Abstract
Folate receptor alpha (FRα) has been described as a factor involved in mediating Ebola virus entry into cells (6). Furthermore, it was suggested that interaction with FRα results in internalization and subsequent viral ingress into the cytoplasm via caveolae (9). Descriptions of cellular receptors for Ebola virus and its entry mechanisms are of fundamental importance, particularly with the advent of vectors bearing Ebola virus glycoprotein (GP) being utilized for gene transfer into cell types such as airway epithelial cells. Thus, the ability of FRα to mediate efficient entry of viral pseudotypes carrying GP was investigated. We identified cell lines and primary cell types such as macrophages that were readily infected by GP pseudotypes despite lacking detectable surface FRα, indicating that this receptor is not essential for Ebola virus infection. Furthermore, we find that T-cell lines stably expressing FRα are not infectible, suggesting that FRα is also not sufficient to mediate entry. T-cell lines lack caveolae, the predominant route of FRα-mediated folate metabolism. However, the coexpression of FRα with caveolin-1, the major structural protein of caveolae, was not able to rescue infectivity in a T-cell line. In addition, other cell types lacking caveolae are fully infectible by GP pseudotypes. Finally, a panel of ligands to and soluble analogues of FRα were unable to inhibit infection on a range of cell lines, questioning the role of FRα as an important factor for Ebola virus entry.
The filoviruses, Ebola virus and Marburg virus, are emerging human pathogens responsible for outbreaks of severe hemorrhagic fever with mortality rates approaching 90%. Filoviruses are negative-stranded RNA viruses consisting of a ribonucleoprotein core surrounded by a matrix of viral proteins VP40 and VP24 and enveloped by a host cell-derived lipid bilayer. Entry into target cells is mediated by lipid bilayer-embedded viral glycoproteins (GP) via binding to cell surface receptors. The distribution of these cellular receptors is likely a major determinant in the cellular and tissue tropism of filoviruses. We and others have shown that retrovirus and rhabdovirus pseudotypes bearing Ebola virus GP have a very broad cell tropism; they infect many cell lines from multiple mammalian and avian species (7, 14, 35, 37). Immortalized B- and T-cell lines, as well as primary lymphocytes (31, 37), represent the only cell lines consistently nontransducible by Ebola virus pseudotypes. Animal studies have revealed that a wide range of cell types are infected by filoviruses, particularly during the later stages of disease (1, 28-30), again with the notable exception of lymphocytes (13, 29). Such a broad cell tropism suggests that filoviruses utilize either a widely expressed and highly conserved surface molecule or a number of alternative molecules for entry into cells.
Chan and colleagues recently used a retrovirus-based selection protocol to identify cDNAs able to confer susceptibility to Jurkat T-cells by pseudotypes bearing Ebola virus or Marburg virus GP (6). Using this method, a number of cDNAs were isolated from a HeLa cell cDNA library with identity to folate receptor alpha (FRα) were identified. FRα is an attractive candidate as a filovirus receptor as it is widely expressed but is not detectable on lymphocytes (12) and is highly conserved between different species. The initial cDNA isolated from the tranduced Jurkat cells, however, lacked the 5′ coding sequence of FRα and initiated translation from an internal in-frame ATG (Met92) (6). This clone of FRα thus lacks an N-terminal signal peptide sequence to target it to the lumen of the endoplasmic reticulum, making it highly unlikely that it is expressed at the cell surface. This inconsistency, together with the fact that a number of cell lines readily infectible by Ebola virus pseudotypes appear to lack FRα, led us to reexamine the role of FRα in Ebola virus infection.
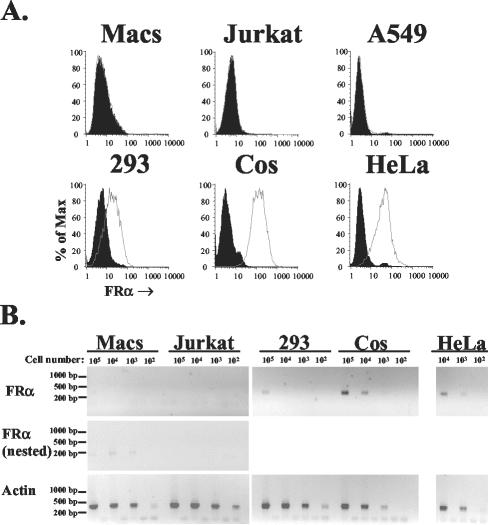
FRα expression on susceptible and nonsusceptible cells.
