.0369 (3) | |
| H1A | 0.5222 | 1.1637 | 0.3938 | 0.044* | |
| O1B | 0.82685 (15) | −0.10351 (6) | −0.01295 (9) | 0.0600 (4) | |
| H1B | 0.8211 | −0.1274 | 0.0149 | 0.072* | |
| O1C | 0.51549 (11) | −0.12665 (6) | 0.14066 (7) | 0.0439 (3) | |
| H1C | 0.5018 | −0.1442 | 0.1072 | 0.053* | |
| O1D | 0.72708 (11) | 1.07763 (5) | 0.40646 (6) | 0.0399 (3) | |
| H1D | 0.7263 | 1.0910 | 0.4422 | 0.048* | |
| O11 | 0.57717 (10) | 0.19361 (6) | 0.53218 (6) | 0.0402 (3) | |
| O12 | 0.63883 (10) | 0.19895 (5) | 0.43294 (5) | 0.0365 (3) | |
| O13 | 0.70470 (11) | 0.12950 (5) | 0.50799 (6) | 0.0411 (3) | |
| O14 | 0.74565 (10) | 0.22713 (5) | 0.52553 (6) | 0.0391 (3) | |
| O21 | 0.70265 (11) | 0.30509 (5) | 0.93705 (6) | 0.0419 (3) | |
| O22 | 0.68867 (11) | 0.24572 (6) | 1.02472 (6) | 0.0435 (3) | |
| O23 | 0.84557 (12) | 0.29237 (7) | 1.01441 (6) | 0.0494 (3) | |
| O24 | 0.78908 (12) | 0.21622 (6) | 0.94591 (7) | 0.0490 (3) | |
| O1W | 0.53785 (14) | 0.66106 (7) | 0.06907 (7) | 0.0520 (4) | |
| H1W1 | 0.5842 (17) | 0.6790 (13) | 0.0585 (16) | 0.078* | |
| H1W2 | 0.4867 (15) | 0.6696 (15) | 0.0473 (15) | 0.078* | |
| O2W | 0.39849 (12) | 0.35357 (7) | 0.38120 (7) | 0.0516 (4) | |
| H2W1 | 0.353 (2) | 0.3742 (12) | 0.3894 (15) | 0.077* | |
| H2W2 | 0.410 (2) | 0.3341 (13) | 0.4128 (12) | 0.077* | |
| O3W | 0.48321 (15) | 0.80476 (10) | 0.04431 (11) | 0.0758 (6) | |
| H3W1 | 0.5343 (19) | 0.802 (2) | 0.0270 (19) | 0.114* | |
| H3W2 | 0.438 (2) | 0.7906 (19) | 0.0191 (17) | 0.114* | |
| O4W | 0.56157 (16) | 0.71447 (10) | 0.51613 (13) | 0.0818 (7) | |
| H4W1 | 0.532 (3) | 0.7452 (12) | 0.515 (2) | 0.123* | |
| H4W2 | 0.6178 (18) | 0.7226 (18) | 0.508 (2) | 0.123* | |
| N1A | 0.34199 (10) | 0.72867 (6) | 0.18977 (6) | 0.0309 (3) | |
| H1AA | 0.3257 | 0.7068 | 0.1575 | 0.037* | |
| N2A | 0.40367 (10) | 0.83468 (6) | 0.33901 (6) | 0.0296 (3) | |
| H2AB | 0.4118 | 0.8217 | 0.3772 | 0.036* | |
| N3A | 0.40772 (10) | 0.89174 (6) | 0.32737 (6) | 0.0310 (3) | |
| N1B | 0.68578 (10) | 0.22189 (6) | 0.31392 (6) | 0.0291 (3) | |
| H1BA | 0.6837 | 0.2300 | 0.3533 | 0.035* | |
| N2B | 0.69915 (10) | 0.17935 (6) | 0.13111 (6) | 0.0279 (3) | |
| H2BB | 0.6984 | 0.2050 | 0.1019 | 0.034* | |
| N3B | 0.70759 (10) | 0.12263 (6) | 0.11726 (6) | 0.0301 (3) | |
| N1C | 0.43842 (10) | 0.31498 (6) | 0.27005 (6) | 0.0295 (3) | |
| H1CA | 0.4313 | 0.3398 | 0.2992 | 0.035* | |
| N2C | 0.44382 (10) | 0.19512 (6) | 0.13377 (6) | 0.0300 (3) | |
| H2CB | 0.4341 | 0.2047 | 0.0944 | 0.036* | |
| N3C | 0.45725 (10) | 0.13929 (6) | 0.15104 (6) | 0.0315 (3) | |
| N1D | 0.60597 (10) | 0.67250 (6) | 0.19298 (6) | 0.0308 (3) | |
| H1DA | 0.5954 | 0.6544 | 0.1575 | 0.037* | |
| N2D | 0.65741 (10) | 0.76047 (5) | 0.35705 (6) | 0.0280 (3) | |
| H2DB | 0.6703 | 0.7429 | 0.3928 | 0.034* | |
| N3D | 0.65647 (10) | 0.81849 (5) | 0.35395 (6) | 0.0290 (3) | |
| C1A | 0.35732 (12) | 0.64644 (7) | 0.25706 (8) | 0.0320 (3) | |
| C2A | 0.38460 (13) | 0.62364 (7) | 0.31475 (8) | 0.0350 (3) | |
| H2AA | 0.3841 | 0.5841 | 0.3201 | 0.042* | |
| C3A | 0.41310 (13) | 0.65853 (8) | 0.36574 (8) | 0.0349 (3) | |
| H3AA | 0.4333 | 0.6425 | 0.4053 | 0.042* | |
| C4A | 0.41202 (12) | 0.71545 (7) | 0.35901 (7) | 0.0305 (3) | |
| H4AA | 0.4284 | 0.7387 | 0.3943 | 0.037* | |
| C5A | 0.38664 (11) | 0.74009 (7) | 0.29990 (7) | 0.0266 (3) | |
| C6A | 0.38709 (11) | 0.80012 (7) | 0.29088 (7) | 0.0274 (3) | |
| C7A | 0.36927 (12) | 0.82069 (7) | 0.22972 (7) | 0.0314 (3) | |
| H7AA | 0.3724 | 0.8598 | 0.2221 | 0.038* | |
| C8A | 0.34752 (13) | 0.78431 (8) | 0.18157 (7) | 0.0328 (3) | |
| H8AA | 0.3358 | 0.7989 | 0.1406 | 0.039* | |
| C9A | 0.36138 (11) | 0.70516 (7) | 0.24806 (7) | 0.0286 (3) | |
| C10A | 0.32063 (13) | 0.60916 (8) | 0.20370 (9) | 0.0369 (4) | |
| C11A | 0.41583 (13) | 0.92246 (7) | 0.37550 (8) | 0.0337 (3) | |
| H11A | 0.4166 | 0.9056 | 0.4152 | 0.040* | |
| C12A | 0.42402 (12) | 0.98322 (7) | 0.37085 (7) | 0.0320 (3) | |
| C13A | 0.41453 (13) | 1.01174 (7) | 0.31441 (7) | 0.0335 (3) | |
| H13A | 0.3953 | 0.9920 | 0.2769 | 0.040* | |
| C14A | 0.43297 (13) | 1.06857 (7) | 0.31306 (7) | 0.0346 (3) | |
| H14A | 0.4256 | 1.0878 | 0.2745 | 0.042* | |
| C15A | 0.46239 (12) | 1.09814 (7) | 0.36776 (7) | 0.0301 (3) | |
| C16A | 0.46704 (15) | 1.07083 (8) | 0.42426 (8) | 0.0397 (4) | |
| H16A | 0.4842 | 1.0908 | 0.4619 | 0.048* | |
| C17A | 0.44635 (16) | 1.01388 (8) | 0.42514 (8) | 0.0419 (4) | |
| H17A | 0.4475 | 0.9953 | 0.4639 | 0.050* | |
| C1B | 0.70261 (11) | 0.32146 (7) | 0.29216 (7) | 0.0309 (3) | |
| C2B | 0.71193 (12) | 0.36314 (7) | 0.24994 (8) | 0.0336 (3) | |
| H2BA | 0.7182 | 0.4009 | 0.2636 | 0.040* | |
| C3B | 0.71234 (12) | 0.35092 (7) | 0.18666 (8) | 0.0326 (3) | |
| H3BA | 0.7181 | 0.3803 | 0.1578 | 0.039* | |
| C4B | 0.70436 (11) | 0.29630 (7) | 0.16667 (7) | 0.0287 (3) | |
| H4BA | 0.7045 | 0.2882 | 0.1238 | 0.034* | |
| C5B | 0.69594 (10) | 0.25208 (7) | 0.20877 (7) | 0.0255 (3) | |
| C6B | 0.69224 (10) | 0.19367 (7) | 0.18988 (7) | 0.0260 (3) | |
