Abstract
Although nucleotide analogs like bromodeoxyuridine have been extensively used to estimate cell proliferation in vivo, precise dynamic parameters are scarce essentially because of the lack of adequate mathematical models. Besides recent developments on T cell dynamics, the turnover rates of B lymphocytes are largely unknown particularly in the context of a virally induced pathological disorder. Here, we aim to resolve this issue by determining the rates of cell proliferation and death during the chronic stage of the bovine leukemia virus (BLV) infection, called bovine persistent lymphocytosis (PL). Our methodology is based on direct intravenous injection of bromodeoxyuridine in association with subsequent flow cytometry. By this in vivo approach, we show that the death rate of PL B lymphocytes is significantly reduced (average death rate, 0.057 day−1 versus 0.156 day−1 in the asymptomatic controls). Concomitantly, proliferation of the PL cells is also significantly restricted compared to the controls (average proliferation rate, 0.0046 day−1 versus 0.0085 day−1). We conclude that bovine PL is characterized by a decreased cell turnover resulting both from a reduction of cell death and an overall impairment of proliferation. The cell dynamic parameters differ from those measured in sheep, an experimental model for BLV infection. Finally, cells expressing p24 major capsid protein ex vivo were not BrdU positive, suggesting an immune selection against proliferating virus-positive lymphocytes. Based on a comparative leukemia approach, these observations might help to understand cell dynamics during other lymphoproliferative disease such as chronic lymphocytic leukemia or human T-cell lymphotropic virus-induced adult T-cell leukemia in humans.
The protracted presence of B lymphocytes in the blood might reflect either the onset of uncontrolled proliferation, the accumulation of cells in which the apoptotic processes are impaired, or a combination of these parameters. Indeed, lymphocyte homeostasis in vivo is the result of a critical balance between cell division and apoptotic death and deregulation of one of these factors (or both) can lead to leukemia. The goal of this study is to precisely quantify the extent of cell proliferation and death during a natural disorder: bovine persistent lymphocytosis (PL) (also called bovine chronic lymphocytic leukemia in reference 23). This disease is induced at reduced frequencies in heterogeneous cattle populations and, after extended and rather benign latency periods, evolves in a minority of cases (about 15%) into more aggressive forms of leukemia or lymphoma (4, 15, 45). The causative agent of these pathologies is bovine leukemia virus (BLV), a betaretrovirus which belongs to a group of pathogens responsible for diverse hematological or neurological disorders in primates and ruminants. The closest relatives of BLV are the human and simian T-lymphotropic viruses types 1 and 2, recently reclassified as primate T-lymphotropic viruses. Based on the sequence homologies between the members of this group, we propose to use BLV as a study model of the related human T-cell lymphotropic viruses.
In this viewpoint, we previously defined the rates of B-cell proliferation and death in sheep infected by BLV (9) and found that B lymphocytes in BLV-infected animals proliferate significantly faster than in the controls. Since the rates of cell death were not significantly different, we concluded that the increase in the number of B lymphocytes during BLV-induced lymphocytosis resulted from higher proliferation rates but was not due to a significant decrease in apoptosis. Although BLV-infected sheep might be a good model system to study a process of leukemogenesis in vivo, this species is not a natural host for BLV. In fact, natural transmission does not occur between sheep and, in terms of pathology, the disease appears to be particularly acute in this species. Indeed, the latency periods preceding the onset of leukemia/lymphoma are significantly shorter and the frequencies are much higher in sheep than in cattle.
Based on ex vivo studies, PL was initially thought to be the result of an increase in cell proliferation (24, 27). This assumption was mainly based upon the increase in tritiated thymidine incorporation observed during ex vivo cell cultures. However, modification of the pool size of a given cell subpopulation depends on the relative ratios at which the cells proliferate and die. Furthermore, short-term cultures are only a faint reflection of the complex mechanisms occurring in vivo in the context of a tightly regulated immune response. We therefore aimed at determining the rates of proliferation and death via a direct in vivo approach in cattle affected by persistent lymphocytosis. Our observations led to the unexpected conclusion that PL is in fact characterized by a decrease in the global B-cell turnover.
MATERIALS AND METHODS
Experimental animals.
All cows were kept under controlled conditions at the National Veterinary Research Institute (Pulawy, Poland). At regular time intervals, the total leukocyte counts were determined and the number of lymphocytes was estimated after examination under the microscope (as described in reference 29). In parallel, the sera from each cow were analyzed for BLV seropositivity with immunodiffusion and enzyme-linked immunosorbent assay.
Isolation of peripheral blood mononuclear cells and cell culture conditions.
Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation over Histopaque 1077 (Sigma Aldrich) and washed three times with PBS (phosphate-buffered saline). After isolation, cell viability was estimated by trypan blue dye exclusion. Four million cells were either directly stained with antibodies or cultivated for 18 h at 37°C in a 5% CO2-air atmosphere in RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 U of penicillin, and 100 μg of streptomycin per ml (InVitrogen). Some samples were cultivated with 200 nM phorbol myristate acetate (PMA) (Sigma Aldrich) and 565 nM ionomycin isolated from Streptomyces conglobatus (Sigma Aldrich). These optimal concentrations for culturing B lymphocytes were obtained by testing serial dilutions.
Immunophenotyping of cows.
PBMCs were labeled with monoclonal antibodies directed against bovine antigens CD4 (CC8, mouse IgG2a), CD5 (CC17, mouse IgG1), CD8 (CACT80C, mouse IgG1), CD11b (CC125, mouse IgG1) and CD14 (CAM36A, mouse IgG1) provided by C. Howard (Institute for Animal Health, Compton, United Kingdom), and by I. Schwartz-Cornil (INRA, Jouy-en-Josas, France) or obtained from VMRD Inc. (Pullman). Cells were then labeled with a rat anti-mouse IgG1 phycoerythrin-antibody (Becton Dickinson Immunocytometry Systems) or with a goat anti-mouse IgG2a fluorescein isothiocyanate conjugate (Caltag Laboratories). Finally, cells were analyzed by flow cytometry on a Beckman Coulter EPICS XL-4C flow cytometer. Ten thousand events were collected for each sample, and data were analyzed with the System II software (Beckman Coulter).
Ex vivo detection of cell apoptosis.
After 18 h of culture, PBMCs were collected, washed twice in PBS supplemented with 10% fetal bovine serum and labeled with 1H4 antibody, which recognizes surface immunoglobulin (sIgM) (26) (provided by K. Walravens, CODA/CERVA, Uccle, Belgium). Then cells were washed twice and incubated with a fluorescein isothiocyanate-conjugated F(ab′)2 fragments of rabbit anti-mouse immunoglobulins (Dako). Next, the labeled cells were fixed with 70% ethanol at −20°C. After two washes, PBMCs were treated with RNase A (50 μg/ml) (Sigma Aldrich), incubated in 20 μg of propidium iodide (Sigma Aldrich), and analyzed by flow cytometry as described (11). The cell doublets were excluded by the FL2a-FL2w gating method. We previously showed that this propidium iodide-labeling technique and the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) methodology yield similar results (10).