Widespread expression in different cell lines appears to support the hypothesis that FRα acts as a cell surface receptor for Ebola virus. However, the published literature indicates that a number of cell types are FRα negative. For example, A549 cells (a human lung carcinoma cell line) lack the ability to bind and internalize folate (36), due to a lack of FRα (21). This was confirmed by both flow cytometry using a mouse monoclonal antibody (LK26) to human FRα (Fig. 1A) and reverse transcription (RT)-PCR (data not shown). A549 cells were 3- to 10-fold more infectible by LacZ-encoding human immunodeficiency virus (HIV) pseudotypes bearing Ebola virus GP (Zaire Mayinga strain) than HeLa or Cos-7 cells, which express substantial levels of FRα (Fig. 1), (7.3 × 105 compared to 5.6 × 104 and 2.7 × 105 focus-forming units [FFU] per ml, respectively). In confirmation of previous findings (7, 37), HeLa cells were also approximately 20-fold-less infectible than 293 cells (1.2 × 106 FFU/ml), which express low levels of FRα (Fig. 1). Thus, FRα is not absolutely required for efficient viral entry mediated by Ebola virus GP, and levels of FRα expression do not appear to correlate with infectivity. Importantly, primary macrophages, the major initial in vivo target for Ebola virus replication in primate animal models (13, 16, 29, 34), were found to express a very small amount of FRα mRNA, with a set of internal nested primers required for detection by RT-PCR (Fig. 1B). No detectable surface expression of FRα on primary macrophages was seen by flow cytometry following 3 to 8 days of culture (Fig. 1A and data not shown). Low levels of FRα message in macrophages may potentially be sufficient to mediate efficient Ebola virus infection, though the lack of detectable surface expression suggests that receptors other than FRα are responsible for infection of this important cell type.
FIG. 1.
FRα expression on common cell lines and primary macrophages. (A) Surface levels of FRα on five cell lines and day 6 primary macrophages were determined by standard flow cytometry (33) with a murine monoclonal antibody directed against human FRα (LK26; Signet) (open histograms) and an isotype control (immunoglobulin G2a; Sigma, St. Louis, Mo.) (filled histograms). Macrophages were isolated from human leukocytes by plastic adherence, as previously described (32). (B) Levels of cellular mRNA for FRα and human β-actin were evaluated by RT-PCR, using an Express direct mRNA capture and RT system (Pierce, Rockford, Ill.). mRNA was extracted and cDNA synthesis was performed in the same well containing serial 10-fold dilutions of each cell type. PCR was performed with 16.5 μl of cDNA by using the conditions 95°C for 30 s followed by 30 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 40 s for FRα (forward primer, TGGGTGGCTGTAGTAGGGGAG; reverse primer, CAGGGGCACGTTCAGTACC) and β-actin (forward primer, CTGGCACCACACCTTCTACAATG; reverse primer, AATGTCACGCACGATTTCCCGC), resulting in 359- and 381-bp products, respectively. Primers were designed so that products spanned intron and exon boundaries, such that the predicted products derived from genomic DNAs were 3,440 and 822 bp for FRα and β-actin, respectively. For cell types negative for FRα in the first round of PCR (macrophages [Macs] and the T-cell line, Jurkat), 5 μl was transferred to new tubes and nested PCR was performed with the same conditions as those described above and the following set of nested primers: forward primer GCCAAGCACCACAAGGAAAAG and reverse primer CCTGGATGAAATGCCGTTTG. This resulted in a 189-bp product with a 3,118-bp predicted genomic DNA product. Both first-round and nested primers recognized all seven isoforms of FRα (8). Gaps between each sample represent controls for which the whole mRNA extraction and RT-PCR procedure was carried out on empty wells (a total of seven controls for the nested PCR). Control experiments with the same reaction mixtures performed on mRNA from each cell line in the absence of reverse transcriptase were all negative (data not shown).
Expression of FRα on FRα-negative cells does not enhance infection.
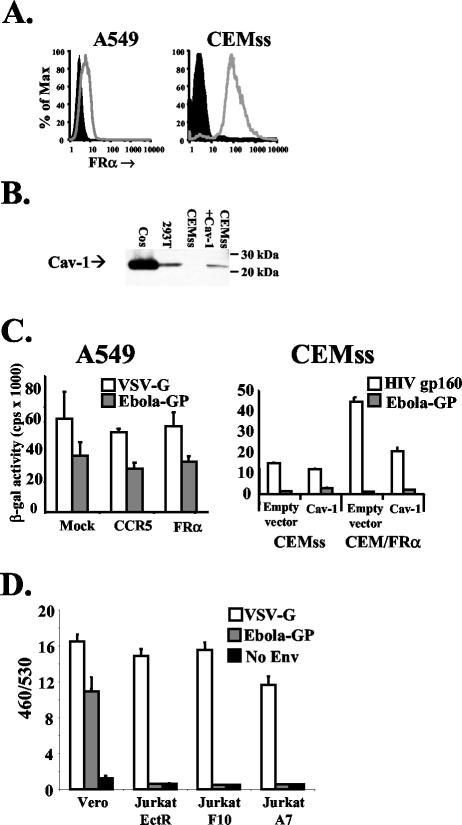
Lentiviral vectors expressing full-length FRα or the human chemokine receptor CCR5 as a control were used to transduce A549 cells, leading to FRα expression on these otherwise FRα-negative cells (Fig. 2A). No discernible differences in levels of either Ebola virus GP or vesicular stomatitis virus G protein (VSV-G) pseudotype infection was seen in mock-transduced or FRα- or CCR5-expressing A549 cells (Fig. 2C). Thus, FRα is not required for and does not enhance infection of A549 cells, suggesting that alternative receptors are sufficient to give maximal infectivity of this cell type.