| C7B | 0.68425 (12) | 0.15244 (7) | 0.23553 (7) | 0.0295 (3) | |
| H7BA | 0.6813 | 0.1139 | 0.2245 | 0.035* | |
| C8B | 0.68081 (11) | 0.16813 (7) | 0.29590 (7) | 0.0298 (3) | |
| H8BA | 0.6747 | 0.1399 | 0.3261 | 0.036* | |
| C9B | 0.69409 (10) | 0.26466 (7) | 0.27264 (7) | 0.0263 (3) | |
| C10B | 0.70130 (13) | 0.33571 (8) | 0.35973 (8) | 0.0370 (4) | |
| C11B | 0.73397 (12) | 0.11286 (7) | 0.06376 (7) | 0.0314 (3) | |
| H11B | 0.7411 | 0.1431 | 0.0362 | 0.038* | |
| C12B | 0.75329 (13) | 0.05546 (7) | 0.04440 (7) | 0.0312 (3) | |
| C13B | 0.72313 (12) | 0.00811 (7) | 0.07544 (7) | 0.0323 (3) | |
| H13B | 0.6866 | 0.0128 | 0.1100 | 0.039* | |
| C14B | 0.74556 (13) | −0.04518 (7) | 0.05668 (8) | 0.0349 (3) | |
| H14B | 0.7228 | −0.0770 | 0.0773 | 0.042* | |
| C15B | 0.80194 (15) | −0.05220 (8) | 0.00714 (9) | 0.0414 (4) | |
| C16B | 0.83228 (18) | −0.00535 (9) | −0.02404 (10) | 0.0491 (5) | |
| H16B | 0.8705 | −0.0100 | −0.0578 | 0.059* | |
| C17B | 0.80718 (16) | 0.04761 (8) | −0.00628 (8) | 0.0416 (4) | |
| H17B | 0.8266 | 0.0792 | −0.0286 | 0.050* | |
| C1C | 0.45406 (12) | 0.39089 (7) | 0.19689 (8) | 0.0331 (3) | |
| C2C | 0.46737 (13) | 0.40745 (7) | 0.13768 (9) | 0.0378 (4) | |
| H2CA | 0.4726 | 0.4462 | 0.1287 | 0.045* | |
| C3C | 0.47331 (14) | 0.36817 (8) | 0.09071 (8) | 0.0387 (4) | |
| H3CA | 0.4841 | 0.3803 | 0.0504 | 0.046* | |
| C4C | 0.46365 (13) | 0.31206 (7) | 0.10262 (8) | 0.0339 (3) | |
| H4CA | 0.4663 | 0.2856 | 0.0701 | 0.041* | |
| C5C | 0.44977 (11) | 0.29300 (7) | 0.16300 (7) | 0.0277 (3) | |
| C6C | 0.44602 (11) | 0.23400 (7) | 0.17822 (7) | 0.0272 (3) | |
| C7C | 0.44761 (11) | 0.21926 (7) | 0.24094 (7) | 0.0280 (3) | |
| H7CA | 0.4531 | 0.1810 | 0.2532 | 0.034* | |
| C8C | 0.44122 (11) | 0.26022 (7) | 0.28428 (7) | 0.0284 (3) | |
| H8CA | 0.4387 | 0.2495 | 0.3262 | 0.034* | |
| C9C | 0.44641 (11) | 0.33293 (7) | 0.21064 (7) | 0.0282 (3) | |
| C10C | 0.45138 (14) | 0.43391 (7) | 0.24691 (10) | 0.0388 (4) | |
| C11C | 0.44711 (13) | 0.10421 (7) | 0.10625 (8) | 0.0338 (3) | |
| H11C | 0.4270 | 0.1174 | 0.0654 | 0.041* | |
| C12C | 0.46553 (12) | 0.04426 (7) | 0.11611 (8) | 0.0329 (3) | |
| C13C | 0.50844 (14) | 0.02223 (8) | 0.17285 (8) | 0.0368 (3) | |
| H13C | 0.5268 | 0.0467 | 0.2068 | 0.044* | |
| C14C | 0.52437 (15) | −0.03457 (8) | 0.18002 (8) | 0.0398 (4) | |
| H14C | 0.5533 | −0.0489 | 0.2189 | 0.048* | |
| C15C | 0.49837 (13) | −0.07113 (7) | 0.13077 (8) | 0.0349 (3) | |
| C16C | 0.45736 (15) | −0.04976 (8) | 0.07377 (9) | 0.0435 (4) | |
| H16C | 0.4403 | −0.0742 | 0.0396 | 0.052* | |
| C17C | 0.44146 (15) | 0.00738 (8) | 0.06702 (9) | 0.0430 (4) | |
| H17C | 0.4135 | 0.0217 | 0.0279 | 0.052* | |
| C1D | 0.64734 (12) | 0.58375 (7) | 0.24630 (8) | 0.0314 (3) | |
| C2D | 0.66576 (13) | 0.55508 (7) | 0.30099 (9) | 0.0357 (3) | |
| H2DA | 0.6749 | 0.5156 | 0.3003 | 0.043* | |
| C3D | 0.67130 (13) | 0.58297 (7) | 0.35787 (8) | 0.0350 (3) | |
| H3DA | 0.6815 | 0.5623 | 0.3955 | 0.042* | |
| C4D | 0.66203 (12) | 0.64020 (7) | 0.35952 (7) | 0.0297 (3) | |
| H4DA | 0.6683 | 0.6590 | 0.3983 | 0.036* | |
| C5D | 0.64323 (11) | 0.67139 (6) | 0.30411 (7) | 0.0264 (3) | |
| C6D | 0.63804 (11) | 0.73205 (6) | 0.30392 (7) | 0.0261 (3) | |
| C7D | 0.61317 (11) | 0.75954 (7) | 0.24666 (7) | 0.0290 (3) | |
| H7DA | 0.6078 | 0.7992 | 0.2451 | 0.035* | |
| C8D | 0.59695 (12) | 0.72870 (7) | 0.19352 (7) | 0.0310 (3) | |
| H8DA | 0.5786 | 0.7476 | 0.1554 | 0.037* | |
| C9D | 0.63124 (11) | 0.64288 (6) | 0.24669 (7) | 0.0273 (3) | |
| C10D | 0.65298 (14) | 0.55395 (8) | 0.18568 (9) | 0.0384 (4) | |
| C11D | 0.67856 (12) | 0.84395 (7) | 0.40515 (7) | 0.0306 (3) | |
| H11D | 0.6895 | 0.8233 | 0.4428 | 0.037* | |
| C12D | 0.68713 (12) | 0.90504 (7) | 0.40613 (7) | 0.0293 (3) | |
| C13D | 0.67608 (13) | 0.93616 (7) | 0.35097 (7) | 0.0325 (3) | |
| H13D | 0.6591 | 0.9176 | 0.3125 | 0.039* | |
| C14D | 0.68964 (14) | 0.99353 (7) | 0.35203 (8) | 0.0354 (3) | |
| H14D | 0.6826 | 1.0141 | 0.3143 | 0.042* | |
| C15D | 0.71355 (13) | 1.02134 (7) | 0.40816 (8) | 0.0320 (3) | |
| C16D | 0.72377 (15) | 0.99121 (7) | 0.46336 (8) | 0.0380 (4) | |
| H16D | 0.7396 | 1.0101 | 0.5018 | 0.046* | |
| C17D | 0.71078 (15) | 0.93350 (7) | 0.46207 (8) | 0.0360 (3) | |
| H17D | 0.7181 | 0.9131 | 0.4999 | 0.043* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0433 (2) | 0.02225 (17) | 0.01919 (17) | −0.00186 (14) | 0.00250 (13) | −0.00207 (12) |
| S2 | 0.0479 (2) | 0.02552 (18) | 0.01916 (16) | 0.00029 (15) | 0.00072 (14) | 0.00022 (13) |
| F1A | 0.0495 (6) | 0.0568 (7) | 0.0428 (6) | 0.0014 (5) | 0.0117 (5) | −0.0161 (5) |
| F2A | 0.0412 (5) | 0.0452 (6) | 0.0479 (6) | 0.0019 (5) | −0.0044 (4) | −0.0139 (5) |
| F3A | 0.0801 (8) | 0.0306 (5) | 0.0525 (7) | −0.0039 (5) | 0.0022 (6) | −0.0054 (5) |
| F1B | 0.0497 (6) | 0.0661 (8) | 0.0330 (5) | 0.0046 (6) | −0.0058 (4) | −0.0057 (5) |
| F2B | 0.0466 (6) | 0.0562 (7) | 0.0360 (5) | −0.0044 (5) | 0.0147 (4) | −0.0098 (5) |
| F3B | 0.0756 (8) | 0.0424 (6) | 0.0409 (6) | −0.0103 (6) | 0.0083 (5) | −0.0164 (5) |