Analysis of 5-bromo-2′-deoxyuridine in vivo.
For each animal, three grams (approximately 5 mg/kg of body weight) of 5-bromo-2′-deoxyuridine (BrdU) (Sigma Aldrich) resuspended in physiologic solution (NaCl 0.9%) were injected intravenously into 6 cows. At regular time intervals (1 to 3 days), 1 ml of blood from each animal was treated with 1× FACS lysing solution (Becton Dickinson Immunocytometry Systems), washed twice with PBS containing 0.5% bovine serum albumin (Sigma Aldrich) and incubated in the presence of biotinylated 1H4 monoclonal antibody for 30 min at 4°C. After two washes, the cells were labeled with streptavidin-phycoerythrin (Becton Dickinson Immunocytometry Systems) and incubated with 1× FACS Permeabilizing Solution (Becton Dickinson Immunocytometry Systems). Finally, leukocytes were stained with 20 μl of anti-BrdU fluorescein isothiocyanate antibody in the presence of DNase (Becton Dickinson Immunocytometry Systems) during 30 min at room temperature and analyzed by flow cytometry. When the experiment was duplicated for confirmation, another uninfected control (no. 109322) was used because of the accidental death of cow BK.
Detection of the BrdU-positive cells expressing viral protein.
After 18 h of culture, PBMCs were collected and washed once with PBS-0.5% bovine serum albumin. The cells were fixed and permeabilized with the IntraStain Reagent (DAKO). Internal detection of the p24 viral protein was performed by sequential incubation with 4′G9 monoclonal antibody and a rat anti-mouse IgG1 phycoerythrin-conjugate (Becton Dickinson Immunocytometry Systems). The cells were permeabilized with 1× FACS Permeabilizing Solution, labeled with anti-BrdU fluorescein isothiocyanate in the presence of DNase (Becton Dickinson Immunocytometry Systems) and analyzed by flow cytometry.
Mathematical modeling.
The calculation of the cell dynamic parameters was performed as described previously (9). Briefly, the rates of proliferation and death within the B-cell population were estimated by fitting the following model to the BrdU incorporation data obtained experimentally: dl/dt = 2σpu + pl − dl, where u denotes the proportion of unlabeled B cells and l the proportion of labeled B cells, p is the average proliferation rate of B cells, and d represents the average death rate of labeled B cells. σ is the probability that a proliferating B cell becomes labeled. The probability that a proliferating B cell becomes labeled is assumed to be an exponentially decreasing function of time, σ = e−α t, reflecting the loss of unincorporated BrdU from the cytoplasm of B cells (i.e., as the time, since the BrdU injection increases the probability of a dividing cell being labeled decreases dramatically because unincorporated BrdU is rapidly cleared). The rate of loss of BrdU was assumed to be the same in all animals, as there is no physiological reason for it to vary and with the same rate improves comparability between the animals. The formula was fitted to the data by nonlinear least squares regression with the program ScoP; standard deviations of the parameters were estimated by calculating the asymptotic covariance matrix.
A number of other plausible models were also developed and fit to the data in order to check that our parameter estimates were robust to changes in the model. In particular, we checked (i) the effect of a time lag between cells dividing and incorporating label in lymphoid organs and their detection in the peripheral blood; (ii) the effect of incomplete distribution of BrdU throughout the body; (iii) the effect of BrdU label dilution; and (iv) the effect of subpopulations of labeled B cells with different kinetics. In every case, these models either failed to fit the data or gave very similar parameters. This indicated that our parameter estimates were robust to the above model changes. Additional details concerning the mathematical model were discussed in a specific paper (2).
RESULTS
Apoptosis and proliferation in ex vivo short-term cultures.
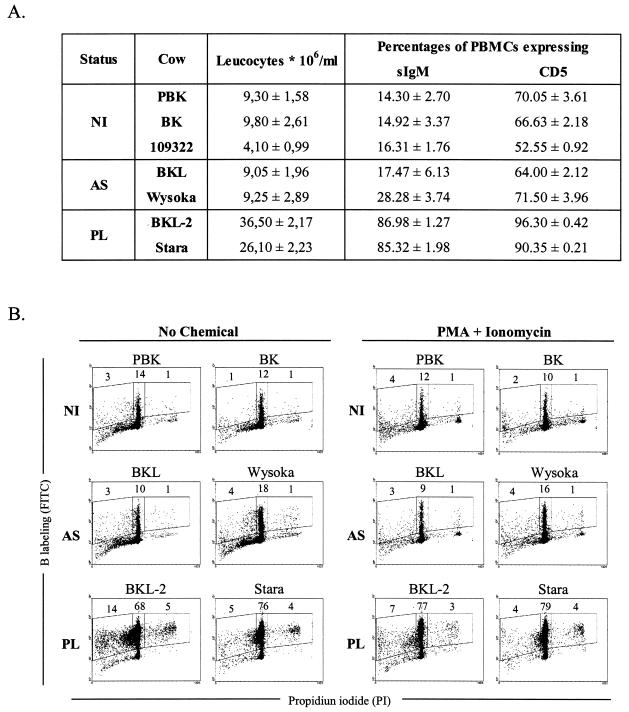
Bovine persistent lymphocytosis naturally occurs in about one third of BLV-infected cattle (4, 15). Besides the presence of antiviral neutralizing antibodies, the disease is diagnosed by high levels of circulating CD5-positive B lymphocytes expressing surface immunoglobulin. Immunophenotyping of two selected animals (BKL-2 and Stara) fit these criteria: 36.4 and 28.2 × 106 leukocytes per ml of blood, respectively, the great majority of which were sIgM and CD5 positive (Fig. 1A). In contrast, asymptomatic but BLV-infected cows (BKL, Wysoka) were in the normal range of the seronegative controls (PBK, BK, 109322). These cows isolated from the field are thus representative of well-defined stages of BLV-associated disorders. In particular, two of them (BKL-2 and Stara) remained chronically lymphocytotic over extended periods of time and were therefore considered typical bovine PL cases.
FIG. 1.
Primary PBMCs undergo apoptosis in short-term cultures. (A) Clinical status of cattle and cell phenotype of their PBMCs. Peripheral blood mononuclear cells were isolated from cows with PL (BKL-2, Stara) and asymptomatic (AS) BLV-infected cattle (BKL, Wysoka), as well as from three seronegative controls (uninfected: NI) (PBK, BK, 109322). The total leukocyte counts were determined and the number of lymphocytes was estimated after examination under the microscope. PBMCs were labeled with monoclonal antibodies directed against sIgM or CD5 and analyzed by flow cytometry. Numbers (± standard deviations), which were deduced from three independent experiments, represent the percentages of positive cells within the total PBMC population. (B) PBMCs were cultivated for 18 h in the absence (no chemical) or in the presence of PMA and ionomycin and labeled with anti-IgM monoclonal antibody 1H4 and a fluorescein isothiocyanate conjugate. After ethanol fixation, the cells were stained with propidium iodide and, after exclusion of the doublets, analyzed by two-color flow cytometry. Results from a representative experiment (10,000 events) are shown as dot plots (x axis: propidium iodide; y axis: B-lymphocyte labeling). Numbers within the plots represent the percentages of positively stained B cells in the PBMC population within each region.