FIG. 2.
Expression of FRα and Cav-1 in cell lines does not enhance Ebola virus pseudotype infection. (A) Surface levels of FRα were assessed as described in the legend to Fig. 1 for A549 cells (left panel) transduced with lentiviral vectors (for protocols, see reference 31) expressing a control of CCR5 (filled histogram) or FRα (open histogram) and for CEMss cells (right panel, filled histogram) or CEM cells stably expressing FRα (open histogram). (B) Total protein levels of Cav-1 were assessed by Western blotting using a directly horseradish peroxidase-conjugated polyclonal rabbit antibody raised against the N terminus of Cav-1 (N-20; Santa Cruz Biotechnology, Santa Cruz, Calif.). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed on cell lysates from 106 cells. CEMss cells were transduced with MLV amphotropic envelope pseudotyped MLV particles carrying the MIGR1 packaging vector encoding human CD8 (27) or MLV MIGR1 encoding human Cav-1. Cos and 293 cells were also tested to determine endogenous levels of Cav-1. (C) Mock-transduced and CCR5- or FRα-transduced A549 cells (left panel) or CEMss and CEM/FRα cells transduced with control or Cav-1-encoding MLV pseudovirions (right panel) were challenged with HIV-based pseudovirions encoding LacZ (18) pseudotyped with Ebola virus GP, VSV-G, or HIV gp160 (HXB2 strain). Two days postchallenge, cells were lysed and soluble β-galactosidase (β-gal) levels were measured with a chemiluminescence kit (Galactostar; Tropix) as per the manufacturer's instructions. Similar results were gained by using pseudovirions encoding luciferase as a reporter gene (data not shown). (D) Vero cells, but not Jurkat cells stably expressing ecotropic MLV receptor or previously described derivates expressing FRα (6), were transducible with VLPs bearing Ebola virus GP. VLPs were made by cotransfecting293T cells with eqimolar amounts of a Bla-VP40 fusion protein and GP, VSV-G, or empty vector. Forty-eight hours posttransfection, filtered supernatant was collected and diluted. Cells (5 × 105) were spin infected at 2,500 rpm for 2 h at 4°C and then incubated for 4 h at 37°C to allow entry. Cells were then labeled with CCF2-AM (Invitrogen, Carlsbad, Calif.) as per the manufacturer's instructions, washed, transferred to clear-bottomed, black-walled microtiter plates, and incubated overnight at room temperature to allow the enzymatic reaction to occur. The following morning, plates were read at 460 and 530 nm (Cytofluor 4000; Applied Biosystems, Foster City, Calif.). Background fluorescence from unlabeled cells was subtracted, and the ratio of fluorescence at 460 nm to that at 530 nm (460/530) was calculated for each well. Means and standard deviations shown are calculated from the results of four replicates, and the results presented are representative of three independent experiments.
To address directly whether FRα expression confers Ebola virus susceptibility to lymphoid cells, a stable population of CEMss cells, a human CD4+-T-cell line, expressing high levels of full-length FRα was generated by transfection and drug selection (termed CEM/FRα). Expression of FRα was demonstrated by fluorescence-activated cell sorting analysis (Fig. 2A), and these cells were challenged with lentiviral vector pseudotypes carrying either Ebola virus GP or, as a control, HIV Env. By both a sensitive chemiluminescent assay for soluble β-galactosidase (Tropix, Bedford, Mass.) (Fig. 2C) and single-cell quantification by β-galactosidase staining, both the FRα-expressing cells and the parental CEMss were resistant to infection by pseudotypes bearing Ebola virus GP (titers of <12 FFU/ml for both parental CEMss and CEM/FRα cells were found by using a viral stock with a titer of 5.0 × 106 FFU/ml on 293 cells). Levels of infectivity were unchanged regardless of whether the infections were carried out in RPMI or M199 medium, which lacks folic acid (see below). Thus, FRα expression on a nonpermissive lymphoid cell line does not confer susceptibility to Ebola virus GP pseudotypes. A small but reproducible increase in infection mediated by HIV Env derived from the CXCR4-tropic strain HXB2 was observed. It is likely that this increase was due merely to the random selection of a slightly more susceptible subpopulation during the cloning process. Importantly, the Jurkat T-cell lines F10 and A9, which contain FRα cDNA as described by Chan et al. (6), were also found not to be infectible by either lacZ or luciferase reporter pseudotypes bearing Ebola virus GP (data not shown).