| F1C | 0.0541 (6) | 0.0421 (6) | 0.0510 (6) | 0.0000 (5) | −0.0020 (5) | −0.0134 (5) |
| F2C | 0.0470 (6) | 0.0404 (6) | 0.0672 (8) | −0.0005 (5) | 0.0204 (5) | −0.0096 (5) |
| F3C | 0.0721 (8) | 0.0244 (5) | 0.0706 (8) | −0.0046 (5) | 0.0192 (6) | 0.0002 (5) |
| F1D | 0.0662 (7) | 0.0610 (8) | 0.0519 (7) | −0.0156 (6) | 0.0271 (6) | −0.0221 (6) |
| F2D | 0.0589 (6) | 0.0400 (6) | 0.0374 (5) | 0.0005 (5) | −0.0047 (5) | −0.0104 (5) |
| F3D | 0.0794 (8) | 0.0325 (5) | 0.0519 (7) | 0.0123 (6) | 0.0008 (6) | −0.0130 (5) |
| O1A | 0.0510 (7) | 0.0273 (6) | 0.0309 (6) | −0.0041 (5) | −0.0018 (5) | 0.0019 (5) |
| O1B | 0.0842 (11) | 0.0310 (7) | 0.0724 (11) | 0.0019 (7) | 0.0401 (9) | −0.0066 (7) |
| O1C | 0.0562 (8) | 0.0296 (6) | 0.0469 (7) | 0.0001 (6) | 0.0101 (6) | 0.0015 (5) |
| O1D | 0.0656 (8) | 0.0214 (5) | 0.0350 (6) | −0.0019 (5) | 0.0162 (5) | −0.0015 (5) |
| O11 | 0.0501 (7) | 0.0436 (7) | 0.0273 (6) | 0.0008 (6) | 0.0064 (5) | 0.0006 (5) |
| O12 | 0.0548 (7) | 0.0327 (6) | 0.0216 (5) | −0.0055 (5) | 0.0032 (5) | 0.0007 (4) |
| O13 | 0.0671 (8) | 0.0250 (6) | 0.0303 (6) | 0.0039 (6) | 0.0025 (5) | −0.0016 (5) |
| O14 | 0.0543 (7) | 0.0307 (6) | 0.0307 (6) | −0.0084 (5) | −0.0015 (5) | −0.0027 (5) |
| O21 | 0.0682 (8) | 0.0303 (6) | 0.0255 (5) | 0.0011 (6) | −0.0020 (5) | 0.0060 (5) |
| O22 | 0.0617 (8) | 0.0409 (7) | 0.0281 (6) | −0.0019 (6) | 0.0061 (5) | 0.0095 (5) |
| O23 | 0.0609 (8) | 0.0509 (8) | 0.0335 (6) | −0.0114 (7) | −0.0063 (6) | −0.0009 (6) |
| O24 | 0.0616 (8) | 0.0462 (8) | 0.0376 (7) | 0.0118 (7) | −0.0007 (6) | −0.0127 (6) |
| O1W | 0.0702 (9) | 0.0519 (8) | 0.0329 (7) | −0.0169 (8) | 0.0026 (6) | 0.0007 (6) |
| O2W | 0.0613 (9) | 0.0580 (9) | 0.0371 (7) | 0.0180 (7) | 0.0129 (6) | −0.0039 (6) |
| O3W | 0.0587 (10) | 0.0836 (13) | 0.0870 (14) | −0.0094 (10) | 0.0159 (9) | −0.0483 (12) |
| O4W | 0.0611 (10) | 0.0841 (15) | 0.1036 (17) | 0.0200 (10) | 0.0237 (10) | 0.0408 (13) |
| N1A | 0.0364 (6) | 0.0333 (7) | 0.0225 (6) | 0.0004 (5) | 0.0015 (5) | −0.0028 (5) |
| N2A | 0.0369 (6) | 0.0275 (6) | 0.0243 (6) | −0.0019 (5) | 0.0033 (5) | 0.0022 (5) |
| N3A | 0.0374 (6) | 0.0266 (6) | 0.0287 (6) | −0.0021 (5) | 0.0033 (5) | 0.0027 (5) |
| N1B | 0.0334 (6) | 0.0340 (7) | 0.0200 (6) | 0.0002 (5) | 0.0041 (4) | −0.0001 (5) |
| N2B | 0.0353 (6) | 0.0252 (6) | 0.0235 (6) | 0.0008 (5) | 0.0042 (5) | 0.0018 (5) |
| N3B | 0.0374 (7) | 0.0258 (6) | 0.0272 (6) | 0.0016 (5) | 0.0043 (5) | −0.0015 (5) |
| N1C | 0.0332 (6) | 0.0267 (6) | 0.0288 (6) | 0.0005 (5) | 0.0045 (5) | −0.0029 (5) |
| N2C | 0.0369 (6) | 0.0273 (6) | 0.0252 (6) | 0.0021 (5) | 0.0018 (5) | 0.0004 (5) |
| N3C | 0.0354 (6) | 0.0272 (6) | 0.0317 (7) | 0.0021 (5) | 0.0033 (5) | 0.0008 (5) |
| N1D | 0.0392 (7) | 0.0290 (6) | 0.0244 (6) | −0.0031 (5) | 0.0045 (5) | −0.0024 (5) |
| N2D | 0.0365 (6) | 0.0207 (6) | 0.0270 (6) | 0.0003 (5) | 0.0041 (5) | 0.0007 (5) |
| N3D | 0.0361 (6) | 0.0200 (6) | 0.0308 (6) | −0.0002 (5) | 0.0042 (5) | 0.0004 (5) |
| C1A | 0.0326 (7) | 0.0311 (8) | 0.0328 (8) | 0.0014 (6) | 0.0063 (6) | −0.0015 (6) |
| C2A | 0.0388 (8) | 0.0294 (7) | 0.0370 (8) | 0.0022 (7) | 0.0063 (6) | 0.0043 (7) |
| C3A | 0.0402 (8) | 0.0359 (8) | 0.0284 (7) | 0.0018 (7) | 0.0029 (6) | 0.0064 (6) |
| C4A | 0.0330 (7) | 0.0338 (8) | 0.0246 (7) | 0.0010 (6) | 0.0026 (5) | 0.0003 (6) |
| C5A | 0.0263 (6) | 0.0295 (7) | 0.0240 (7) | 0.0005 (6) | 0.0038 (5) | 0.0006 (6) |
| C6A | 0.0276 (7) | 0.0304 (7) | 0.0244 (7) | 0.0005 (6) | 0.0036 (5) | 0.0014 (6) |
| C7A | 0.0380 (8) | 0.0301 (7) | 0.0259 (7) | 0.0012 (6) | 0.0036 (6) | 0.0029 (6) |
| C8A | 0.0391 (8) | 0.0373 (8) | 0.0218 (7) | 0.0024 (7) | 0.0028 (5) | 0.0037 (6) |
| C9A | 0.0285 (7) | 0.0316 (8) | 0.0260 (7) | 0.0021 (6) | 0.0048 (5) | −0.0004 (6) |
| C10A | 0.0399 (8) | 0.0321 (8) | 0.0389 (9) | 0.0015 (7) | 0.0059 (7) | −0.0047 (7) |
| C11A | 0.0423 (8) | 0.0315 (8) | 0.0269 (7) | −0.0073 (7) | 0.0029 (6) | 0.0032 (6) |
| C12A | 0.0379 (8) | 0.0302 (8) | 0.0280 (7) | −0.0058 (6) | 0.0043 (6) | 0.0003 (6) |
| C13A | 0.0442 (8) | 0.0315 (8) | 0.0243 (7) | −0.0018 (7) | 0.0027 (6) | −0.0022 (6) |
| C14A | 0.0471 (9) | 0.0327 (8) | 0.0238 (7) | 0.0030 (7) | 0.0034 (6) | 0.0031 (6) |
| C15A | 0.0343 (7) | 0.0265 (7) | 0.0294 (7) | −0.0009 (6) | 0.0032 (5) | 0.0002 (6) |
| C16A | 0.0585 (10) | 0.0357 (9) | 0.0241 (7) | −0.0109 (8) | 0.0018 (7) | −0.0002 (6) |
| C17A | 0.0632 (11) | 0.0371 (9) | 0.0246 (7) | −0.0122 (8) | 0.0018 (7) | 0.0043 (7) |
| C1B | 0.0293 (7) | 0.0346 (8) | 0.0289 (8) | 0.0007 (6) | 0.0033 (5) | −0.0048 (6) |
| C2B | 0.0340 (8) | 0.0296 (7) | 0.0375 (8) | −0.0007 (6) | 0.0053 (6) | −0.0039 (6) |
| C3B | 0.0329 (7) | 0.0304 (8) | 0.0348 (8) | 0.0004 (6) | 0.0052 (6) | 0.0048 (6) |
| C4B | 0.0294 (7) | 0.0315 (7) | 0.0256 (7) | −0.0001 (6) | 0.0047 (5) | 0.0010 (6) |
| C5B | 0.0239 (6) | 0.0287 (7) | 0.0238 (7) | 0.0008 (5) | 0.0030 (5) | 0.0008 (6) |
| C6B | 0.0245 (6) | 0.0285 (7) | 0.0251 (7) | 0.0021 (5) | 0.0026 (5) | 0.0008 (6) |