We first aimed to determine the extent of apoptosis as well as the proliferative capacity of lymphocytes isolated from these animals. To this end, purified PBMCs were transiently cultivated, labeled with anti-immunoglobulin M antibody, stained with propidium iodide (propidium iodide) after ethanol fixation and analyzed by two-color flow cytometry to evaluate the proportion of the B cells in the different phases of the cell cycle (illustrated in Fig. 1B: x axis = propidium iodide; y axis = B-cell labeling). Under these culture conditions, apoptotic B lymphocytes staining in sub-G0/G1 represented for example 14% of the total PBMC population of PL cow BKL-2 (Fig. 1B, left region). The majority of the lymphocytes were resting in G0/G1 (68%, middle region) and some initiated the S phase of the cell cycle (5%, right region). The distribution of the cells throughout the cycle was measured for each animal yielding very reproducible and consistent results.
Levels of spontaneous apoptosis were low (between 1 and 14%) and very few cells underwent proliferation (less than 5%), the highest ratios being observed in the PL samples (14/5 and 5/4, respectively, for BKL-2 and Stara, Fig. 1B; No chemical). With the aim of further enhancing cell viability and triggering proliferation, two chemicals (PMA, a PKC activator and ionomycin, a calcium ionophore) were added to the culture medium. Under these optimized conditions for B-lymphocyte cultivation, apoptosis was only marginally affected in the controls and reduced in the PL samples, whereas proliferation could not be further stimulated (Fig. 1B; PMA + ionomycin).
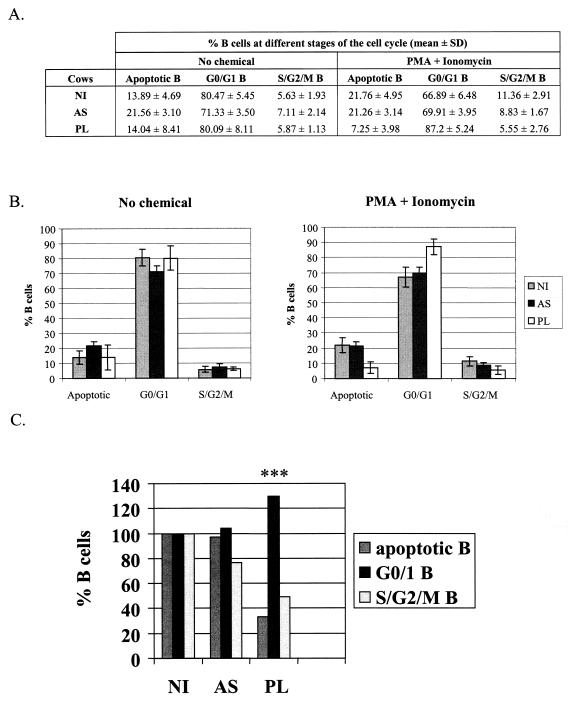
We conclude that the number of apoptotic B cells within the total PBMC population as well as the ability of B lymphocytes to proliferate are increased in the PL cases. In a quadruplicate experiment, however, the relative percentages of B cells in sub-G1, G0/G1 and S/G2/M were similar in all three categories of samples, i.e., in uninfected, asymptomatic and PL cells (Fig. 2A and B, no chemical). Proportionally, the relative rates of apoptosis and proliferation within the B-lymphocyte pool were thus not significantly altered under spontaneous conditions. However, it appeared that the PMA and ionomycin activators were more efficient at rescuing PL B lymphocytes from apoptosis (7.25% versus 21.76 and 21.26% in the uninfected and asymptomatic controls; P < 0.001 according to the Student t test), the majority of cells being in the G0/G1 phase of the cell cycle (87.2%, Fig. 2A and B). Importantly, the PL cells were less prone to enter the S/G2/M phase (5.55% versus 11.36/8.83% in the uninfected/asymptomatic controls), the relative decrease in cell proliferation being highly statistically significant (***, P < 0.001 according to the Student t test).
FIG. 2.
Relative rates of apoptosis as well as the levels of proliferation depend on the culture conditions. (A) PBMCs were isolated from PL, asymptomatic (AS) or noninfected animals (NI) and cultivated ex vivo under spontaneous (no chemical) or optimized conditions (in the presence of PMA and ionomycin). Cells were then labeled with anti-IgM monoclonal antibody and stained with propidium iodide (as described for Fig. 1B). Mean percentages (± standard deviations) of B lymphocytes at different stages of the cell cycle (sub-G1/apoptotic, G0/G1, S and G2/M) were calculated from four independent experiments. (B) Graphic representation of the mean values and standard deviations from panel A. (C) Relative proportions of the B lymphocytes at various stages of the cell cycle under optimized conditions (in the presence of PMA and ionomycin). ***, highly statistically significant, P < 0.001 according to the Student t test.
We conclude that, under optimized (but not spontaneous) ex vivo cell culture conditions, the relative proportions of apoptosis as well as the levels of proliferation are significantly reduced in PL cows (schematized in Fig. 2C).
BrdU incorporation into B lymphocytes in vivo.
To unravel the biological relevance of these ex vivo studies, we next aimed to analyze cell proliferation and renewal in vivo. To this end, we used a very direct approach based on intravenous injection of BrdU, which permits, after its incorporation into DNA, identification by flow cytometry of cells that have proliferated. A single dose of 3 g of BrdU was injected into six cows: two with PL (BKL-2, Stara), two aleukemic BLV-infected animals (BKL, Wysoka), and two seronegative controls (PBK, BK). In order to evaluate the kinetics of BrdU incorporation, an aliquot of blood from each cow was collected at regular time intervals after injection. After lysis of the red blood cells, the leukocytes were labeled with a mixture of biotinylated anti-IgM 1H4 monoclonal antibody and a streptavidin-phycoerythrin (PE) conjugate. The cells were then permeabilized, stained with anti-BrdU/fluorescein isothiocyanate in the presence of DNase, and analyzed by two-color flow cytometry.
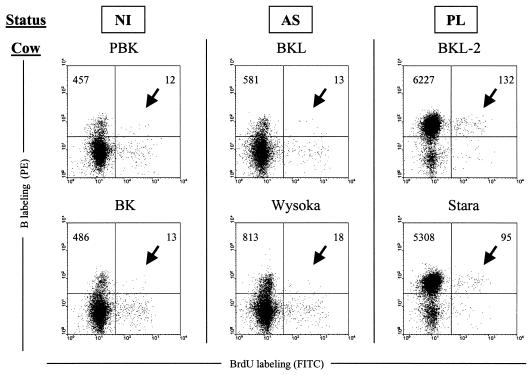
Based on their size and granularity, the lymphocyte and monocyte populations were selected in order to exclude the granulocytes from the analysis. An example of IgM + BrdU dual flow cytometry analysis performed at day 6 postinjection is illustrated in Fig. 3. It appeared that more B BrdU double positive cells (arrows) were stained in samples from the cows with PL (absolute cell counts of 132 and 95 among 10,000 events in BKL-2 and Stara) compared to the aleukemic (n = 13 and 18) and to the controls (n = 12 and 13). The background levels (corresponding to the samples preceding injection of BrdU) were below the limit of detection (data not shown).