Although extensive analysis(7, 37, 38) has yet to identify any differences in viral entry between infectious Ebola virus and retroviral pseudotypes bearing Ebola virus GP, it has been suggested that the lack of similarity between long, filamentous Ebola virus particles and small, spherical retroviral particles may give rise to artifactual results. To address this concern, a unique assay to determine the entry of filamentous Ebola virus-like particle (VLP) has been developed (G. Simmons and P. Bates, unpublished data). This assay relies on the published observation that expression of Ebola virus VP40 matrix protein leads to budding of VLPs in the absence of any other viral proteins (15). Furthermore, these VLPs are filamentous and closely resemble infectious Ebola virus particles by electron microscopy (15). Coexpression of Ebola virus GP with VP40 gives rise to VLPs studded with GP on their surfaces (2, 25). To monitor the cellular entry of these VLPs, a chimeric β-lactamase (Bla)-VP40 protein was produced. We have found that N-terminal tagging of VP40 with Bla does not adversely effect budding but rather produces particles able to deliver Bla enzymatic activity to target cells, such as Vero cells, upon membrane fusion (Fig. 2D). Bla-VP40 particles budding from transfected 293T cells in the presence or absence of GP formed long, filamentous particles as judged by electron microscopy (data not shown) and closely resembled previously described particles (2, 25). GP-mediated transfer of functional Bla enzymatic activity to the cytoplasm of target cells is measured by labeling these cells with CCF2-AM, a substrate capable of fluorescing at a wavelength of 530 nm when excited at 409 nm. Hydrolysis of a β-lactam ring in CCF2 by Bla creates a product that, when excited at 409 nm, fluoresces at a lower emission wavelength of 460 nm (4, 19). Thus, the degree of Bla enzymatic activity can be ascertained by plotting a ratio of the fluorescence at 460 nm to that at 530 nm (Fig. 2D). Vero cells were efficiently transduced by Bla+ VLPs bearing either Ebola virus GP or VSV-G but not by particles containing no envelope (Fig. 2D). VLP transduction of Bla mediated by Ebola virus GP, as in wild-type Ebola virus infection, is pH dependent and can be inhibited by neutralizing anti-Ebola virus GP sera (Simmons and Bates, unpublished). In accordance with the results of the Ebola virus GP pseudovirion experiments described above, neither parental Jurkat cells expressing the ectotropic murine leukemia virus (MLV) receptor (Jurkat EctR cells) nor derivatives expressing FRα (Jurkat A7 and F10 cells) (6) were transduced above background levels by Bla+ VLPs bearing Ebola virus GP. For both the Vero and Jurkat cell lines, background levels of Bla+ VLPs with no envelope proteins were similar to those of CCF2-AM-labeled cells with no particles added (data not shown). In contrast to the Ebola virus GP results, VLPs bearing VSV-G exhibited levels of entry into all of the Jurkat cell lines that were similar to levels of entry into Vero cells (Fig. 2D). Overall, these experiments with filamentous particles confirm the pseudovirion results, demonstrating that FRα does not affect T-cell susceptibility to Ebola virus GP-mediated entry.
Caveolin-1 expression does not enhance lymphocyte infection.
FRα either inhabits or is induced by folate or antibody binding to cluster into cholesterol-rich, flask-like invaginations in the plasma membrane termed caveolae (3). Upon binding with folate, FRα's internalization of the caveolae occurs as part of a process termed potocytosis. An H+-pump-mediated decrease in vesicular pH then leads to folate release, and FRα is trafficked back to the cell surface (17). Ebola virus, a pH-dependent virus (37), likely requires endocytosis and trafficking to low-pH vesicles in order to efficiently infect cells. Therefore, if FRα does function as a receptor for Ebola virus, then a pathway similar to that utilized for folic acid metabolism may be employed by the virus for entry. This hypothesis is supported by the finding that caveolin-1, the key structural component of caveolae, appears to colocalize with internalized Ebola virus GP, but not VSV-G, pseudotypes following infection (9). Lymphocytes, including T-cell lines such as CEMss (Fig. 2B), lack expression of caveolin-1 (Cav-1) and hence fail to form caveolae (10, 11), although introduction of Cav-1 into lymphocytes establishes caveolae formation in these cells (10). Thus, Chan and colleagues argue that the failure of Ebola virus GP pseudotypes to efficiently infect T-cells expressing FRα may be due to the lack of caveolae in such cells (6, 9). Other cell types, such as a number of rat thyroid epithelial cell lines (including FRT, WRT, and PCC13 cells), however, are also devoid of caveolae (20, 23, 39; data not shown). To address whether caveolae are essential for infection, these cell lines were analyzed for infection by Ebola virus pseudotypes and found to be susceptible (titers of 1.5 × 105, 2.8 × 105, and 3.5 × 105 FFU/ml on FRT, WRT, and PCC13 cells, respectively). FRT cells stably expressing Cav-1 efficiently form caveolae (23). To further assess the role of caveolae in Ebola virus entry, parental FRT cells and cells expressing Cav-1 were challenged with Ebola virus GP pseudotypes. A roughly twofold increase in infection by Ebola virus GP pseudotypes was seen in the cells expressing Cav-1 (1.5 × 105 and 3.1 × 105 FFU/ml for FRT and FRT/Cav-1 cells, respectively). Pseudotypes bearing VSV-G, however, showed similar increases on these cells (1.3 × 107 and 2.7 × 107 FFU/ml for FRT and FRT/Cav-1 cells, respectively), suggesting that the observed increase may be due to selection of a subpopulation which is more permissive for expression of the HIV vector.