| C7B | 0.0341 (7) | 0.0273 (7) | 0.0273 (7) | 0.0004 (6) | 0.0043 (5) | 0.0026 (6) |
| C8B | 0.0316 (7) | 0.0316 (7) | 0.0262 (7) | 0.0019 (6) | 0.0034 (5) | 0.0065 (6) |
| C9B | 0.0238 (6) | 0.0308 (7) | 0.0240 (7) | 0.0006 (5) | 0.0015 (5) | −0.0003 (6) |
| C10B | 0.0403 (8) | 0.0382 (9) | 0.0323 (8) | −0.0021 (7) | 0.0039 (6) | −0.0088 (7) |
| C11B | 0.0406 (8) | 0.0288 (7) | 0.0246 (7) | −0.0009 (6) | 0.0034 (6) | 0.0014 (6) |
| C12B | 0.0411 (8) | 0.0283 (7) | 0.0238 (7) | 0.0001 (6) | 0.0026 (6) | 0.0008 (6) |
| C13B | 0.0400 (8) | 0.0328 (8) | 0.0249 (7) | 0.0023 (7) | 0.0069 (6) | 0.0037 (6) |
| C14B | 0.0426 (8) | 0.0305 (8) | 0.0320 (8) | 0.0009 (7) | 0.0067 (6) | 0.0045 (6) |
| C15B | 0.0529 (10) | 0.0298 (8) | 0.0440 (9) | 0.0010 (8) | 0.0155 (8) | −0.0049 (7) |
| C16B | 0.0717 (13) | 0.0392 (10) | 0.0419 (10) | −0.0042 (9) | 0.0294 (9) | −0.0043 (8) |
| C17B | 0.0637 (11) | 0.0326 (8) | 0.0313 (8) | −0.0057 (8) | 0.0173 (8) | 0.0007 (7) |
| C1C | 0.0301 (7) | 0.0285 (8) | 0.0404 (9) | −0.0013 (6) | 0.0024 (6) | 0.0026 (7) |
| C2C | 0.0395 (8) | 0.0297 (8) | 0.0434 (9) | −0.0041 (7) | 0.0012 (7) | 0.0088 (7) |
| C3C | 0.0422 (9) | 0.0405 (9) | 0.0330 (8) | −0.0029 (7) | 0.0028 (6) | 0.0105 (7) |
| C4C | 0.0384 (8) | 0.0347 (8) | 0.0284 (7) | −0.0005 (7) | 0.0030 (6) | 0.0024 (6) |
| C5C | 0.0255 (6) | 0.0282 (7) | 0.0288 (7) | 0.0000 (6) | 0.0013 (5) | 0.0019 (6) |
| C6C | 0.0248 (6) | 0.0282 (7) | 0.0284 (7) | 0.0012 (5) | 0.0030 (5) | 0.0007 (6) |
| C7C | 0.0300 (7) | 0.0255 (7) | 0.0283 (7) | −0.0011 (6) | 0.0030 (5) | 0.0014 (6) |
| C8C | 0.0292 (7) | 0.0301 (7) | 0.0261 (7) | −0.0015 (6) | 0.0039 (5) | 0.0022 (6) |
| C9C | 0.0261 (6) | 0.0275 (7) | 0.0307 (7) | 0.0008 (6) | 0.0028 (5) | 0.0016 (6) |
| C10C | 0.0404 (9) | 0.0254 (7) | 0.0516 (10) | −0.0023 (7) | 0.0095 (7) | 0.0002 (7) |
| C11C | 0.0415 (8) | 0.0319 (8) | 0.0272 (7) | 0.0038 (7) | 0.0007 (6) | −0.0019 (6) |
| C12C | 0.0379 (8) | 0.0308 (8) | 0.0298 (8) | 0.0027 (6) | 0.0030 (6) | −0.0036 (6) |
| C13C | 0.0486 (9) | 0.0344 (8) | 0.0274 (7) | 0.0004 (7) | 0.0049 (6) | −0.0041 (6) |
| C14C | 0.0523 (10) | 0.0374 (9) | 0.0297 (8) | 0.0039 (8) | 0.0043 (7) | 0.0025 (7) |
| C15C | 0.0375 (8) | 0.0299 (8) | 0.0386 (8) | −0.0002 (7) | 0.0105 (6) | −0.0004 (7) |
| C16C | 0.0522 (10) | 0.0344 (9) | 0.0414 (9) | 0.0016 (8) | −0.0046 (7) | −0.0109 (8) |
| C17C | 0.0575 (10) | 0.0373 (9) | 0.0315 (8) | 0.0082 (8) | −0.0066 (7) | −0.0047 (7) |
| C1D | 0.0349 (7) | 0.0265 (7) | 0.0326 (8) | −0.0029 (6) | 0.0035 (6) | −0.0045 (6) |
| C2D | 0.0421 (8) | 0.0215 (7) | 0.0423 (9) | −0.0004 (6) | −0.0003 (7) | −0.0015 (7) |
| C3D | 0.0447 (8) | 0.0267 (8) | 0.0323 (8) | −0.0007 (7) | −0.0003 (6) | 0.0050 (6) |
| C4D | 0.0357 (7) | 0.0261 (7) | 0.0270 (7) | −0.0016 (6) | 0.0027 (5) | −0.0004 (6) |
| C5D | 0.0273 (6) | 0.0246 (7) | 0.0275 (7) | −0.0020 (6) | 0.0047 (5) | −0.0008 (6) |
| C6D | 0.0264 (6) | 0.0244 (7) | 0.0281 (7) | −0.0014 (5) | 0.0054 (5) | −0.0002 (6) |
| C7D | 0.0331 (7) | 0.0240 (7) | 0.0299 (7) | 0.0000 (6) | 0.0039 (5) | 0.0031 (6) |
| C8D | 0.0361 (7) | 0.0310 (8) | 0.0259 (7) | −0.0007 (6) | 0.0043 (5) | 0.0039 (6) |
| C9D | 0.0287 (7) | 0.0253 (7) | 0.0282 (7) | −0.0031 (6) | 0.0042 (5) | −0.0012 (6) |
| C10D | 0.0473 (9) | 0.0297 (8) | 0.0381 (9) | −0.0012 (7) | 0.0052 (7) | −0.0086 (7) |
| C11D | 0.0412 (8) | 0.0231 (7) | 0.0282 (7) | 0.0018 (6) | 0.0067 (6) | 0.0020 (6) |
| C12D | 0.0381 (7) | 0.0221 (7) | 0.0282 (7) | 0.0016 (6) | 0.0060 (6) | −0.0008 (6) |
| C13D | 0.0476 (9) | 0.0250 (7) | 0.0251 (7) | 0.0030 (6) | 0.0043 (6) | −0.0019 (6) |
| C14D | 0.0544 (9) | 0.0247 (7) | 0.0280 (7) | 0.0043 (7) | 0.0096 (6) | 0.0034 (6) |
| C15D | 0.0415 (8) | 0.0224 (7) | 0.0339 (8) | 0.0023 (6) | 0.0120 (6) | −0.0005 (6) |
| C16D | 0.0606 (10) | 0.0267 (8) | 0.0268 (7) | −0.0016 (7) | 0.0055 (7) | −0.0047 (6) |
| C17D | 0.0560 (10) | 0.0269 (8) | 0.0248 (7) | 0.0002 (7) | 0.0034 (6) | 0.0025 (6) |
Geometric parameters (Å, °)
| S1—O12 | 1.4750 (11) | C14A—H14A | 0.9500 |
| S1—O11 | 1.4767 (13) | C15A—C16A | 1.384 (2) |
| S1—O14 | 1.4782 (13) | C16A—C17A | 1.387 (3) |
| S1—O13 | 1.4808 (13) | C16A—H16A | 0.9500 |
| S2—O24 | 1.4636 (14) | C17A—H17A | 0.9500 |
| S2—O23 | 1.4786 (15) | C1B—C2B | 1.369 (2) |
| S2—O21 | 1.4794 (13) | C1B—C9B | 1.420 (2) |
| S2—O22 | 1.4838 (13) | C1B—C10B | 1.509 (2) |
| F1A—C10A | 1.338 (2) | C2B—C3B | 1.405 (2) |
| F2A—C10A | 1.345 (2) | C2B—H2BA | 0.9500 |
| F3A—C10A | 1.326 (2) | C3B—C4B | 1.374 (2) |
| F1B—C10B | 1.339 (2) | C3B—H3BA | 0.9500 |
| F2B—C10B | 1.337 (2) | C4B—C5B | 1.410 (2) |
| F3B—C10B | 1.337 (2) | C4B—H4BA | 0.9500 |
| F1C—C10C | 1.343 (2) | C5B—C9B | 1.422 (2) |
| F2C—C10C | 1.337 (2) | C5B—C6B | 1.452 (2) |
| F3C—C10C | 1.329 (2) | C6B—C7B | 1.410 (2) |
| F1D—C10D | 1.335 (2) | C7B—C8B | 1.370 (2) |
| F2D—C10D | 1.332 (2) | C7B—H7BA | 0.9500 |
| F3D—C10D | 1.337 (2) | C8B—H8BA | 0.9500 |
| O1A—C15A | 1.357 (2) | C11B—C12B | 1.465 (2) |
| O1A—H1A | 0.8400 | C11B—H11B | 0.9500 |