FIG. 3.
Bromodeoxyuridine incorporates into B lymphocytes in vivo. Two PL (BKL-2, Stara) and aleukemic (BKL, Wysoka) BLV-infected cattle and three controls (PBK, BK, 109322) (PBK and 109322 are represented) were injected intravenously with 3 g of BrdU, and an aliquot of blood (1 ml) was collected 6 days later. After lysis of the red blood cells, B cells were labeled with biotinylated 1H4 monoclonal antibody and streptavidin-phycoerythrin (PE) conjugate. Then, the cells were stained with anti-BrdU fluorescein isothiocyanate antibody in the presence of DNase and analyzed by two-color flow cytometry (x axis = BrdU; y axis = B lymphocytes). Ten thousand cells (lymphocytes, monocytes, and granulocytes) were acquired and PBMCs were selected by the forward/side scatter gating method. The total numbers of B cells are indicated in the upper quadrants.
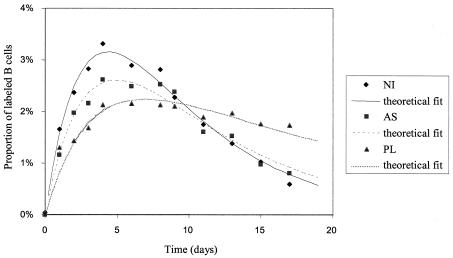
At first glance, one might conclude that PL cows have proportionally more B lymphocytes in the blood that underwent proliferation after BrdU injection. However, since PL cows have more B cells, we calculated the percentage of BrdU-positive cells within the sIgM-positive cell population. Furthermore, to evaluate the reproducibility of the data, the experiment was duplicated two months later with the same animals (except for a noninfected control BK that died accidentally and was replaced by 109322). The mean values of BrdU incorporation rates into the B-cell population were determined experimentally at regular intervals of time after the BrdU pulse (Fig. 4). The data corresponding to the measured incorporation rates (triangles, squares, and lozenges for PLs, asymptomatic, and uninfected animals, respectively) were fitted to a mathematical model that includes (i) the rate of cell proliferation and the death of labeled lymphocytes, (ii) the probability for a cell to become labeled, which declines exponentially with time reflecting the loss of unincorporated label after a single injection, (iii) the dilution of the BrdU label upon division (see Materials and Methods and reference 2 for details on the methodology). The model assumes that the duration of the S phase was constant and that the size of the global B-cell population remained stable throughout the experiment. With these criteria and parameters, the theoretical model fit the experimental data well (curves in Fig. 4), further supporting the validity of our approach.
FIG. 4.
Altered kinetics of BrdU incorporation in PL cows. Blood samples from cows (see Fig. 3) were collected at different days after a single pulse of BrdU injection. The percentage of BrdU-positive cells within the total B-lymphocyte population was determined and the data corresponding to the measured incorporation rates were fitted to a mathematical model, yielding theoretical fit curves (see Materials and Methods). Figure shows the average data for three groups of cows within two experiments. PL, AS, and NI are, respectively, persistently lymphocytic (triangles), asymptomatic (squares), and noninfected (lozenges) animals.
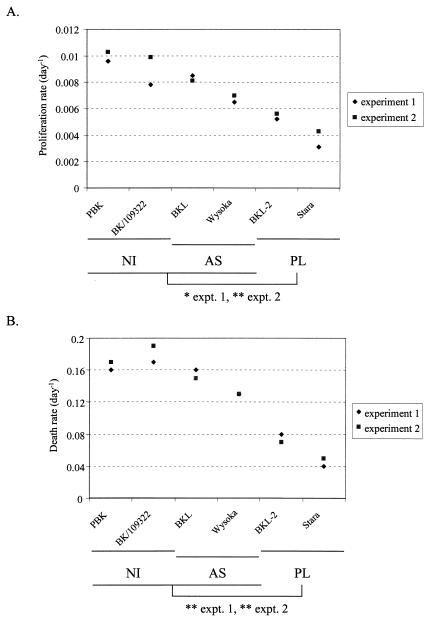
Fitting the model (dl/dt = 2σpu+ pl − dl) to the data enables the estimation of the minimal average proliferation rate of B lymphocytes (p) and average death rate of labeled B lymphocytes (d). It appears that the mean death rate in PL (d = 0.057 day−1) is decreased compared to the controls (uninfected and asymptomatic; d = 0.156 day−1) (Table 1 and Fig. 5). In other words, bovine PL is characterized by a significant reduction in B-cell death in vivo. In terms of B-cell dynamics, these rates imply that 5.73% of BrdU-labeled B cells die every day in animals with PL whereas the normal level in the controls is 15.63%. Concomitantly, the minimal proliferation rate (p = 0.0046 day−1 in PL versus 0.0085 day−1 in controls) is also significantly decreased during PL, allowing the maintenance of a constant number of B lymphocytes in the blood. This rate p relates to the proportion of new cells that are produced by proliferation every day. A proliferation rate of 0.0046 day−1 in PLs thus corresponds to 0.46% of peripheral blood B cells proliferating daily (Table 1). Statistical analysis revealed that both the proliferation and death rates were significantly reduced in PL cows compared to uninfected and asymptomatic (*, ** measured by two-tailed Student t test, 90% and 95% confidence levels, respectively), no difference being observed in the other categories of animals. Finally, as clearly illustrated on Fig. 5, overlapping of the calculated death and proliferation parameters supported the reproducibility of the experiment, even in the noninfected controls where two different animals had to be used (BK and 109322).
TABLE 1.
Proliferation and death ratesa
| Expt. | Status | Cow | Death rate (day−1 ± SD) | Proliferation rate (day−1 ± SD) |
|---|---|---|---|---|
| 1 | NI | PBK | 0.163 ± 0.055 | 0.0096 ± 0.0013 |
| BK | 0.175 ± 0.064 | 0.0078 ± 0.0012 | ||
| AS | BKL | 0.161 ± 0.057 | 0.0085 ± 0.0013 | |
| Wysoka | 0.125 ± 0.048 | 0.0065 ± 0.0011 | ||
| PL | BKL2 | 0.076 ± 0.034 | 0.0052 ± 0.0010 | |
| Stara | 0.037 ± 0.032 | 0.0031 ± 0.0008 | ||
| 2 | NI | PBK | 0.169 ± 0.028 | 0.0103 ± 0.0006 |
| 109322 | 0.186 ± 0.031 | 0.0099 ± 0.0006 | ||
| AS | BKL | 0.146 ± 0.025 | 0.0081 ± 0.0006 | |
| Wysoka | 0.125 ± 0.023 | 0.0070 ± 0.0006 | ||
| PL | BKL2 | 0.070 ± 0.016 | 0.0056 ± 0.0005 | |
| Stara | 0.046 ± 0.015 | 0.0043 ± 0.0004 | ||
| Means | NI + AS | 0.1563 ± 0.0224 | 0.0085 ± 0.0014 | |
| PL | 0.0573 ± 0.0187 | 0.0046 ± 0.0011 |
The minimal B-lymphocyte proliferation and death rates (± standard deviations) were estimated from fitting the model to the data deduced from two independent experiments. PL, AS, and NI are, respectively, persistently lymphocytic, asymptomatic, and noninfected animals.
