Abstract
In the molecule of the title compound, C15H10N2O, the dihedral angle between the two benzene rings is 65.49 (9)°.
Related literature
For the synthesis of a related compound, see: Sharman & van Lier (2003 ▶). For the crystal structure of an isomer of the title compound see: Ocak Ískeleli (2007 ▶). For related literature, see: Atalay et al. (2003 ▶, 2004 ▶); Cave et al. (1986 ▶); Koysal et al. (2004 ▶); Leznoff & Lever (1989–1996 ▶); McKeown (1998 ▶); Ocak et al. (2003 ▶).
Experimental
Crystal data
C15H10N2O
M r = 234.25
Orthorhombic,
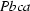
a = 25.514 (3) Å
b = 14.6064 (18) Å
c = 6.6109 (6) Å
V = 2463.7 (5) Å3
Z = 8
Mo Kα radiation
μ = 0.08 mm−1
T = 295 (2) K
0.6 × 0.5 × 0.3 mm
Data collection
Bruker P4 diffractometer
Absorption correction: none
3094 measured reflections
2254 independent reflections
1372 reflections with I > 2σ(I)
R int = 0.041
3 standard reflections every 97 reflections intensity decay: none
Refinement
R[F 2 > 2σ(F 2)] = 0.054
wR(F 2) = 0.109
S = 1.04
2254 reflections
165 parameters
H-atom parameters constrained
Δρmax = 0.21 e Å−3
Δρmin = −0.23 e Å−3
Data collection: XSCANS (Bruker, 1997 ▶); cell refinement: XSCANS; data reduction: XSCANS; program(s) used to solve structure: SHELXTL (Bruker, 1997 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536807068225/rk2070sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536807068225/rk2070Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors thank the HBUST for financial support.
supplementary crystallographic information
Comment
Phthalonitriles are among the most important precusors of phthalocyanine materials (Leznoff, 1989–1986). Mono phenyloxyphthalonitriles have been used for preparing symetrical phthalocyanines and subphthalocyanines which have been applied in many areas, such as laser printing, photocopying, optical data storage, catalyst etc. (McKeown, 1998). The 3–(m–tolyloxy)phthalonitrile (I), which contains an electron–donating moiety and a strong electron–accepting fragment linked by an oxygen atom, is also a good model suitable for the study of photoinduced electron transfer between the short linked donor and acceptor. The rate of such electron transfer process and the lifetime of the resultant charge separation state, however, are highly dependent on the relative orientation between the donor and the acceptor (Cave, 1986). The crystal structure of the title compound, (I), can therefore provide very helpful information for it.
The triple bond lengths between C and N, both 1.140 (3)Å and 1.133 (3) Å, as shown in Fig. 1, agree with literature values (Ocak et al., 2003). The geometry around the O atoms is in good agreement with the literature (Atalay et al., 2003, 2004; Koysal et al., 2004). The dihedral angle between the two aromatic rings planes is 65.49 (9)°. The crystal structure of compound involves extensive intermolecular π–π interactions, as can be seen from the packing diagram (Fig. 2). Phthalonitrile moieties are packed shoulder by shoulder along the a–axis which is stablized by the intermolecular dipole–dipole interactions and partial face–to–face π–π overlaping along the c–axis, while the toluene moieties are arranged by face to face π–π stacking along the b–axis and shouler by shoulder along the c–axis within the distance 4.15–4.20 Å. It is worth noting that the structure of the isomeric 4–(m–tolyloxy)phthalonitrile is monoclinic (Ocak Ískeleli, 2007) while the title compound report herein is orthorhombic.
Experimental
The m–cresol (1.56 g, 14.4 mmol) and 3–nitrophthalonitrile (1.60 g, 9.3 mmol) were dissolved in dry DMF (30 ml) with stirring under N2. Dry fine–powdered potassium carbonate (2.5 g, 18.1 mmol) was added in portions evenly every 10 min. The reaction mixture was stirred for 48 h at room temperature and poured into iced water (150 g). The product was filtered off and washed with (10% w/w) NaOH solution and water until the filtrate was neutral. Recrystallization from ethanol gave a white product (yield 1.2 g, 55%). Single crystals were obtained from absolute ethanol at room temperature via slow evaporation (m.p. 374–375 K). IR data (νmax/cm-1): 3086 (Ar—H), 2980–2950 (CH3), 2229 (CN). 1H NMR data (p.p.m.): 2.34 (s,3H), 7.00–7.05 (d, 1H), 7.07 (s, 1H), 7.12–7.17 (d, 1H), 7.24–7.29 (dd, 1H), 7.35–7.42 (t, 1H), 7.81–7.84 (d, 1H), 7.84–7.85 (d, 1H).