| O1B—C15B | 1.355 (2) | C12B—C13B | 1.400 (2) |
| O1B—H1B | 0.8400 | C12B—C17B | 1.400 (2) |
| O1C—C15C | 1.357 (2) | C13B—C14B | 1.379 (2) |
| O1C—H1C | 0.8400 | C13B—H13B | 0.9500 |
| O1D—C15D | 1.3564 (19) | C14B—C15B | 1.398 (2) |
| O1D—H1D | 0.8400 | C14B—H14B | 0.9500 |
| O1W—H1W1 | 0.810 (17) | C15B—C16B | 1.393 (3) |
| O1W—H1W2 | 0.812 (17) | C16B—C17B | 1.375 (3) |
| O2W—H2W1 | 0.821 (17) | C16B—H16B | 0.9500 |
| O2W—H2W2 | 0.828 (17) | C17B—H17B | 0.9500 |
| O3W—H3W1 | 0.824 (18) | C1C—C2C | 1.377 (3) |
| O3W—H3W2 | 0.841 (18) | C1C—C9C | 1.421 (2) |
| O4W—H4W1 | 0.835 (18) | C1C—C10C | 1.498 (3) |
| O4W—H4W2 | 0.820 (19) | C2C—C3C | 1.395 (3) |
| N1A—C8A | 1.343 (2) | C2C—H2CA | 0.9500 |
| N1A—C9A | 1.381 (2) | C3C—C4C | 1.373 (3) |
| N1A—H1AA | 0.8800 | C3C—H3CA | 0.9500 |
| N2A—C6A | 1.329 (2) | C4C—C5C | 1.421 (2) |
| N2A—N3A | 1.3870 (19) | C4C—H4CA | 0.9500 |
| N2A—H2AB | 0.8800 | C5C—C9C | 1.411 (2) |
| N3A—C11A | 1.271 (2) | C5C—C6C | 1.448 (2) |
| N1B—C8B | 1.340 (2) | C6C—C7C | 1.405 (2) |
| N1B—C9B | 1.372 (2) | C7C—C8C | 1.367 (2) |
| N1B—H1BA | 0.8800 | C7C—H7CA | 0.9500 |
| N2B—C6B | 1.335 (2) | C8C—H8CA | 0.9500 |
| N2B—N3B | 1.3938 (18) | C11C—C12C | 1.463 (2) |
| N2B—H2BB | 0.8800 | C11C—H11C | 0.9500 |
| N3B—C11B | 1.276 (2) | C12C—C17C | 1.390 (2) |
| N1C—C8C | 1.342 (2) | C12C—C13C | 1.399 (2) |
| N1C—C9C | 1.376 (2) | C13C—C14C | 1.378 (3) |
| N1C—H1CA | 0.8800 | C13C—H13C | 0.9500 |
| N2C—C6C | 1.337 (2) | C14C—C15C | 1.392 (3) |
| N2C—N3C | 1.3898 (19) | C14C—H14C | 0.9500 |
| N2C—H2CB | 0.8800 | C15C—C16C | 1.390 (3) |
| N3C—C11C | 1.278 (2) | C16C—C17C | 1.385 (3) |
| N1D—C8D | 1.347 (2) | C16C—H16C | 0.9500 |
| N1D—C9D | 1.371 (2) | C17C—H17C | 0.9500 |
| N1D—H1DA | 0.8800 | C1D—C2D | 1.367 (3) |
| N2D—C6D | 1.337 (2) | C1D—C9D | 1.428 (2) |
| N2D—N3D | 1.3859 (17) | C1D—C10D | 1.506 (2) |
| N2D—H2DB | 0.8800 | C2D—C3D | 1.397 (3) |
| N3D—C11D | 1.271 (2) | C2D—H2DA | 0.9500 |
| C1A—C2A | 1.375 (2) | C3D—C4D | 1.372 (2) |
| C1A—C9A | 1.416 (2) | C3D—H3DA | 0.9500 |
| C1A—C10A | 1.497 (2) | C4D—C5D | 1.412 (2) |
| C2A—C3A | 1.402 (3) | C4D—H4DA | 0.9500 |
| C2A—H2AA | 0.9500 | C5D—C9D | 1.413 (2) |
| C3A—C4A | 1.366 (2) | C5D—C6D | 1.449 (2) |
| C3A—H3AA | 0.9500 | C6D—C7D | 1.410 (2) |
| C4A—C5A | 1.416 (2) | C7D—C8D | 1.364 (2) |
| C4A—H4AA | 0.9500 | C7D—H7DA | 0.9500 |
| C5A—C9A | 1.409 (2) | C8D—H8DA | 0.9500 |
| C5A—C6A | 1.446 (2) | C11D—C12D | 1.462 (2) |
| C6A—C7A | 1.410 (2) | C11D—H11D | 0.9500 |
| C7A—C8A | 1.363 (2) | C12D—C17D | 1.395 (2) |
| C7A—H7AA | 0.9500 | C12D—C13D | 1.402 (2) |
| C8A—H8AA | 0.9500 | C13D—C14D | 1.381 (2) |
| C11A—C12A | 1.458 (2) | C13D—H13D | 0.9500 |
| C11A—H11A | 0.9500 | C14D—C15D | 1.392 (2) |
| C12A—C17A | 1.390 (2) | C14D—H14D | 0.9500 |
| C12A—C13A | 1.395 (2) | C15D—C16D | 1.390 (2) |
| C13A—C14A | 1.379 (2) | C16D—C17D | 1.388 (2) |
| C13A—H13A | 0.9500 | C16D—H16D | 0.9500 |
| C14A—C15A | 1.398 (2) | C17D—H17D | 0.9500 |
| O12—S1—O11 | 108.99 (8) | F3B—C10B—C1B | 112.13 (16) |
| O12—S1—O14 | 110.19 (7) | F2B—C10B—C1B | 111.89 (14) |
| O11—S1—O14 | 110.05 (8) | F1B—C10B—C1B | 112.10 (15) |
| O12—S1—O13 | 109.70 (7) | N3B—C11B—C12B | 120.67 (15) |
| O11—S1—O13 | 108.58 (8) | N3B—C11B—H11B | 119.7 |
| O14—S1—O13 | 109.30 (8) | C12B—C11B—H11B | 119.7 |
| O24—S2—O23 | 110.16 (9) | C13B—C12B—C17B | 118.49 (16) |
| O24—S2—O21 | 110.23 (8) | C13B—C12B—C11B | 123.02 (15) |
| O23—S2—O21 | 109.45 (9) | C17B—C12B—C11B | 118.46 (15) |
| O24—S2—O22 | 109.52 (9) | C14B—C13B—C12B | 121.04 (15) |
| O23—S2—O22 | 109.39 (8) | C14B—C13B—H13B | 119.5 |
| O21—S2—O22 | 108.07 (8) | C12B—C13B—H13B | 119.5 |
| C15A—O1A—H1A | 109.5 | C13B—C14B—C15B | 119.69 (16) |
| C15B—O1B—H1B | 109.5 | C13B—C14B—H14B | 120.2 |
| C15C—O1C—H1C | 109.5 | C15B—C14B—H14B | 120.2 |
| C15D—O1D—H1D | 109.5 | O1B—C15B—C16B | 118.08 (17) |
| H1W1—O1W—H1W2 | 109 (3) | O1B—C15B—C14B | 122.24 (18) |
| H2W1—O2W—H2W2 | 103 (2) | C16B—C15B—C14B | 119.67 (17) |
| H3W1—O3W—H3W2 | 104 (3) | C17B—C16B—C15B | 120.35 (16) |
| H4W1—O4W—H4W2 | 104 (3) | C17B—C16B—H16B | 119.8 |
| C8A—N1A—C9A | 121.06 (14) | C15B—C16B—H16B | 119.8 |
| C8A—N1A—H1AA | 119.5 | C16B—C17B—C12B | 120.71 (17) |
| C9A—N1A—H1AA | 119.5 | C16B—C17B—H17B | 119.6 |
| C6A—N2A—N3A | 118.18 (13) | C12B—C17B—H17B | 119.6 |
| C6A—N2A—H2AB | 120.9 | C2C—C1C—C9C | 119.74 (16) |
| N3A—N2A—H2AB | 120.9 | C2C—C1C—C10C | 119.85 (16) |
| C11A—N3A—N2A | 114.69 (13) | C9C—C1C—C10C | 120.38 (16) |
| C8B—N1B—C9B | 121.76 (13) | C1C—C2C—C3C | 121.03 (16) |
| C8B—N1B—H1BA | 119.1 | C1C—C2C—H2CA | 119.5 |
| C9B—N1B—H1BA | 119.1 | C3C—C2C—H2CA | 119.5 |
| C6B—N2B—N3B | 118.13 (13) | C4C—C3C—C2C | 120.17 (16) |
| C6B—N2B—H2BB | 120.9 | C4C—C3C—H3CA | 119.9 |
| N3B—N2B—H2BB | 120.9 | C2C—C3C—H3CA | 119.9 |
| C11B—N3B—N2B | 114.36 (14) | C3C—C4C—C5C | 120.78 (16) |
| C8C—N1C—C9C | 121.06 (14) | C3C—C4C—H4CA | 119.6 |
| C8C—N1C—H1CA | 119.5 | C5C—C4C—H4CA | 119.6 |
| C9C—N1C—H1CA | 119.5 | C9C—C5C—C4C | 118.67 (15) |