FIG. 5.
Proliferation and death rates are reduced in PL cows. The minimal proliferation (panel A) and death (panel B) rates were estimated from fitting the model to the data deduced from two independent experiments. PL, AS, and NI are, respectively, persistently lymphocytic, asymptomatic, and noninfected animals. Statistical analysis reveals that the proliferation and death rates are significantly reduced in PL cows compared to uninfected and asymptomatic in both experiments: average proliferation rates of B cells in PL cows lower than in uninfected and asymptomatic, confidence level: 90% in experiment 1 (*), 95% in experiment 2 (**). Average death rates of labeled B cells in PL cows were lower than in uninfected and asymptomatic, confidence level: 95% in experiment 1 (**), 95% in experiment 2 (**) (two-tailed Student t test in each case).
Together, these data demonstrate that the in vivo B-cell turnover is decreased in cows with PL.
Proliferation and viral expression.
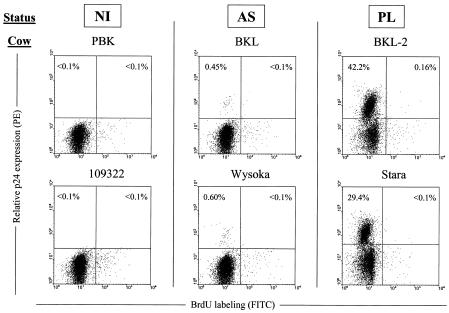
With the aim of correlating cell turnover and viral infection, cells from six animals involved in the study were isolated at three days post-BrdU injection. This time point is close to the maximal level of BrdU incorporation (see Fig. 4). Since the virus is apparently silent in the vast majority of circulating lymphocytes, peripheral blood mononuclear cells were transiently cultivated to trigger viral expression, labeled with anti-capsid (p24) and BrdU antibodies and analyzed by flow cytometry. In the asymptomatic samples (BKL and Wysoka), a very small but distinct population of p24-positive cells (0.45% and 0.60%, respectively) could clearly be seen above the background (Fig. 6). In contrast, as expected, the short-term cultures from the PL cows contained a large proportion of p24-positive lymphocytes (42.2 and 29.4% in BKL-2 and Stara, respectively). Besides the confirmation of the clinical status of the PL cows (i.e., many virus-positive cells in the blood), dual flow cytometry also revealed an interesting observation after staining for p24 expression and BrdU incorporation. Indeed, very few cells, if any (<0.16% for BKL-2 and < 0.1% for Stara), stained as double positives reaching the background levels set by the negative controls. We conclude that BrdU-labeled PBMCs that were circulating 3 days after a pulse of BrdU included almost no cells that spontaneously expressed p24 capsid protein ex vivo. Thus, transient virus expression ex vivo and proliferation 3 days earlier in vivo are mutually exclusive.
FIG. 6.
Cell proliferation and viral expression appear mutually exclusive. Three days post-BrdU injection, PBMCs from noninfected (PBK, 109322), aleukemic (BKL, Wysoka), and PL (BKL-2, Stara) cows were isolated and cultivated for 18 h. The cells were then fixed and incubated with anti-p24 antibody 4′G9, which recognizes the viral capsid protein, and with a phycoerythrin-conjugated secondary antibody. Finally, cells were stained with anti-BrdU fluorescein isothiocyanate conjugate containing DNase and analyzed by flow cytometry. A representative experiment (out of three) is represented as dot plots (10,000 gated events). Numbers represent the percentages of positively stained cells in each quadrant.
DISCUSSION
The goal of this study was to precisely quantify the extent of cell proliferation and death in the context of a natural hematological disorder called bovine PL. Paradoxically, PL has been qualified as a lymphoproliferative disease based on short-term cultures (4, 10, 15, 40, 42) and we demonstrate here that the main parameter that accounts for the high levels of peripheral B lymphocytes is a reduction in cell death (0.057 day−1 versus 0.156 day−1) associated with a decreased proliferation (0.0046 day−1 versus 0.0085 day−1). In fact, a main contribution of this report is to show that conclusions drawn from ex vivo cultures are greatly dependent on the experimental conditions used. Indeed, the reduced ability of PL B lymphocytes to proliferate or undergo apoptosis is revealed only in the presence of suitable polyclonal activators (PMA and ionomycin) in the culture medium (as illustrated in Fig. 2). Under spontaneous conditions (RPMI medium containing 10% of fetal calf serum), no effect is observed, the relative proportion of cells undergoing either apoptosis or proliferation being preserved in PL cultures. Importantly, in the absence of short-term culture, all (or almost all) peripheral blood lymphocytes are resting in the G0/G1 phase of the cell cycle (data not shown), indicating that proliferation occurs in other sites (bone marrow, lymph nodes or Peyer's patches).
Another reassessment that should be mentioned in this work concerns the link between viral expression and inhibition of apoptosis. We and others have previously reported that ex vivo, cells in which the virus is expressed are not prone to undergo apoptosis (10, 11, 38). This conclusion was based on flow cytometric analyses of labeled B lymphocytes double-stained for viral protein synthesis as well as for apoptotic markers. Since all BLV-expressing cells were nonapoptotic, the most straightforward interpretation was that the virus efficiently inhibits cell death. An alternate explanation, which we initially considered to be less likely, is that the virus-positive cells were already eliminated in vivo and, therefore, cannot be detected as apoptotic ex vivo. Indeed, dual flow cytometry (Fig. 6) demonstrates an almost complete absence of p24 and BrdU double positive cells, revealing the mutually exclusive presence of both markers. In other words, among all infected cells proliferating in vivo as measured by BrdU uptake, none of them was found to express viral proteins ex vivo, a phenotype that was also observed in BLV-infected sheep (9).
Since most p24-positive cells are spared from apoptosis ex vivo, p24 and BrdU and double positives were not lost during the culture but were rather eliminated in vivo. If we postulate that viral expression and cell activation are closely linked, as largely illustrated in the literature (6, 7, 22), the lack of p24 and BrdU and double positive cells reveals a very efficient negative selection taking place in vivo. Another nonexclusive interpretation would be that only a subpopulation of cells harboring an integrated provirus is allowed to proliferate (i.e., incorporate BrdU), provided that no viral proteins are expressed. In any case, our direct in vivo approach casts some light onto a very active process of selection against infected cells.