While caveolae are clearly not necessary for efficient infection in these cell types, it is possible that caveolae represent only one of a number of potential routes of cellular entry. Therefore, as Cav-1 expression in lymphocytes reconstitutes caveolae formation (10), CEMss and CEM/FRα cells were transduced with MLV pseudotypes encoding human Cav-1. SDS-PAGE analysis of transduced CEMss cells demonstrated Cav-1 expression at levels similar to endogenous Cav-1 seen in 293T cells (Fig. 2B and data not shown). Only a slight increase in Ebola virus-GP-mediated infection of both CEMss and CEM/FRα cells transduced with Cav-1 compared to cells transduced with empty vector was noted (Fig. 2C). CEM/FRα cells expressing Cav-1 were no more infectible by Ebola virus-GP pseudotypes than parental CEMss with Cav-1 (Fig. 2C). Thus, caveolae formation does not facilitate FRα-mediated infection of T-cell lines, suggesting that lack of caveolae is not the reason for inefficient Ebola virus-GP pseudotype infection in T-cell lines expressing FRα, but rather either other missing components are required for viral entry or FRα does not function as an efficient mediator of entry for Ebola virus.
Viral pseudotypes expressing a membrane-bound form of protein A can bind to cell surface molecules via captured monoclonal antibodies (26). A mutant form of influenza virus hemagglutinin (HA) functional for membrane fusion but not sialic acid binding (22) can then be coincorporated into these viral pseudotypes and used to mediate viral entry (N. Chai and P. Bates, unpublished results). In this manner, lentiviral particles targeted to FRα with an anti-FRα monoclonal antibody (LK26; Signet, Dedham, Mass.) were able to infect the FRα-positive cell lines 293T and CEM/FRα (7.3 × 105 and 2.1 × 102 FFU/ml, respectively) but not CEMss cells (<12 FFU/ml). Thus, FRα on T cells is able to internalize and direct virus to suitable low-pH endosomes for triggering of HA. Complementation of Ebola virus GP with coincorporated HA, however, did not lead to either CEMss nor CEM/FRα cell infection (<12 FFU/ml for both cell lines compared to 5.6 × 106 on 293T cells), suggesting a lack of sufficient interactions between GP and FRα to allow viral internalization.
Folic acid fails to inhibit Ebola virus pseudotype infection.
Folic acid, the natural ligand for FRα, was tested for the ability to inhibit Ebola virus GP bearing pseudotype infection of GHOST cells. GHOST cells are a derivative of HOS, a human osteosarcoma cell line, that contain green fluorescent protein (GFP) under the control of an HIV long terminal repeat (LTR). Thus, upon infection by HIV pseudotypes, GFP expression is activated by Tat protein(5). Many formulations of growth media, such as Dulbecco's modified Eagle's medium (DMEM), contain higher levels of folic acid than that reported by Chan et al. to give a 90% reduction in infection on HOS cells (6). Thus, M199 medium, together with charcoal-stripped fetal bovine serum, was used due to its low folic acid content. No differences in Ebola virus GP pseudotype titers were observed in GHOST cells, regardless of whether infections were carried out in M199 medium or in DMEM (data not shown), suggesting that the high levels of folate in DMEM do not affect basal levels of Ebola virus GP pseudotype infection. M199 medium supplemented with concentrations of folic acid up to 2.2 mM also had no effect on Ebola virus pseudotype infection (Fig. 3A). Due to the use of sodium hydroxide for complete solubility of folic acid, the medium-alone controls were adjusted to the same pH (8.5). This adjustment caused a small decrease in levels of infection for both Ebola virus GP and VSV-G pseudotypes compared to levels in neutral-pH M199 medium (data not shown). Thus, contrary to the findings published by Chan et al. (6), folic acid did not significantly reduce Ebola virus GP-mediated entry into a cell line derived from HOS cells. Similar findings with LacZ-encoding pseudotypes were also found in Vero cells (Fig. 3B). In addition, 5-methyltetrahydrofolic acid, an analogue of folic acid that is readily soluble in water and thus can be used at neutral pH, also showed no specific inhibition of Ebola virus GP pseudotype infection of GHOST or Vero cells at concentrations up to 5 mM (Fig. 3B and data not shown). Similarly, a number of polyclonal and monoclonal antibodies directed against FRα (LK26 [Signet], polyclonal rabbit anti-bovine folate binding protein [Biogenesis, Brentwood, N.H.], and goat anti-bovine folate binding protein [Rockland, Gilbertsville, Pa.]), as well as soluble versions of folate binding protein (purified bovine folate binding protein [Biogenesis]), failed to specifically inhibit Ebola virus pseudotype infection in HOS and HeLa cells (data not shown). It is possible that neither folic acid nor the antibodies and other ligands tested utilize the same binding sites as Ebola virus GP on FRα; however, some of these same reagents were reported previously to inhibit infection on HOS and Vero cells (6). The inhibitory effects of cholesterol depletion on Ebola virus infection (2, 9) suggest a role for lipid rafts in viral entry. It is possible that in experiments by others, ligands to glycosylphosphatidylinositol-anchoredproteins (such as FRα) that cluster in rafts may give weak, nonspecific inhibition of viral entry. Monoclonal antibodies to at least three separate GPI-anchored, lipid raft-specific proteins are able to relatively weakly inhibit human T-cell leukemia virus type 1 infection (24). It is unlikely that all of these proteins play a direct role in human T-cell leukemia virus type 1 entry, suggesting that, indeed, viral entry through lipid rafts may be adversely affected nonspecifically by ligands to raft-associated proteins.