Refinement
All H atoms were positioned geometrically and refined using a riding model, with C—H = 0.96 Å (CH3) and C—H = 0.93 Å (CH) with Uiso(H) = 1.2Ueq (parent C) (for CH) or Uiso(H) = 1.5Ueq (parent C) (for CH3).
Figures
Fig. 1.
The molecular structure of title compound with the atom numbering scheme. Displacement ellipsoids are drawn at 35% probability level. Hydrogen atoms are presented as spheres of arbitrary radius.
Fig. 2.
The packing of (I), viewed down the c–axis.
Crystal data
| C15H10N2O | Dx = 1.263 Mg m−3 |
| Mr = 234.25 | Melting point: 374 K |
| Orthorhombic, Pbca | Mo Kα radiation λ = 0.71073 Å |
| Hall symbol: -P 2ac 2ab | Cell parameters from 58 reflections |
| a = 25.514 (3) Å | θ = 2.8–25.5º |
| b = 14.6064 (18) Å | µ = 0.08 mm−1 |
| c = 6.6109 (6) Å | T = 295 (2) K |
| V = 2463.7 (5) Å3 | Prism, colourless |
| Z = 8 | 0.6 × 0.5 × 0.3 mm |
| F000 = 976 |
Data collection
| Bruker P4 diffractometer | Rint = 0.041 |
| Radiation source: fine–focus sealed tube | θmax = 25.5º |
| Monochromator: graphite | θmin = 2.1º |
| T = 295(2) K | h = −30→1 |
| ω scans | k = −17→1 |
| Absorption correction: none | l = −1→8 |
| 3094 measured reflections | 3 standard reflections |
| 2254 independent reflections | every 97 reflections |
| 1372 reflections with I > 2σ(I) | intensity decay: none |
Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.054 | w = 1/[σ2(Fo2) + (0.001P)2 + 2.2P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.109 | (Δ/σ)max < 0.001 |
| S = 1.04 | Δρmax = 0.21 e Å−3 |
| 2254 reflections | Δρmin = −0.23 e Å−3 |
| 165 parameters | Extinction correction: SHELXTL (Bruker, 1997), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.00028 (6) |
| Secondary atom site location: difference Fourier map |
Special details
| Geometry. All s.u.'s (except the s.u.'s in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell s.u.'s are taken into account individually in the estimation of s.u.'s in distances, angles and torsion angles; correlations between s.u.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell s.u.'s is used for estimating s.u.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R–factor wR and goodness of fit S are based on F2, conventional R–factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2σ(F2) is used only for calculating R–factors(gt) etc. and is not relevant to the choice of reflections for refinement. R–factors based on F2 are statistically about twice as large as those based on F, and R–factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.65134 (6) | 0.53717 (13) | 0.7428 (3) | 0.0755 (6) | |
| N1 | 0.43352 (8) | 0.42711 (17) | 0.7588 (4) | 0.0682 (7) | |
| N2 | 0.57804 (9) | 0.33811 (18) | 0.7422 (5) | 0.0842 (8) | |
| C1 | 0.46678 (9) | 0.47844 (18) | 0.7550 (4) | 0.0532 (6) | |
| C2 | 0.57161 (9) | 0.41537 (19) | 0.7437 (4) | 0.0570 (7) | |
| C3 | 0.50933 (9) | 0.54397 (17) | 0.7508 (4) | 0.0510 (6) | |
| C4 | 0.56143 (9) | 0.51093 (16) | 0.7447 (4) | 0.0499 (6) | |