As mentioned previously, PL in cattle is a naturally occurring disorder induced by a retrovirus called BLV. Experimentally, however, this virus can also be transmitted to sheep in which it induces leukemia after shorter latency periods (45). In other words, BLV-associated pathogenesis in sheep is more acute than in cattle. We have recently demonstrated that progressive lymphocytosis and subsequent leukemia in sheep result from an increased cell proliferation rather than a defect in apoptosis (9). The net increase in proliferation in the absence of compensating cell death creates an imbalance in the numbers of lymphocytes that can largely account for the occurrence of leukemia. Interestingly, BLV-infected sheep do not develop a stable and chronic lymphocytosis that arises promptly after an asymptomatic phase, as observed in a fraction of BLV-infected cows (i.e., PL), but rather harbor gradually increasing numbers of neoplastic cells. However, it remains possible that the short transition period preceding the onset of bovine PL or even the occurrence of the leukemic stage is associated with an increase of the proliferation rates. Since eradication programs have drastically diminished the incidence of naturally occurring BLV-associated leukemia cases, this question is however very difficult to address.
In terms of pathology and in the context of a comparative approach, bovine PL (or bovine chronic lymphocytic leukemia) (23) shares similarities with chronic lymphocytic leukemia in humans. Human chronic lymphocytic leukemia is the most frequent form of leukemia in Western countries to occur in middle-aged and elderly individuals (reviewed in references 1, 13, 17, 30, 31, 35-37, 43, and 44). The hallmark of this leukemia is an increase in the absolute number of peripheral blood mononuclear cells (above 10,000 per mm3), most of which are small and mature B lymphocytes. Phenotypically, these cells are CD5-positive B lymphocytes (so-called B-1A cells) expressing relatively low levels of surface membrane immunoglobulins (mostly IgM and IgD) as well as the typical pan-B markers (CD19 and CD20).
Another characteristic of chronic lymphocytic leukemia is the membrane instability of CD23 (or FcɛRII, the low affinity receptor for immunoglobulin E), which is rapidly cleaved from the cell surface and overexpressed as a soluble form. The metabolic processes leading to the onset of chronic lymphocytic leukemia are presently unknown. The most commonly agreed mechanism postulates that neoplastic CD5 and lymphocytes accumulate because of the inhibition of apoptosis (3, 5, 8, 34). A series of reports have indeed extensively documented the relative resistance of chronic lymphocytic leukemia cells to undergo spontaneous programmed cell death upon long-term ex vivo cultivation. Simultaneously, the cells are also refractory to activation and proliferation, even in the presence of efficient polyclonal activators such as phytohemagglutinin (39). However, these conclusions obtained from ex vivo cultures are in conflict with indicators of cell proliferation in vivo: induction of Myc synthesis, expression of proliferating cell nuclear antigen, presence of the Ki67-specific epitope, and release of high doses of soluble CD23 possibly indicative of cellular activation (14, 19, 20, 25, 46).
In fact, experiments based on ex vivo cultivation are highly dependent on the culture conditions (cell concentration, type of medium, amount of serum) and might lead to misinterpretations. Although bovine PL is clearly a distinct clinical entity, the similarities with chronic lymphocytic leukemia in human are nevertheless remarkably striking: (i) increased leukocyte counts (above 10,000 per mm3) due to high levels of mature B lymphocytes (ii) cell phenotype characterized by surface IgMs, CD5 and CD11c (iii) overexpression of proliferation markers (Myc, PCNA, Ki-67 epitope) and cytokines (IL-6, IL-10, and TNF-α); (iiii) modulation of apoptosis (increased Bcl2/Bax ratio, mutations of p53 in a proportion of the lymphoid tumors, involvement of reduced glutathione in inhibition of cell death) (12, 16, 28, 33, 41, 47; A. Sanchez-Alcaraz et al., submitted for publication); (iiii) disease transformation into lymphoma or lymphosarcoma in about 10 to 15% of the PL cases (although PL is not a prerequisite for tumor formation).
Similarly, human chronic lymphocytic leukemia disease in clinically healthy patients turns into prolymphocytic leukemia (10% of cases) or large-cell lymphoma (Richter's syndrome) (2 to 5% of cases) (36). However, similarity does not imply identity and the bovine model exhibits some unique characteristics: (i) PL is associated with infection by a retrovirus that does not infect humans, (ii) the disease is polyclonal in terms of cell populations, (iii) bovine PL cells appear to be less anergy-like in short-term cultures, (iv) the IgM molecule seems to be consistently expressed at normal levels in vivo (although its synthesis is frequently reduced ex vivo [our unpublished observations]). Despite these differences and in the absence of corresponding information in humans, we speculate that chronic lymphocytic leukemia might be characterized by a reduced cell turnover as observed in bovine PL. This hypothesis thus supports the current dogma, which postulates that human chronic lymphocytic leukemia is due to an accumulation of cells exhibiting a defect in apoptosis.
In terms of comparative leukemia, BLV shares tight genomic homologies as well as functional similarities with the related human T-cell lymphotropic virus type 1. This virus infects about 20 million people worldwide and induces a fatal disease called adult T-cell leukemia (21, 48). A still unanswered question concerns the dynamic process that governs the occurrence of leukemia. Defining whether adult T-cell leukemia is due to increased proliferation or inhibition of apoptosis (or both) is an essential point that must be considered to design adequate therapeutic strategies against this still incurable disease. This type of experiment would, however, be extremely difficult to realize directly on patients with acute adult T-cell leukemia essentially because of its rare occurrence and high severity. Although the cell types are clearly distinct (CD4 and CD8 for human T-cell lymphotropic virus and B for BLV) (18, 32), dynamic parameters deduced from the PL model might contribute to the understanding of adult T-cell leukemia development in humans. Perhaps, the most trivial and direct inference is that ex vivo studies may not correctly reflect in vivo cell turnover.
To summarize, we have determined two main parameters that characterize the maintenance of homeostasis in bovine PL. We defined the death rate of PL B lymphocytes (d = 0.057 day−1 versus 0.156 day−1 in the asymptomatic and noninfected controls) as well as their levels of proliferation (p = 0.0046 day−1 versus 0.0085 day−1, respectively). These numbers thus precisely cipher the dynamics of PL, which is characterized by a concomitant reduction of apoptosis and cell proliferation.
Acknowledgments
We thank the “Fortis Bank Assurance,” the “Belgian Federation against Cancer,” the “FNRS,” the “Loterie Nationale,” the “Pôles d'attraction interuniversitaires pour le compte de l'Etat belge, Services fédéraux des affaires scientifiques, techniques et culturelles,” the “Actions de Recherche Concertées du Ministère de la Communauté Française,” the “Commissariat Général aux Relations Internationales/Direction Générale des Relations Extérieures (Région Wallonne),” and the “Wellcome Trust” for financial support. R.K. and L.W. are research directors of the “Fonds National de la Recherche Scientifique” (FNRS), whereas C.D. is a fellow of the “Action de Recherche Concertée du Ministère de la Communauté Française” and of the “Télévie” (FNRS).
The antibodies were kindly provided by K. Walravens (CODA/CERVA, Uccle, Belgium), J. J. Letesson (FUNDP, Namur, Belgium), D. Portetelle (FSAGx, Gembloux, Belgium), C. Howard (Institute for Animal Health, Compton, United Kingdom), and I. Schwartz-Cornil (INRA, Jouy-en-Josas, France). We are grateful to M. Nuttinck and M. Zaborna for excellent technical help and we thank C. R. M. Bangham, D. Bednarek, and L. Lagneaux for helpful discussions and careful reading of the manuscript.
