Abstract
A potential barrier to the development of genetically targeted adenovirus (Ad) vectors for cell-specific delivery of gene therapeutics lies in the fact that several types of targeting protein ligands require posttranslational modifications, such as the formation of disulfide bonds, which are not available to Ad capsid proteins due to their nuclear localization during assembly of the virion. To overcome this problem, we developed a new targeting strategy, which combines genetic modifications of the Ad capsid with a protein bridge approach, resulting in a vector-ligand targeting complex. The components of the complex associate by virtue of genetic modifications to both the Ad capsid and the targeting ligand. One component of this mechanism of association, the Fc-binding domain of Staphylococcus aureus protein A, is genetically incorporated into the Ad fiber protein. The ligand is comprised of a targeting component fused with the Fc domain of immunoglobulin, which serves as a docking moiety to bind to these genetically modified fibers during the formation of the Ad-ligand complex. The modular design of the ligand solves the problem of structural and biosynthetic compatibility with the Ad and thus facilitates targeting of the vector to a variety of cellular receptors. Our study shows that targeting ligands incorporating the Fc domain and either an anti-CD40 single-chain antibody or CD40L form stable complexes with protein A-modified Ad vectors, resulting in significant augmentation of gene delivery to CD40-positive target cells. Since this gene transfer is independent of the expression of the native Ad5 receptor by the target cells, this strategy results in the derivation of truly targeted Ad vectors suitable for tissue-specific gene therapy.
Adenoviruses (Ads) are a family of over 50 viral mammalian pathogens, whose nonenveloped protein capsids embody a single copy of double-stranded DNA genome (36). Based on their ability to agglutinate red blood cells and the homology of their genomes, Ads have been classified into species A through F. The vast majority of the studies of Ad biology have been done on human Ad serotype 2 (Ad2) and Ad5, respectively, both belonging to species C.
The well-understood life cycle of these viruses, combined with relatively simple methods for the generation, propagation, and purification of recombinants derived from Ad2 and Ad5, made them attractive candidates as gene delivery vectors for human gene therapy. However, two decades of the extensive use of Ad-based vectors as prototypes of future gene therapeutics has revealed a number of limitations of this vector system, which have hampered its rapid transition into the clinic. One of these drawbacks is the relative inefficiency of gene delivery by Ad vectors to certain types of diseased human tissues. On the other hand, the susceptibility of many normal tissues to Ad infection makes them random targets for Ad vectors and results in the suboptimal distribution of the viruses upon administration to patients.
Attempts to rectify this deficiency of Ad vectors have been rationalized by the identification of the molecular determinants of the virus tropism. A typical Ad capsid is an icosahedron, whose planes are formed by the Ad hexon protein, whereas the vertices are occupied by a penton assembly formed by the penton base and protruding fiber proteins (9). The cell entry mechanism used by the majority of human Ad serotypes involves two sequential interactions between an Ad particle and a cell. According to this concept, the first of the two contacts involves the Ad fiber protein (17, 26) and the so-called coxsackievirus and Ad receptor (CAR) (4, 39). Specifically, the carboxy-terminal knob domain of the fiber binds to the immunoglobulin-like D1 domain of CAR (5, 13), resulting in the tight association of the virus with the cell. The presence of CAR on a target cell is thus recognized as a critical prerequisite of efficient infection. This binding step is followed by the secondary contact, which involves the arginine-glycine-aspartic acid (RGD) sequence found in the Ad penton base protein and the cellular integrins αvβ3 and αvβ5 (45, 46). This interaction triggers the internalization of the virion within a clathrin-coated endosome (44). Acidification of the endosome is believed to lead to the release of the virus into the cytoplasm, followed by its translocation to the nucleus, where the replication of the virus begins. It has been reported that, whereas CAR is used by the majority of human Ads as a primary receptor (34), other cell surface molecules are also exploited in this capacity by certain Ad serotypes (1, 10, 24, 35). This observation suggests that the receptor specificity of a given Ad serotype may be modified by redirecting the virus to alternative cellular receptors.
This targeting concept has been realized by using the following strategies. In adapter-mediated targeting, the tropism of the virus is modified by an extraneous targeting moiety, the ligand, which associates with the Ad virion either covalently or noncovalently. Adapters or adapter-ligand complexes successfully used for Ad targeting include bispecific antibody (Ab) conjugates, genetic fusions of single-chain Ab (scFv) with CAR, or scFv-scFv diabodies (reviewed in reference 21). Adapter-mediated targeting is rather versatile and technically simple, it may use a wide range of targeting ligands, and it allows for the rapid generation of analytical amounts of targeted complexes and their fast validation. However, it requires the production and purification of at least two different components (the virus and targeting ligand), their subsequent conjugation in a targeting complex, and the purification of that complex from nonreacted components.These requirements substantially complicate the large-scale production of the vector complex, which may result in significant batch-to-batch variations and complicate the regulatory approval of the vector for clinical use.