| C5 | 0.60184 (9) | 0.57428 (18) | 0.7405 (4) | 0.0568 (6) | |
| C6 | 0.59127 (11) | 0.66675 (19) | 0.7441 (5) | 0.0678 (8) | |
| H6A | 0.6187 | 0.7086 | 0.7429 | 0.081* | |
| C7 | 0.54050 (11) | 0.69742 (19) | 0.7495 (5) | 0.0708 (8) | |
| H7A | 0.5338 | 0.7600 | 0.7514 | 0.085* | |
| C8 | 0.49907 (11) | 0.63599 (19) | 0.7523 (4) | 0.0634 (7) | |
| H8A | 0.4647 | 0.6571 | 0.7551 | 0.076* | |
| C9 | 0.69296 (10) | 0.58418 (18) | 0.6486 (5) | 0.0641 (8) | |
| C10 | 0.74003 (9) | 0.58177 (18) | 0.7517 (5) | 0.0647 (7) | |
| H10A | 0.7423 | 0.5552 | 0.8792 | 0.078* | |
| C11 | 0.78408 (10) | 0.62010 (19) | 0.6599 (6) | 0.0757 (9) | |
| C12 | 0.77921 (11) | 0.6595 (2) | 0.4711 (6) | 0.0835 (10) | |
| H12A | 0.8085 | 0.6851 | 0.4096 | 0.100* | |
| C13 | 0.73158 (12) | 0.6617 (2) | 0.3718 (6) | 0.0822 (10) | |
| H13A | 0.7289 | 0.6891 | 0.2452 | 0.099* | |
| C14 | 0.68779 (11) | 0.6230 (2) | 0.4610 (5) | 0.0725 (8) | |
| H14A | 0.6556 | 0.6233 | 0.3951 | 0.087* | |
| C15 | 0.83646 (11) | 0.6162 (2) | 0.7674 (7) | 0.1120 (16) | |
| H15A | 0.8590 | 0.6632 | 0.7152 | 0.168* | |
| H15B | 0.8523 | 0.5574 | 0.7455 | 0.168* | |
| H15C | 0.8313 | 0.6255 | 0.9098 | 0.168* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0412 (9) | 0.0688 (12) | 0.1166 (18) | −0.0042 (8) | 0.0010 (11) | 0.0251 (12) |
| N1 | 0.0486 (12) | 0.0869 (17) | 0.0691 (16) | −0.0053 (12) | −0.0033 (12) | 0.0028 (14) |
| N2 | 0.0641 (15) | 0.0659 (16) | 0.123 (3) | 0.0005 (12) | 0.0043 (16) | 0.0054 (17) |
| C1 | 0.0453 (13) | 0.0675 (16) | 0.0469 (14) | 0.0045 (12) | −0.0021 (12) | 0.0014 (13) |
| C2 | 0.0411 (12) | 0.0611 (16) | 0.0688 (18) | −0.0028 (12) | 0.0024 (13) | 0.0046 (15) |
| C3 | 0.0461 (12) | 0.0618 (15) | 0.0452 (14) | 0.0005 (11) | −0.0002 (12) | 0.0005 (12) |
| C4 | 0.0433 (12) | 0.0564 (14) | 0.0500 (14) | 0.0003 (11) | 0.0015 (12) | 0.0024 (13) |
| C5 | 0.0447 (12) | 0.0627 (15) | 0.0630 (17) | −0.0007 (11) | −0.0008 (13) | 0.0029 (14) |
| C6 | 0.0603 (16) | 0.0609 (16) | 0.082 (2) | −0.0092 (13) | 0.0012 (16) | 0.0005 (16) |
| C7 | 0.0757 (18) | 0.0554 (15) | 0.081 (2) | 0.0070 (14) | 0.0064 (17) | −0.0022 (16) |
| C8 | 0.0555 (14) | 0.0682 (17) | 0.0665 (17) | 0.0106 (13) | 0.0057 (14) | 0.0006 (15) |
| C9 | 0.0456 (13) | 0.0553 (15) | 0.091 (2) | −0.0074 (12) | 0.0025 (15) | 0.0055 (16) |
| C10 | 0.0458 (13) | 0.0570 (15) | 0.091 (2) | 0.0012 (12) | −0.0044 (15) | 0.0047 (16) |
| C11 | 0.0420 (14) | 0.0559 (16) | 0.129 (3) | 0.0002 (12) | −0.0001 (17) | −0.0025 (19) |
| C12 | 0.0565 (17) | 0.0668 (19) | 0.127 (3) | −0.0029 (14) | 0.0243 (19) | 0.012 (2) |
| C13 | 0.075 (2) | 0.075 (2) | 0.097 (3) | −0.0020 (16) | 0.0121 (19) | 0.0110 (19) |
| C14 | 0.0572 (16) | 0.0723 (19) | 0.088 (2) | −0.0070 (14) | −0.0036 (16) | 0.0037 (18) |
| C15 | 0.0446 (15) | 0.090 (2) | 0.202 (5) | −0.0048 (15) | −0.022 (2) | 0.013 (3) |
Geometric parameters (Å, °)
| O1—C5 | 1.375 (3) | C9—C14 | 1.370 (4) |