REFERENCES
- 1.Andritsos, L., and H. Khoury. 2002. Chronic lymphocytic leukemia. Curr. Treat. Options Oncol. 3:225-231. [DOI] [PubMed] [Google Scholar]
- 2.Asquith, B., C. Debacq, D. Macallan, L. Willems, and C. Bangham. 2002. Lymphocyte kinetics: the interpretation of labelling data. Trends Immunol. 23:596-601. [DOI] [PubMed] [Google Scholar]
- 3.Blaise, R., P. Masdehors, A. Lauge, D. Stoppa-Lyonnet, C. Alapetite, H. Merle-Beral, J. L. Binet, S. Omura, H. Magdelenat, L. Sabatier, and J. Delic. 2001. Chromosomal DNA and p53 stability, ubiquitin system and apoptosis in B-chronic lymphocytic leukemia lymphocytes. Leuk. Lymphoma 42:1173-1180. [DOI] [PubMed] [Google Scholar]
- 4.Burny, A., F. Bex, H. Chantrenne, Y. Cleuter, D. Dekegel, J. Ghysdael, R. Kettmann, M. Leclercq, J. Leunen, M. Mammerickx, and D. Portetelle. 1978. Bovine leukemia virus involvement in enzootic bovine leukosis. Adv. Cancer Res. 28:251-311. [DOI] [PubMed] [Google Scholar]
- 5.Caligaris-Cappio, F. 2000. Biology of chronic lymphocytic leukemia. Rev. Clin. Exp. Hematol. 4:5-21. [DOI] [PubMed] [Google Scholar]
- 6.Copeland, K. F., and J. L. Heeney. 1996. T helper cell activation and human retroviral pathogenesis. Microbiol. Rev. 60:722-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corley, R. B., F. E. Lund, T. D. Randall, L. B. King, S. Doerre, and D. L. Woodland. 1992. Mouse mammary tumor proviral gene expression in cells of the B lineage. Semin. Immunol. 4:287-296. [PubMed] [Google Scholar]
- 8.Dameshek, W. 1967. Chronic lymphocytic leukemia—an accumulative disease of immunologically incompetent lymphocytes. Blood Suppl. 29:82-84. [PubMed] [Google Scholar]
- 9.Debacq, C., B. Asquith, P. Kerkhofs, D. Portetelle, A. Burny, R. Kettmann, and L. Willems. 2002. Increased cell proliferation, but not reduced cell death, induces lymphocytosis in bovine leukemia virus-infected sheep. Proc. Natl. Acad. Sci. USA 99:10048-10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dequiedt, F., G. H. Cantor, V. T. Hamilton, S. M. Pritchard, W. C. Davis, P. Kerkhofs, A. Burny, R. Kettmann, and L. Willems. 1999. Bovine leukemia virus-induced persistent lymphocytosis in cattle does not correlate with increased ex vivo survival of B lymphocytes. J. Virol. 73:1127-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dequiedt, F., E. Hanon, P. Kerkhofs, P. P. Pastoret, D. Portetelle, A. Burny, R. Kettmann, and L. Willems. 1997. Both wild-type and strongly attenuated bovine leukemia viruses protect peripheral blood mononuclear cells from apoptosis. J. Virol. 71:630-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dequiedt, F., R. Kettmann, A. Burny, and L. Willems. 1995. Mutations in the p53 tumor-suppressor gene are frequently associated with bovine leukemia virus-induced leukemogenesis in cattle but not in sheep. Virology 209:676-683. [DOI] [PubMed] [Google Scholar]
- 13.Egerer, G., M. Hensel, and A. D. Ho. 2001. Infectious complications in chronic lymphoid malignancy. Curr. Treat. Options Oncol. 2:237-244. [DOI] [PubMed] [Google Scholar]
- 14.Faderl, S., M. J. Keating, K. A. Do, S. Y. Liang, H. M. Kantarjian, S. O'Brien, G. Garcia-Manero, T. Manshouri, and M. Albitar. 2002. Expression profile of 11 proteins and their prognostic significance in patients with chronic lymphocytic leukemia (chronic lymphocytic leukemia). Leukemia 16:1045-1052. [DOI] [PubMed] [Google Scholar]
- 15.Ferrer, J. F., R. R. Marshak, D. A. Abt, and S. J. Kenyon. 1979. Relationship between lymphosarcoma and persistent lymphocytosis in cattle: a review. J. Am. Vet. Med. Assoc. 175:705-708. [PubMed] [Google Scholar]
- 16.Gentile, G., M. Cipone, C. Tassi, S. Pileri, and P. Tazzari. 1992. Ki-67 antigen expression in lymphocytes of cattle infected with bovine leukemia virus (BLV). Dtsch. Tierarztl. Wochenschr. 99:206-208. [PubMed] [Google Scholar]
- 17.Hamblin, T. 2002. Chronic lymphocytic leukaemia: one disease or two? Ann. Hematol. 81:299-303. [DOI] [PubMed] [Google Scholar]
- 18.Hanon, E., J. C. Stinchcombe, M. Saito, B. E. Asquith, G. P. Taylor, Y. Tanaka, J. N. Weber, G. M. Griffiths, and C. R. Bangham. 2000. Fratricide among CD8(and) T lymphocytes naturally infected with human T cell lymphotropic virus type I. Immunity 13:657-664. [DOI] [PubMed] [Google Scholar]
- 19.Hjalmar, V., R. Hast, and E. Kimby. 2002. Cell surface expression of CD25, CD54, and CD95. Eur. J. Haematol. 68:127-134. [DOI] [PubMed] [Google Scholar]
- 20.Hubmann, R., J. D. Schwarzmeier, M. Shehata, M. Hilgarth, M. Duechler, M. Dettke, and R. Berger. 2002. Notch2 is involved in the overexpression of CD23 in B-cell chronic lymphocytic leukemia. Blood 99:3742-3747. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson, S. 2002. Immunopathogenesis of human T cell lymphotropic virus type I-associated neurologic disease. J. Infect. Dis. 186(Suppl. 2):S187-S192. [DOI] [PubMed] [Google Scholar]
- 22.Kagi, D., B. Ledermann, K. Burki, R. M. Zinkernagel, and H. Hengartner. 1996. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu. Rev. Immunol. 14:207-232. [DOI] [PubMed] [Google Scholar]
- 23.Kalvelyte, A. V., and L. C. Pabrezaite. 1998. Proto-oncogene expression in bovine peripheral blood leukemic lymphocytes during their spontaneous proliferation, differentiation and apoptosis in vitro. Leuk. Res. 22:135-143. [DOI] [PubMed] [Google Scholar]
- 24.Kenyon, S. J., and C. E. Piper. 1977. Cellular basis of persistent lymphocytosis in cattle infected with bovine leukemia virus. Infect. Immun. 16:891-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korz, C., A. Pscherer, A. Benner, D. Mertens, C. Schaffner, E. Leupolt, H. Dohner, S. Stilgenbauer, and P. Lichter. 2002. Evidence for distinct pathomechanisms in B-cell chronic lymphocytic leukemia and mantle cell lymphoma by quantitative expression analysis of cell cycle and apoptosis-associated genes. Blood 99:4554-4561. [DOI] [PubMed] [Google Scholar]