FIG. 3.
Inhibition of Ebola virus pseudotype infection of GHOST cells by folic acid. (A) GHOST cells were incubated at 4°C for 30 min with M199 medium containing 5% charcoal-stripped fetal bovine serum alone (approximate basal folic acid concentration in M199 medium is 0.02 mM) or supplemented with various concentrations of folic acid dissolved in sodium hydroxide (1 M). The pH of all solutions was adjusted to 8.5. An equal volume of HIV-based pseudovirions encoding GFP pseudotyped with Ebola virus GP or VSV-G was then added to give final concentrations of folic acid of 0.02, 0.22, 0.73, and 2.2 mM. Cells were incubated for 6 h, the medium was replaced, and the cells were incubated for a further 48 h. GFP expression was then analyzed by flow cytometry, and results are displayed as percentages of the expression in the control (M199 medium alone [pH 8.5], i.e., 0.02 mM folic acid). The results of the experiment shown are representative of three independent experiments. Error bars represent standard deviations of results from three replicates. (B) Vero cells were incubated as described above with M199 medium supplemented with folic acid (broken lines) or 5-methyltetrafolic acid (solid lines). An equal volume of HIV-based pseudovirions encoding LacZ pseudotyped with Ebola virus GP (squares) or MLV amphotropic envelope (circles) was then added to give the plotted concentrations. Cells were incubated for 6 h, the medium was replaced, and the cells were incubated for a further 48 h. lacZ expression was then analyzed by quantification of X-galactosidase-stained cells as previously described (32), and results are displayed as percentages of the expression level in the control.
In this report, we describe a lack of evidence for the role of FRα as a major component in Ebola virus GP-mediated cell entry. While it is clear that a number of cell types, including primary macrophages, lack FRα and thus must require other molecules for efficient entry, it is more difficult to definitively rule out a role for FRα in all cell types. However, a number of ligands to FRα, including folic acid and antibodies previously reported to inhibit Ebola virus GP pseudotype infection (6), were not found to significantly affect infection on the cell lines tested. As demonstrated with FRα-deficient A549 cells, receptors other than FRα that are capable of mediating efficient infection of Ebola virus exist. It is possible that these receptors are also present on the cell lines used to analyze FRα ligand inhibition and thus allow continued efficient infection even in the presence of complete FRα occlusion. The fact that expression of FRα, even in the presence of Cav-1, was unable to mediate infection of a T-cell line, however, questions the role that FRα plays in Ebola virus infection. Thus, we believe that the receptor(s) for filoviruses remains to be elucidated. Ebola virus GP pseudotyped retroviruses have been used to efficiently transduce cell types, such as airway epithelial cells, that are refractory to the more commonly used glycoprotein from VSV (18). Thus, Ebola virus GP may be utilized as an alternative for gene transfer protocols and highlights the need for a clearer understanding of the mechanisms of Ebola virus entry. In addition, the description of Ebola virus-specific receptors will facilitate the design and testing of inhibitors of Ebola virus infection as potential therapeutics.
Acknowledgments
We thank R. Mora and E. Rodriguez-Boulan (Weill Medical College of Cornell University, New York, N.Y.) for FRT and FRT/CAV-1 cells, Judy Meinkoth (University of Pennsylvania) for PCC13 and WRT cells, and Mark Goldsmith (Gladstone Institute, UCSF) for Jurkat cell lines and derivates. We also thank Robert Doms and Sean Amberg for critical reading of the manuscript.
This work was supported by grant RO1 AI43455 from the NIH. G.S. was supported by a long-term EMBO fellowship.