| C6C—N2C—N3C | 118.65 (13) | C9C—C5C—C6C | 118.94 (14) |
| C6C—N2C—H2CB | 120.7 | C4C—C5C—C6C | 122.24 (15) |
| N3C—N2C—H2CB | 120.7 | N2C—C6C—C7C | 121.53 (14) |
| C11C—N3C—N2C | 115.06 (14) | N2C—C6C—C5C | 120.55 (14) |
| C8D—N1D—C9D | 121.28 (14) | C7C—C6C—C5C | 117.90 (14) |
| C8D—N1D—H1DA | 119.4 | C8C—C7C—C6C | 119.59 (14) |
| C9D—N1D—H1DA | 119.4 | C8C—C7C—H7CA | 120.2 |
| C6D—N2D—N3D | 117.71 (13) | C6C—C7C—H7CA | 120.2 |
| C6D—N2D—H2DB | 121.1 | N1C—C8C—C7C | 122.68 (14) |
| N3D—N2D—H2DB | 121.1 | N1C—C8C—H8CA | 118.7 |
| C11D—N3D—N2D | 115.80 (13) | C7C—C8C—H8CA | 118.7 |
| C2A—C1A—C9A | 120.45 (15) | N1C—C9C—C5C | 119.35 (14) |
| C2A—C1A—C10A | 119.84 (16) | N1C—C9C—C1C | 121.05 (15) |
| C9A—C1A—C10A | 119.70 (15) | C5C—C9C—C1C | 119.57 (15) |
| C1A—C2A—C3A | 120.19 (16) | F3C—C10C—F2C | 107.13 (15) |
| C1A—C2A—H2AA | 119.9 | F3C—C10C—F1C | 106.28 (15) |
| C3A—C2A—H2AA | 119.9 | F2C—C10C—F1C | 105.80 (16) |
| C4A—C3A—C2A | 120.50 (15) | F3C—C10C—C1C | 112.48 (16) |
| C4A—C3A—H3AA | 119.8 | F2C—C10C—C1C | 112.50 (15) |
| C2A—C3A—H3AA | 119.8 | F1C—C10C—C1C | 112.18 (15) |
| C3A—C4A—C5A | 120.54 (15) | N3C—C11C—C12C | 121.87 (15) |
| C3A—C4A—H4AA | 119.7 | N3C—C11C—H11C | 119.1 |
| C5A—C4A—H4AA | 119.7 | C12C—C11C—H11C | 119.1 |
| C9A—C5A—C4A | 119.19 (15) | C17C—C12C—C13C | 118.11 (17) |
| C9A—C5A—C6A | 118.83 (14) | C17C—C12C—C11C | 119.13 (16) |
| C4A—C5A—C6A | 121.98 (14) | C13C—C12C—C11C | 122.75 (15) |
| N2A—C6A—C7A | 121.25 (15) | C14C—C13C—C12C | 120.75 (16) |
| N2A—C6A—C5A | 120.74 (14) | C14C—C13C—H13C | 119.6 |
| C7A—C6A—C5A | 118.01 (14) | C12C—C13C—H13C | 119.6 |
| C8A—C7A—C6A | 119.85 (15) | C13C—C14C—C15C | 120.54 (17) |
| C8A—C7A—H7AA | 120.1 | C13C—C14C—H14C | 119.7 |
| C6A—C7A—H7AA | 120.1 | C15C—C14C—H14C | 119.7 |
| N1A—C8A—C7A | 122.55 (15) | O1C—C15C—C16C | 122.71 (17) |
| N1A—C8A—H8AA | 118.7 | O1C—C15C—C14C | 117.90 (17) |
| C7A—C8A—H8AA | 118.7 | C16C—C15C—C14C | 119.39 (17) |
| N1A—C9A—C5A | 119.55 (15) | C17C—C16C—C15C | 119.66 (17) |
| N1A—C9A—C1A | 121.49 (15) | C17C—C16C—H16C | 120.2 |
| C5A—C9A—C1A | 118.96 (14) | C15C—C16C—H16C | 120.2 |
| F3A—C10A—F1A | 107.99 (15) | C16C—C17C—C12C | 121.54 (17) |
| F3A—C10A—F2A | 106.30 (15) | C16C—C17C—H17C | 119.2 |
| F1A—C10A—F2A | 106.34 (15) | C12C—C17C—H17C | 119.2 |
| F3A—C10A—C1A | 112.91 (15) | C2D—C1D—C9D | 120.07 (15) |
| F1A—C10A—C1A | 112.11 (15) | C2D—C1D—C10D | 120.03 (15) |
| F2A—C10A—C1A | 110.80 (14) | C9D—C1D—C10D | 119.68 (15) |
| N3A—C11A—C12A | 121.08 (15) | C1D—C2D—C3D | 120.98 (15) |
| N3A—C11A—H11A | 119.5 | C1D—C2D—H2DA | 119.5 |
| C12A—C11A—H11A | 119.5 | C3D—C2D—H2DA | 119.5 |
| C17A—C12A—C13A | 118.46 (16) | C4D—C3D—C2D | 120.11 (15) |
| C17A—C12A—C11A | 118.37 (15) | C4D—C3D—H3DA | 119.9 |
| C13A—C12A—C11A | 123.12 (15) | C2D—C3D—H3DA | 119.9 |
| C14A—C13A—C12A | 120.05 (15) | C3D—C4D—C5D | 120.67 (15) |
| C14A—C13A—H13A | 120.0 | C3D—C4D—H4DA | 119.7 |
| C12A—C13A—H13A | 120.0 | C5D—C4D—H4DA | 119.7 |
| C13A—C14A—C15A | 120.76 (15) | C4D—C5D—C9D | 119.23 (14) |
| C13A—C14A—H14A | 119.6 | C4D—C5D—C6D | 122.20 (14) |
| C15A—C14A—H14A | 119.6 | C9D—C5D—C6D | 118.56 (14) |
| O1A—C15A—C16A | 122.69 (15) | N2D—C6D—C7D | 121.76 (14) |
| O1A—C15A—C14A | 117.67 (14) | N2D—C6D—C5D | 119.98 (14) |
| C16A—C15A—C14A | 119.61 (16) | C7D—C6D—C5D | 118.25 (14) |
| C15A—C16A—C17A | 119.03 (16) | C8D—C7D—C6D | 119.49 (15) |
| C15A—C16A—H16A | 120.5 | C8D—C7D—H7DA | 120.3 |
| C17A—C16A—H16A | 120.5 | C6D—C7D—H7DA | 120.3 |
| C16A—C17A—C12A | 121.83 (16) | N1D—C8D—C7D | 122.62 (15) |
| C16A—C17A—H17A | 119.1 | N1D—C8D—H8DA | 118.7 |
| C12A—C17A—H17A | 119.1 | C7D—C8D—H8DA | 118.7 |
| C2B—C1B—C9B | 120.26 (15) | N1D—C9D—C5D | 119.54 (14) |
| C2B—C1B—C10B | 120.03 (16) | N1D—C9D—C1D | 121.78 (14) |
| C9B—C1B—C10B | 119.71 (15) | C5D—C9D—C1D | 118.67 (14) |
| C1B—C2B—C3B | 121.04 (16) | F2D—C10D—F1D | 106.48 (16) |
| C1B—C2B—H2BA | 119.5 | F2D—C10D—F3D | 106.44 (15) |
| C3B—C2B—H2BA | 119.5 | F1D—C10D—F3D | 107.05 (16) |
| C4B—C3B—C2B | 119.78 (15) | F2D—C10D—C1D | 113.44 (15) |
| C4B—C3B—H3BA | 120.1 | F1D—C10D—C1D | 111.08 (14) |
| C2B—C3B—H3BA | 120.1 | F3D—C10D—C1D | 111.95 (16) |
| C3B—C4B—C5B | 120.95 (14) | N3D—C11D—C12D | 119.94 (14) |
| C3B—C4B—H4BA | 119.5 | N3D—C11D—H11D | 120.0 |
| C5B—C4B—H4BA | 119.5 | C12D—C11D—H11D | 120.0 |
| C4B—C5B—C9B | 119.12 (14) | C17D—C12D—C13D | 118.45 (15) |
| C4B—C5B—C6B | 122.54 (14) | C17D—C12D—C11D | 120.48 (14) |
| C9B—C5B—C6B | 118.31 (14) | C13D—C12D—C11D | 121.02 (14) |
| N2B—C6B—C7B | 120.89 (14) | C14D—C13D—C12D | 120.69 (15) |
| N2B—C6B—C5B | 120.79 (14) | C14D—C13D—H13D | 119.7 |
| C7B—C6B—C5B | 118.29 (14) | C12D—C13D—H13D | 119.7 |
| C8B—C7B—C6B | 119.77 (15) | C13D—C14D—C15D | 120.25 (15) |
| C8B—C7B—H7BA | 120.1 | C13D—C14D—H14D | 119.9 |
| C6B—C7B—H7BA | 120.1 | C15D—C14D—H14D | 119.9 |
| N1B—C8B—C7B | 122.33 (15) | O1D—C15D—C16D | 122.39 (15) |
| N1B—C8B—H8BA | 118.8 | O1D—C15D—C14D | 117.78 (15) |
| C7B—C8B—H8BA | 118.8 | C16D—C15D—C14D | 119.82 (15) |