| O1—C9 | 1.409 (3) | C9—C10 | 1.381 (4) |
| N1—C1 | 1.133 (3) | C10—C11 | 1.394 (4) |
| N2—C2 | 1.140 (3) | C10—H10A | 0.9300 |
| C1—C3 | 1.448 (3) | C11—C12 | 1.380 (5) |
| C2—C4 | 1.420 (4) | C11—C15 | 1.515 (4) |
| C3—C8 | 1.369 (4) | C12—C13 | 1.382 (4) |
| C3—C4 | 1.415 (3) | C12—H12A | 0.9300 |
| C4—C5 | 1.386 (3) | C13—C14 | 1.384 (4) |
| C5—C6 | 1.377 (4) | C13—H13A | 0.9300 |
| C6—C7 | 1.371 (4) | C14—H14A | 0.9300 |
| C6—H6A | 0.9300 | C15—H15A | 0.9600 |
| C7—C8 | 1.387 (4) | C15—H15B | 0.9600 |
| C7—H7A | 0.9300 | C15—H15C | 0.9600 |
| C8—H8A | 0.9300 | ||
| C5—O1—C9 | 119.7 (2) | C10—C9—O1 | 115.2 (3) |
| N1—C1—C3 | 179.8 (3) | C9—C10—C11 | 118.4 (3) |
| N2—C2—C4 | 177.7 (3) | C9—C10—H10A | 120.8 |
| C8—C3—C4 | 121.0 (2) | C11—C10—H10A | 120.8 |
| C8—C3—C1 | 120.4 (2) | C12—C11—C10 | 119.2 (3) |
| C4—C3—C1 | 118.7 (2) | C12—C11—C15 | 121.3 (3) |
| C5—C4—C3 | 118.2 (2) | C10—C11—C15 | 119.5 (3) |
| C5—C4—C2 | 121.3 (2) | C11—C12—C13 | 121.2 (3) |
| C3—C4—C2 | 120.5 (2) | C11—C12—H12A | 119.4 |
| O1—C5—C6 | 124.5 (2) | C13—C12—H12A | 119.4 |
| O1—C5—C4 | 114.8 (2) | C12—C13—C14 | 119.9 (3) |
| C6—C5—C4 | 120.6 (2) | C12—C13—H13A | 120.1 |
| C7—C6—C5 | 120.4 (2) | C14—C13—H13A | 120.1 |
| C7—C6—H6A | 119.8 | C9—C14—C13 | 118.5 (3) |
| C5—C6—H6A | 119.8 | C9—C14—H14A | 120.8 |
| C6—C7—C8 | 120.6 (3) | C13—C14—H14A | 120.8 |
| C6—C7—H7A | 119.7 | C11—C15—H15A | 109.5 |
| C8—C7—H7A | 119.7 | C11—C15—H15B | 109.5 |
| C3—C8—C7 | 119.3 (2) | H15A—C15—H15B | 109.5 |
| C3—C8—H8A | 120.4 | C11—C15—H15C | 109.5 |
| C7—C8—H8A | 120.4 | H15A—C15—H15C | 109.5 |
| C14—C9—C10 | 122.7 (3) | H15B—C15—H15C | 109.5 |
| C14—C9—O1 | 121.9 (3) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: RK2070).
References
- Atalay, Ş., Ağar, A., Akdemir, N. & Ağar, E. (2003). Acta Cryst. E59, o1111–o1112.
- Atalay, Ş., Çoruh, U., Akdemir, N. & Ağar, E. (2004). Acta Cryst. E60, o303–o305.
- Bruker. (1997). SHELXTL and XSCANS Bruker AXS Inc., Madison, Wisconsin, USA.
- Cave, R. J., Siders, P. & Marcus, R. A. (1986). J. Phys. Chem.90, 1436–1439.
- Köysal, Y., Işık, Ş., Akdemir, N., Ağar, E. & Kantar, C. (2004). Acta Cryst. E60, o930–o931.
- Leznoff, C. C. & Lever, A. B. P. (1989–1996). Phthalocyanines: Properties and Applications , Vol. 1. pp. 1–20. Weinheim/New York: VCH Publishers Inc.
- McKeown, N. B. (1998). Phthalocyanine Materials: Synthesis, Structure and Function pp. 12–30. Cambridge University Press.
- Ocak Ískeleli, N. (2007). Acta Cryst. E63, o997–o998.
- Ocak, N., Ağar, A., Akdemir, N., Ağar, E., García-Granda, S. & Erdönmez, A. (2003). Acta Cryst. E59, o1000–o1001.
- Sharman, W. M. & van Lier, J. E. (2003). The Porphyrin Handbook, Vol. 15, edited by K. M. Kadish, K. M. Smith & G. Guilard, pp. 1–60. New York: Academic Press.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536807068225/rk2070sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536807068225/rk2070Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