REFERENCES
- 1.Baskerville, A., S. P. Fisher-Hoch, G. H. Neild, and A. B. Dowsett. 1985. Ultrastructural pathology of experimental Ebola haemorrhagic fever virus infection. J. Pathol. 147:199-209. [DOI] [PubMed] [Google Scholar]
- 2.Bavari, S., C. M. Bosio, E. Wiegand, G. Ruthel, A. B. Will, T. W. Geisbert, M. Hevey, C. Schmaljohn, A. Schmaljohn, and M. J. Aman. 2002. Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J. Exp. Med. 195:593-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brzezinska, A., P. Winska, and M. Balinska. 2000. Cellular aspects of folate and antifolate membrane transport. Acta Biochim. Pol. 47:735-749. [PubMed] [Google Scholar]
- 4.Cavrois, M., C. De Noronha, and W. C. Greene. 2002. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat. Biotechnol. 20:1151-1154. [DOI] [PubMed] [Google Scholar]
- 5.Cecilia, D., V. N. KewalRamani, J. O'Leary, B. Volsky, P. Nyambi, S. Burda, S. Xu, D. R. Littman, and S. Zolla-Pazner. 1998. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J. Virol. 72:6988-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, S. Y., C. J. Empig, F. J. Welte, R. F. Speck, A. Schmaljohn, J. F. Kreisberg, and M. A. Goldsmith. 2001. Folate receptor-alpha is a cofactor for cellular entry by Marburg and Ebola viruses. Cell 106:117-126. [DOI] [PubMed] [Google Scholar]
- 7.Chan, S. Y., R. F. Speck, M. C. Ma, and M. A. Goldsmith. 2000. Distinct mechanisms of entry by envelope glycoproteins of Marburg and Ebola (Zaire) viruses. J. Virol. 74:4933-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elwood, P. C., K. Nachmanoff, Y. Saikawa, S. T. Page, P. Pacheco, S. Roberts, and K. N. Chung. 1997. The divergent 5′ termini of the alpha human folate receptor (hFR) mRNAs originate from two tissue-specific promoters and alternative splicing: characterization of the alpha hFR gene structure. Biochemistry 36:1467-1478. [DOI] [PubMed] [Google Scholar]
- 9.Empig, C. J., and M. A. Goldsmith. 2002. Association of the caveola vesicular system with cellular entry by filoviruses. J. Virol. 76:5266-5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fra, A. M., E. Williamson, K. Simons, and R. G. Parton. 1995. De novo formation of caveolae in lymphocytes by expression of VIP21- caveolin. Proc. Natl. Acad. Sci. USA 92:8655-8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fra, A. M., E. Williamson, K. Simons, and R. G. Parton. 1994. Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J. Biol. Chem. 269:30745-30748. [PubMed] [Google Scholar]
- 12.Franklin, W. A., M. Waintrub, D. Edwards, K. Christensen, P. Prendegrast, J. Woods, P. A. Bunn, and J. F. Kolhouse. 1994. New anti-lung-cancer antibody cluster 12 reacts with human folate receptors present on adenocarcinoma. Int. J. Cancer Suppl. 8:89-95. [DOI] [PubMed] [Google Scholar]
- 13.Geisbert, T. W., L. E. Hensley, T. R. Gibb, K. E. Steele, N. K. Jaax, and P. B. Jahrling. 2000. Apoptosis induced in vitro and in vivo during infection by Ebola and Marburg viruses. Lab. Investig. 80:171-186. [DOI] [PubMed] [Google Scholar]
- 14.Ito, H., S. Watanabe, A. Takada, and Y. Kawaoka. 2001. Ebola virus glycoprotein: proteolytic processing, acylation, cell tropism, and detection of neutralizing antibodies. J. Virol. 75:1576-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jasenosky, L. D., G. Neumann, I. Lukashevich, and Y. Kawaoka. 2001. Ebola virus VP40-induced particle formation and association with the lipid bilayer. J. Virol. 75:5205-5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, E., N. Jaax, J. White, and P. Jahrling. 1995. Lethal experimental infections of rhesus monkeys by aerosolized Ebola virus. Int. J. Exp. Pathol. 76:227-236. [PMC free article] [PubMed] [Google Scholar]
- 17.Kamen, B. A., M. T. Wang, A. J. Streckfuss, X. Peryea, and R. G. Anderson. 1988. Delivery of folates to the cytoplasm of MA104 cells is mediated by a surface membrane receptor that recycles. J. Biol. Chem. 263:13602-13609. [PubMed] [Google Scholar]
- 18.Kobinger, G. P., D. J. Weiner, Q. C. Yu, and J. M. Wilson. 2001. Filovirus-pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo. Nat. Biotechnol. 19:225-230. [DOI] [PubMed] [Google Scholar]
- 19.Lineberger, J. E., R. Danzeisen, D. J. Hazuda, A. J. Simon, and M. D. Miller. 2002. Altering expression levels of human immunodeficiency virus type 1 gp120-gp41 affects efficiency but not kinetics of cell-cell fusion. J. Virol. 76:3522-3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipardi, C., R. Mora, V. Colomer, S. Paladino, L. Nitsch, E. Rodriguez-Boulan, and C. Zurzolo. 1998. Caveolin transfection results in caveolae formation but not apical sorting of glycosylphosphatidylinositol (GPI)-anchored proteins in epithelial cells. J. Cell Biol. 140:617-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu, J. Y., D. A. Lowe, M. D. Kennedy, and P. S. Low. 1999. Folate-targeted enzyme prodrug cancer therapy utilizing penicillin-V amidase and a doxorubicin prodrug. J. Drug Target. 7:43-53. [DOI] [PubMed] [Google Scholar]