| N1B—C9B—C1B | 121.65 (14) | C17D—C16D—C15D | 119.76 (16) |
| N1B—C9B—C5B | 119.50 (14) | C17D—C16D—H16D | 120.1 |
| C1B—C9B—C5B | 118.84 (14) | C15D—C16D—H16D | 120.1 |
| F3B—C10B—F2B | 107.05 (15) | C16D—C17D—C12D | 121.03 (15) |
| F3B—C10B—F1B | 107.03 (15) | C16D—C17D—H17D | 119.5 |
| F2B—C10B—F1B | 106.26 (16) | C12D—C17D—H17D | 119.5 |
| C6A—N2A—N3A—C11A | 174.68 (15) | C13B—C12B—C17B—C16B | −1.8 (3) |
| C6B—N2B—N3B—C11B | −166.53 (14) | C11B—C12B—C17B—C16B | 176.22 (19) |
| C6C—N2C—N3C—C11C | 175.23 (15) | C9C—C1C—C2C—C3C | 0.1 (3) |
| C6D—N2D—N3D—C11D | −177.70 (14) | C10C—C1C—C2C—C3C | 177.96 (16) |
| C9A—C1A—C2A—C3A | 2.4 (2) | C1C—C2C—C3C—C4C | 1.5 (3) |
| C10A—C1A—C2A—C3A | −176.03 (16) | C2C—C3C—C4C—C5C | −1.4 (3) |
| C1A—C2A—C3A—C4A | 1.4 (3) | C3C—C4C—C5C—C9C | −0.2 (2) |
| C2A—C3A—C4A—C5A | −3.0 (2) | C3C—C4C—C5C—C6C | −175.78 (15) |
| C3A—C4A—C5A—C9A | 0.7 (2) | N3C—N2C—C6C—C7C | −8.9 (2) |
| C3A—C4A—C5A—C6A | −178.49 (15) | N3C—N2C—C6C—C5C | 169.30 (13) |
| N3A—N2A—C6A—C7A | −3.0 (2) | C9C—C5C—C6C—N2C | 176.53 (14) |
| N3A—N2A—C6A—C5A | 177.14 (13) | C4C—C5C—C6C—N2C | −7.9 (2) |
| C9A—C5A—C6A—N2A | 175.32 (13) | C9C—C5C—C6C—C7C | −5.2 (2) |
| C4A—C5A—C6A—N2A | −5.5 (2) | C4C—C5C—C6C—C7C | 170.37 (14) |
| C9A—C5A—C6A—C7A | −4.6 (2) | N2C—C6C—C7C—C8C | −174.45 (14) |
| C4A—C5A—C6A—C7A | 174.61 (14) | C5C—C6C—C7C—C8C | 7.3 (2) |
| N2A—C6A—C7A—C8A | −176.70 (15) | C9C—N1C—C8C—C7C | −2.9 (2) |
| C5A—C6A—C7A—C8A | 3.2 (2) | C6C—C7C—C8C—N1C | −3.4 (2) |
| C9A—N1A—C8A—C7A | −2.0 (2) | C8C—N1C—C9C—C5C | 5.0 (2) |
| C6A—C7A—C8A—N1A | 0.1 (3) | C8C—N1C—C9C—C1C | −173.31 (14) |
| C8A—N1A—C9A—C5A | 0.5 (2) | C4C—C5C—C9C—N1C | −176.54 (14) |
| C8A—N1A—C9A—C1A | −178.97 (15) | C6C—C5C—C9C—N1C | −0.8 (2) |
| C4A—C5A—C9A—N1A | −176.41 (13) | C4C—C5C—C9C—C1C | 1.8 (2) |
| C6A—C5A—C9A—N1A | 2.8 (2) | C6C—C5C—C9C—C1C | 177.50 (13) |
| C4A—C5A—C9A—C1A | 3.1 (2) | C2C—C1C—C9C—N1C | 176.53 (15) |
| C6A—C5A—C9A—C1A | −177.73 (13) | C10C—C1C—C9C—N1C | −1.3 (2) |
| C2A—C1A—C9A—N1A | 174.83 (15) | C2C—C1C—C9C—C5C | −1.7 (2) |
| C10A—C1A—C9A—N1A | −6.7 (2) | C10C—C1C—C9C—C5C | −179.57 (15) |
| C2A—C1A—C9A—C5A | −4.6 (2) | C2C—C1C—C10C—F3C | −1.2 (2) |
| C10A—C1A—C9A—C5A | 173.83 (14) | C9C—C1C—C10C—F3C | 176.61 (15) |
| C2A—C1A—C10A—F3A | 7.6 (2) | C2C—C1C—C10C—F2C | 119.88 (18) |
| C9A—C1A—C10A—F3A | −170.92 (15) | C9C—C1C—C10C—F2C | −62.3 (2) |
| C2A—C1A—C10A—F1A | −114.69 (18) | C2C—C1C—C10C—F1C | −120.97 (18) |
| C9A—C1A—C10A—F1A | 66.8 (2) | C9C—C1C—C10C—F1C | 56.9 (2) |
| C2A—C1A—C10A—F2A | 126.68 (17) | N2C—N3C—C11C—C12C | 175.75 (14) |
| C9A—C1A—C10A—F2A | −51.8 (2) | N3C—C11C—C12C—C17C | 172.15 (17) |
| N2A—N3A—C11A—C12A | 178.14 (14) | N3C—C11C—C12C—C13C | −9.1 (3) |
| N3A—C11A—C12A—C17A | −171.37 (17) | C17C—C12C—C13C—C14C | −1.3 (3) |
| N3A—C11A—C12A—C13A | 6.0 (3) | C11C—C12C—C13C—C14C | 179.97 (17) |
| C17A—C12A—C13A—C14A | 3.8 (3) | C12C—C13C—C14C—C15C | 0.3 (3) |
| C11A—C12A—C13A—C14A | −173.53 (17) | C13C—C14C—C15C—O1C | −179.77 (16) |
| C12A—C13A—C14A—C15A | 0.7 (3) | C13C—C14C—C15C—C16C | 0.9 (3) |
| C13A—C14A—C15A—O1A | 177.59 (16) | O1C—C15C—C16C—C17C | 179.70 (18) |
| C13A—C14A—C15A—C16A | −4.1 (3) | C14C—C15C—C16C—C17C | −1.0 (3) |
| O1A—C15A—C16A—C17A | −179.04 (17) | C15C—C16C—C17C—C12C | 0.0 (3) |
| C14A—C15A—C16A—C17A | 2.7 (3) | C13C—C12C—C17C—C16C | 1.2 (3) |
| C15A—C16A—C17A—C12A | 1.9 (3) | C11C—C12C—C17C—C16C | 179.97 (18) |
| C13A—C12A—C17A—C16A | −5.2 (3) | C9D—C1D—C2D—C3D | −1.4 (3) |
| C11A—C12A—C17A—C16A | 172.26 (18) | C10D—C1D—C2D—C3D | 173.24 (16) |
| C9B—C1B—C2B—C3B | 0.6 (2) | C1D—C2D—C3D—C4D | −2.5 (3) |
| C10B—C1B—C2B—C3B | −179.37 (15) | C2D—C3D—C4D—C5D | 2.3 (3) |
| C1B—C2B—C3B—C4B | −0.7 (2) | C3D—C4D—C5D—C9D | 1.7 (2) |
| C2B—C3B—C4B—C5B | −0.1 (2) | C3D—C4D—C5D—C6D | −176.80 (15) |
| C3B—C4B—C5B—C9B | 1.0 (2) | N3D—N2D—C6D—C7D | −2.7 (2) |
| C3B—C4B—C5B—C6B | −176.94 (14) | N3D—N2D—C6D—C5D | 176.75 (12) |
| N3B—N2B—C6B—C7B | −5.8 (2) | C4D—C5D—C6D—N2D | 4.2 (2) |
| N3B—N2B—C6B—C5B | 172.22 (13) | C9D—C5D—C6D—N2D | −174.39 (13) |
| C4B—C5B—C6B—N2B | 1.6 (2) | C4D—C5D—C6D—C7D | −176.38 (14) |
| C9B—C5B—C6B—N2B | −176.40 (13) | C9D—C5D—C6D—C7D | 5.1 (2) |
| C4B—C5B—C6B—C7B | 179.70 (14) | N2D—C6D—C7D—C8D | 178.09 (14) |
| C9B—C5B—C6B—C7B | 1.7 (2) | C5D—C6D—C7D—C8D | −1.4 (2) |
| N2B—C6B—C7B—C8B | 177.71 (14) | C9D—N1D—C8D—C7D | 0.7 (2) |
| C5B—C6B—C7B—C8B | −0.4 (2) | C6D—C7D—C8D—N1D | −1.6 (2) |
| C9B—N1B—C8B—C7B | 0.4 (2) | C8D—N1D—C9D—C5D | 3.2 (2) |
| C6B—C7B—C8B—N1B | −0.7 (2) | C8D—N1D—C9D—C1D | −175.89 (14) |
| C8B—N1B—C9B—C1B | −177.93 (14) | C4D—C5D—C9D—N1D | 175.41 (14) |
| C8B—N1B—C9B—C5B | 1.0 (2) | C6D—C5D—C9D—N1D | −6.0 (2) |
| C2B—C1B—C9B—N1B | 179.26 (14) | C4D—C5D—C9D—C1D | −5.5 (2) |
| C10B—C1B—C9B—N1B | −0.8 (2) | C6D—C5D—C9D—C1D | 173.12 (13) |
| C2B—C1B—C9B—C5B | 0.3 (2) | C2D—C1D—C9D—N1D | −175.57 (15) |
| C10B—C1B—C9B—C5B | −179.72 (14) | C10D—C1D—C9D—N1D | 9.8 (2) |
| C4B—C5B—C9B—N1B | 179.91 (13) | C2D—C1D—C9D—C5D | 5.3 (2) |