- 26.Letesson, J. J., A. Van den Broecke, Y. Marbaix-Cleuter, M. Delcommenne, A. Mager, M. Mammerickx, A. Burny, and A. Depelchin. 1991. FACS analysis of bovine leukemia virus (BLV)-infected cell lines with monoclonal antibodies (mAbs) to B cells and to monocytes/macrophages. Vet. Immunol. Immunopathol. 27:207-213. [DOI] [PubMed] [Google Scholar]
- 27.Matheise, J. P., M. Delcommenne, A. Mager, C. H. Didembourg, and J. J. Letesson. 1992. CD5 and B cells from bovine leukemia virus infected cows are activated cycling cells responsive to interleukin 2. Leukemia 6:304-309. [PubMed] [Google Scholar]
- 28.Meirom, R., S. Moss, J. Brenner, D. Heller, and Z. Trainin. 1997. Levels and role of cytokines in bovine leukemia virus (BLV) infection. Leukemia 11(Suppl. 3):219-220. [PubMed] [Google Scholar]
- 29.Merezak, C., M. Reichert, C. Van Lint, P. Kerkhofs, D. Portetelle, L. Willems, and R. Kettmann. 2002. Inhibition of histone deacetylases induces bovine leukemia virus expression in vitro and in vivo. J. Virol. 76:5034-5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molica, S. 1997. Prognostic value of biological variables in B-cell chronic lymphocytic leukemia. Can we improve upon clinical parameters? Haematologica 82:705-709. [PubMed] [Google Scholar]
- 31.Montserrat, E., F. Bosch, and C. Rozman. 1997. Treatment of B-cell chronic lymphocytic leukaemia: current status and future perspectives. J. Intern. Med. Suppl. 740:63-67. [PubMed] [Google Scholar]
- 32.Nagai, M., M. B. Brennan, J. A. Sakai, C. A. Mora, and S. Jacobson. 2001. CD8(+) T cells are an in vivo reservoir for human T-cell lymphotropic virus type I. Blood 98:1858-1861. [DOI] [PubMed] [Google Scholar]
- 33.Pyeon, D., K. L. O'Reilly, and G. A. Splitter. 1996. Increased interleukin-10 mRNA expression in tumor-bearing or persistently lymphocytotic animals infected with bovine leukemia virus. J. Virol. 70:5706-5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed, J. C., S. Kitada, Y. Kim, and J. Byrd. 2002. Modulating apoptosis pathways in low-grade B-cell malignancies with biological response modifiers. Semin. Oncol. 29:10-24. [DOI] [PubMed] [Google Scholar]
- 35.Rosenwald, A., and L. M. Staudt. 2002. Clinical translation of gene expression profiling in lymphomas and leukemias. Semin. Oncol. 29:258-263. [DOI] [PubMed] [Google Scholar]
- 36.Rozman, C., and E. Montserrat. 1995. Chronic lymphocytic leukemia. N. Engl. J. Med. 333:1052-1057. [DOI] [PubMed] [Google Scholar]
- 37.Schattner, E. J. 2002. Apoptosis in lymphocytic leukemias and lymphomas. Cancer Investig. 20:737-748. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz-Cornil, I., N. Chevallier, C. Belloc, D. Le Rhun, V. Laine, M. Berthelemy, A. Mateo, and D. Levy. 1997. Bovine leukaemia virus-induced lymphocytosis in sheep is associated with reduction of spontaneous B-cell apoptosis. J. Gen. Virol. 78:153-162. [DOI] [PubMed] [Google Scholar]
- 39.Stephenson, C. F., Z. R. Desai, and J. M. Bridges. 1991. The proliferative activity of B-chronic lymphocytic leukaemia lymphocytes prior to and after stimulation with TPA and PHA. Leuk. Res. 15:1005-1012. [DOI] [PubMed] [Google Scholar]
- 40.Stone, D. M., L. K. Norton, and W. C. Davis. 2000. Spontaneously proliferating lymphocytes from bovine leukaemia virus-infected, lymphocytotic cattle are not the virus-expressing lymphocytes, as these cells are delayed in G(0)/G(1) of the cell cycle and are spared from apoptosis. J. Gen. Virol. 81:971-981. [DOI] [PubMed] [Google Scholar]
- 41.Stone, D. M., L. K. Norton, N. S. Magnuson, and W. C. Davis. 1996. Elevated pim-1 and c-myc proto-oncogene induction in B lymphocytes from BLV-infected cows with persistent B lymphocytosis. Leukemia 10:1629-1638. [PubMed] [Google Scholar]
- 42.Trueblood, E. S., W. C. Brown, G. H. Palmer, W. C. Davis, D. M. Stone, and T. F. McElwain. 1998. B-lymphocyte proliferation during bovine leukemia virus-induced persistent lymphocytosis is enhanced by T-lymphocyte-derived interleukin-2. J. Virol. 72:3169-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward, J. H. 2001. Autoimmunity in chronic lymphocytic leukemia. Curr. Treat. Options Oncol. 2:253-257. [DOI] [PubMed] [Google Scholar]
- 44.Wierda, W. G. and S. O'Brien. 2001. Immunotherapy of chronic lymphocytic leukemia. Expert Rev. Anticancer Ther. 1:73-83. [DOI] [PubMed] [Google Scholar]
- 45.Willems, L., A. Burny, D. Collete, O. Dangoisse, F. Dequiedt, J. S. Gatot, P. Kerkhofs, L. Lefebvre, C. Merezak, T. Peremans, D. Portetelle, J. C. Twizere, and R. Kettmann. 2000. Genetic determinants of bovine leukemia virus pathogenesis. AIDS Res. Hum. Retrovir. 16:1787-1795. [DOI] [PubMed] [Google Scholar]
- 46.Wolowiec, D., L. Ciszak, A. Kosmaczewska, D. Bocko, R. Teodorowska, I. Frydecka, and K. Kuliczkowski. 2001. Cell cycle regulatory proteins and apoptosis in B-cell chronic lymphocytic leukemia. Haematologica 86:1296-1304. [PubMed] [Google Scholar]
- 47.Yakobson, B., J. Brenner, H. Ungar-Waron, and Z. Trainin. 2000. Cellular immune response cytokine expression during the initial stage of bovine leukemia virus (BLV) infection determines the disease progression to persistent lymphocytosis. Comp. Immunol. Microbiol. Infect. Dis. 23:197-208. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida, M. 1996. Molecular biology of human T-cell lymphotropic virus-I: recent progress. J. Acquir. Immune. Defic. Syndr. Hum. Retrovirol. 13(Suppl. 1):S63-S68. [DOI] [PubMed] [Google Scholar]