- 22.Martin, J., S. A. Wharton, Y. P. Lin, D. K. Takemoto, J. J. Skehel, D. C. Wiley, and D. A. Steinhauer. 1998. Studies of the binding properties of influenza hemagglutinin receptor-site mutants. Virology 241:101-111. [DOI] [PubMed] [Google Scholar]
- 23.Mora, R., V. L. Bonilha, A. Marmorstein, P. E. Scherer, D. Brown, M. P. Lisanti, and E. Rodriguez-Boulan. 1999. Caveolin-2 localizes to the golgi complex but redistributes to plasma membrane, caveolae, and rafts when co-expressed with caveolin-1. J. Biol. Chem. 274:25708-25717. [DOI] [PubMed] [Google Scholar]
- 24.Niyogi, K., and J. E. Hildreth. 2001. Characterization of new syncytium-inhibiting monoclonal antibodies implicates lipid rafts in human T-cell leukemia virus type 1 syncytium formation. J. Virol. 75:7351-7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noda, T., H. Sagara, E. Suzuki, A. Takada, H. Kida, and Y. Kawaoka. 2002. Ebola virus VP40 drives the formation of virus-like filamentous particles along with GP. J. Virol. 76:4855-4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohno, K., K. Sawai, Y. Iijima, B. Levin, and D. Meruelo. 1997. Cell-specific targeting of Sindbis virus vectors displaying IgG-binding domains of protein A. Nat. Biotechnol. 15:763-767. [DOI] [PubMed] [Google Scholar]
- 27.Pear, W. S., G. P. Nolan, M. L. Scott, and D. Baltimore. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90:8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pereboeva, L. A., V. K. Tkachev, L. V. Kolesnikova, L. Krendeleva, E. I. Riabchikova, and M. P. Smolina. 1993. The ultrastructural changes in guinea pig organs during the serial passage of the Ebola virus. Vopr. Virusol. 38:179-182. [PubMed] [Google Scholar]
- 29.Ryabchikova, E. I., L. V. Kolesnikova, and S. V. Luchko. 1999. An analysis of features of pathogenesis in two animal models of Ebola virus infection. J. Infect. Dis. 179(Suppl. 1):S199-S202. [DOI] [PubMed]
- 30.Ryabchikova, E. I., L. V. Kolesnikova, and S. V. Netesov. 1999. Animal pathology of filoviral infections. Curr. Top. Microbiol. Immunol. 235:145-173. [DOI] [PubMed] [Google Scholar]
- 31.Simmons, G., J. D. Reeves, C. C. Grogan, L. H. Vandenberghe, F. Baribaud, J. C. Whitbeck, E. Burke, M. J. Buchmeier, E. J. Soilleux, J. L. Riley, R. W. Doms, P. Bates, and S. Pohlmann. 2003. DC-SIGN and DC-SIGNR bind Ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology 305:115-123. [DOI] [PubMed] [Google Scholar]
- 32.Simmons, G., J. D. Reeves, A. McKnight, N. Dejucq, S. Hibbitts, C. A. Power, E. Aarons, D. Schols, E. De Clercq, A. E. Proudfoot, and P. R. Clapham. 1998. CXCR4 as a functional coreceptor for human immunodeficiency virus type 1 infection of primary macrophages. J. Virol. 72:8453-8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simmons, G., R. J. Wool-Lewis, F. Baribaud, R. C. Netter, and P. Bates. 2002. Ebola virus glycoproteins induce global surface protein down-modulation and loss of cell adherence. J. Virol. 76:2518-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stroher, U., E. West, H. Bugany, H. D. Klenk, H. J. Schnittler, and H. Feldmann. 2001. Infection and activation of monocytes by Marburg and Ebola viruses. J. Virol. 75:11025-11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takada, A., C. Robison, H. Goto, A. Sanchez, K. G. Murti, M. A. Whitt, and Y. Kawaoka. 1997. A system for functional analysis of Ebola virus glycoprotein. Proc. Natl. Acad. Sci. USA 94:14764-14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang, S., J. Luo, D. A. Lantrip, D. J. Waters, C. J. Mathias, M. A. Green, P. L. Fuchs, and P. S. Low. 1997. Design and synthesis of [111In]DTPA-folate for use as a tumor-targeted radiopharmaceutical. Bioconjug. Chem. 8:673-679. [DOI] [PubMed] [Google Scholar]
- 37.Wool-Lewis, R. J., and P. Bates. 1998. Characterization of Ebola virus entry by using pseudotyped viruses: identification of receptor-deficient cell lines. J. Virol. 72:3155-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wool-Lewis, R. J., and P. Bates. 1999. Endoproteolytic processing of the Ebola virus envelope glycoprotein: cleavage is not required for function. J. Virol. 73:1419-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zurzolo, C., W. van't Hof, G. van Meer, and E. Rodriguez-Boulan. 1994. VIP21/caveolin, glycosphingolipid clusters and the sorting of glycosylphosphatidylinositol-anchored proteins in epithelial cells. EMBO J. 13:42-53. [DOI] [PMC free article] [PubMed] [Google Scholar]