| C6B—C5B—C9B—N1B | −2.0 (2) | C10D—C1D—C9D—C5D | −169.30 (14) |
| C4B—C5B—C9B—C1B | −1.1 (2) | C2D—C1D—C10D—F2D | 122.03 (18) |
| C6B—C5B—C9B—C1B | 176.96 (13) | C9D—C1D—C10D—F2D | −63.3 (2) |
| C2B—C1B—C10B—F3B | −1.7 (2) | C2D—C1D—C10D—F1D | −118.07 (19) |
| C9B—C1B—C10B—F3B | 178.35 (14) | C9D—C1D—C10D—F1D | 56.6 (2) |
| C2B—C1B—C10B—F2B | 118.63 (18) | C2D—C1D—C10D—F3D | 1.5 (2) |
| C9B—C1B—C10B—F2B | −61.3 (2) | C9D—C1D—C10D—F3D | 176.19 (15) |
| C2B—C1B—C10B—F1B | −122.11 (18) | N2D—N3D—C11D—C12D | 175.34 (13) |
| C9B—C1B—C10B—F1B | 57.9 (2) | N3D—C11D—C12D—C17D | 179.64 (16) |
| N2B—N3B—C11B—C12B | 175.40 (14) | N3D—C11D—C12D—C13D | −3.1 (2) |
| N3B—C11B—C12B—C13B | 15.0 (3) | C17D—C12D—C13D—C14D | 0.9 (3) |
| N3B—C11B—C12B—C17B | −162.93 (18) | C11D—C12D—C13D—C14D | −176.46 (16) |
| C17B—C12B—C13B—C14B | −0.1 (3) | C12D—C13D—C14D—C15D | −0.7 (3) |
| C11B—C12B—C13B—C14B | −178.04 (16) | C13D—C14D—C15D—O1D | 179.32 (16) |
| C12B—C13B—C14B—C15B | 2.0 (3) | C13D—C14D—C15D—C16D | 0.0 (3) |
| C13B—C14B—C15B—O1B | 179.48 (19) | O1D—C15D—C16D—C17D | −178.83 (17) |
| C13B—C14B—C15B—C16B | −2.0 (3) | C14D—C15D—C16D—C17D | 0.5 (3) |
| O1B—C15B—C16B—C17B | 178.7 (2) | C15D—C16D—C17D—C12D | −0.3 (3) |
| C14B—C15B—C16B—C17B | 0.1 (3) | C13D—C12D—C17D—C16D | −0.4 (3) |
| C15B—C16B—C17B—C12B | 1.8 (4) | C11D—C12D—C17D—C16D | 176.94 (17) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O4W—H4W1···O11i | 0.835 (18) | 2.23 (3) | 2.986 (2) | 151 (5) |
| N2A—H2AB···O11i | 0.88 | 1.99 | 2.8592 (18) | 170 |
| C4A—H4AA···O11i | 0.95 | 2.28 | 3.199 (2) | 163 |
| O1A—H1A···O12ii | 0.84 | 1.89 | 2.6677 (18) | 154 |
| O1D—H1D···O13ii | 0.84 | 1.75 | 2.5771 (17) | 167 |
| O4W—H4W2···O14iii | 0.820 (19) | 2.06 (2) | 2.860 (2) | 167 (5) |
| C4D—H4DA···O14iii | 0.95 | 2.50 | 3.367 (2) | 152 |
| C11D—H11D···O14iii | 0.95 | 2.52 | 3.272 (2) | 136 |
| N1A—H1AA···O21i | 0.88 | 2.06 | 2.8636 (18) | 151 |
| O3W—H3W2···O22i | 0.841 (18) | 2.039 (19) | 2.864 (2) | 167 (4) |
| N2B—H2BB···O22iv | 0.88 | 1.93 | 2.7909 (18) | 166 |
| C4B—H4BA···O22iv | 0.95 | 2.37 | 3.294 (2) | 165 |
| C11B—H11B···O22iv | 0.95 | 2.55 | 3.319 (2) | 138 |
| O3W—H3W1···O23iii | 0.824 (18) | 1.952 (19) | 2.776 (2) | 179 (5) |
| O1W—H1W1···O24iii | 0.810 (17) | 1.933 (17) | 2.725 (2) | 165 (3) |
| C8A—H8AA···O24i | 0.95 | 2.39 | 3.134 (2) | 135 |
| C7C—H7CA···O1Av | 0.95 | 2.46 | 3.0647 (19) | 122 |
| C8C—H8CA···O1Av | 0.95 | 2.47 | 3.078 (2) | 122 |
| O2W—H2W1···O1Dvi | 0.821 (17) | 2.114 (18) | 2.933 (2) | 175 (4) |
| C8B—H8BA···O1Dv | 0.95 | 2.34 | 3.234 (2) | 157 |
| O1C—H1C···O3Wv | 0.84 | 1.82 | 2.651 (2) | 168 |
| O2W—H2W2···O4Wi | 0.828 (17) | 1.931 (17) | 2.759 (3) | 177 (3) |
Symmetry codes: (i) −x+1, −y+1, −z+1; (ii) x, y+1, z; (iii) −x+3/2, y+1/2, −z+1; (iv) x, y, z−1; (v) x, y−1, z; (vi) x−1/2, −y+3/2, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: NC2084).
References
- Chen, Y. L., Fang, K. C., Sheu, J. Y., Hsu, S. L. & Tzeng, C. C. (2001). J. Med. Chem.44, 2374–2377. [DOI] [PubMed]
- Desai, S. B., Desai, P. B. & Desai, K. R. (2001). Heterocycl. Commun. 7, 83–90.
- El-Masry, A. H., Fahmy, H. H. & Abdelwahed, S. H. A. (2000). Molecules, 5, 1429–1438.
- Fun, H.-K., Chinnakali, K., Razak, I. A., Lu, Z.-L. & Kang, B.-S. (1999). Acta Cryst. C55, 574–576.
- Hodnett, E. M. & Dunn, W. J. (1970). J. Med. Chem.13, 768–770. [DOI] [PubMed]
- Kahwa, I. A., Selbin, J., Hsieh, T. C.-Y. & Laine, R. A. (1986). Inorg. Chim. Acta, 118, 179–185.
- Maguire, M. P., Sheets, K. R., McVety, K., Spada, A. P. & Zilberstein, A. (1994). J. Med. Chem.37, 2129–2137. [DOI] [PubMed]
- Misra, V. S., Singh, S., Agarwal, R. & Chaudhary, K. C. (1981). J. Chem. Soc. Pak.3, 209–213.
- Oxford Diffraction (2007). CrysAlisPro and CrysAlis RED. Versions 1.171.31.8. Oxford Diffraction Ltd, Abingdon, Oxfordshire, England.
- Pandey, S. N., Sriram, D., Nath, G. & De Clercq, E. (1999). Il Farmaco, 54, 624–628. [DOI] [PubMed]
- Roma, G., Braccio, M. D., Grossi, G., Mattioli, F. & Ghia, M. (2000). Eur. J. Med. Chem.35, 1021–1035. [DOI] [PubMed]
- Sadık, G., Necmi, D., Ibrahim, Y., Alaaddin, Ç. & Dinçer, M. (2004). Acta Cryst. E60, o889–o891.
- Saim, O., Dincer, U., Leyla, T. Y., Nermin, B. & Bahattin, G. (2004). J. Mol. Struct.688, 207–211.
- Santos, M. L. P., Bagatin, I. A., Pereira, E. M. & Ferreira, A. M. D. C. (2001). J. Chem. Soc. Dalton Trans. pp. 838–844.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Singh, W. M. & Dash, B. C. (1988). Pesticides, 22, 33–37.
- Varma, R. S., Prakash, R., Khan, M. M. & Ali, A. (1986). Indian Drugs, 23, 345–349.
- Wang, Z., Jian, F., Duan, C., Bai, Z. & You, X. (1998). Acta Cryst. C54, 1927–1929.
- Yathirajan, H. S., Sarojini, B. K., Narayana, B., Sunil, K. & Bolte, M. (2007). Acta Cryst. E63, o2720–o2721.
- Zhang, X. & Jenekhe, S. A. (2000). Macromolecules, 33, 2069–2082.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808000561/nc2084sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808000561/nc2084Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report