In contrast, genetic targeting, which is based on the genetic incorporation of the ligand into the Ad capsid (reviewed in reference 22) results in a one-component, self-assembling, and self-replicating vector, which, once made and validated, may be amplified to any desired scale. The choice of ligands in this strategy, however, is limited to proteins only. Furthermore, additional limitations may be imposed by the potential structural or biosynthetic incompatibility of the ligand with the protein components of Ad capsid. For instance, recent studies by Magnusson et al. (27) have shown that protein ligands, such as the epidermal growth factor or scFvs, whose correct folding requires the formation of disulfide bonds, cannot be used for genetic targeting of Ad.
To overcome the limitations of these targeting strategies, we sought to develop a new approach, which combines elements of the genetic modification of the Ad capsid with the adapter-mediated targeting. We establish here the feasibility and efficacy of this strategy to target Ad vectors. Specifically, we show that by incorporation of the immunoglobulin (Ig)-binding domain of Staphylococcus aureus protein A into the Ad fiber protein, a virus vector capable of associating with the Fc domain of Ig can be derived. Furthermore, we genetically fused the Ig Fc domain with a targeting scFv ligand and showed that this domain can serve as a docking moiety during the formation of the Ad-ligand targeting complex. Most important, we have shown that, upon self-assembly, this complex retains its stability during purification and storage and can efficiently deliver transgenes to target cells by using the cell entry pathway determined by its ligand component.
MATERIALS AND METHODS
Cell lines.
293 human embryonal kidney cells, their derivative 293T/17 (which expresses the simian virus 40 large T antigen), and Namalwa Burkitt's lymphoma human cells were purchased from the American Type Culture Collection (Manassas, Va.). The generation of 293.CD40 cells stably expressing human CD40 will be described elsewhere (unpublished data). Namalwa cells were cultured in RPMI medium adjusted to contain 1.5 g of sodium bicarbonate/liter supplemented with 2 mM l-glutamine, 4.5 g of glucose/liter, 1.0 mM sodium pyruvate, and 7.5% fetal bovine serum (FBS). 293 and 293T/17 cells were propagated in Dulbecco modified Eagle medium (DMEM)-F-12 medium with 10% FBS, 2 mM glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. FBS was purchased from HyClone (Logan, Utah), and media and supplements were from Mediatech (Herndon, Va.). All cells were propagated at 37°C in a 5% CO2 atmosphere.
Dendritic cells (DCs) were derived from the peripheral blood of normal donors, by using a protocol approved by the UAB Institutional Review Board. Peripheral blood mononuclear cells were purified with gradient centrifugation by using Histopaque (Sigma Diagnostics, St. Louis, Mo.). CD14+ monocytes were then isolated by using CD14 Microbeads and magnetic cell sorting (Miltenyi Biotec, Auburn, Calif.). They were cultured for 6 days in RPMI 1640 medium with 10% FBS, 2 mM glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 50 μM 2-mercaptoethanol containing 100 ng of recombinant human interleukin-4 (R&D Systems, Minneapolis, Minn.) and 100 ng of recombinant human granulocyte-macrophage colony-stimulating factor (Immunex, Seattle, Wash.)/ml (40). Expression of molecular markers typical of immature DCs (CD14− CD11c+ CD40+ CD86+ HLADR+) was confirmed by staining with relevant monoclonal antibodies (MAbs).
Antibodies.
Rabbit anti-Ad2 polyclonal antibodies were purchased from the National Institute of Allergy and Infection Diseases (Bethesda, Md.). Anti-mouse and anti-rabbit immunoglobulin polyclonal antibodies conjugated with horseradish peroxidase were from Amersham Pharmacia Biotech, Inc. (Piscataway, N.J.) and Dako (Carpinteria, Calif.), respectively. 4D2 anti-fiber (18) mouse MAb was provided by Jeffrey Engler (University of Alabama at Birmingham). Penta-His MAb, which binds a five-histidine sequence, was purchased from Qiagen (Valencia, Calif.).
Genetic engineering.
Restriction endonucleases and T4 DNA ligase were purchased from New England Biolabs (Beverly, Mass.). The PCR was performed with Pfu DNA polymerase (Stratagene, La Jolla, Calif.).
To facilitate the modifications of the HI-loop of Ad5 fiber, the shuttle vector pKanHI-BaeI carrying the Ad5 fiber gene with flanking regions of Ad genomic DNA, and the recognition sequence for the restriction endonuclease BaeI within the HI-loop was constructed by a two-step cloning strategy. First, the shuttle vector pKanΔHI was generated by subcloning of the 3.1-kb PmeI-EcoRI fragment of pXKΔHI (3), whose ends were filled in with the Klenow fragment of DNA polymerase I of Escherichia coli, into ApoI-AflIII-digested pZErO-2 (Invitrogen, Carlsbad, Calif.). Next, a BaeI recognition site within the HI-loop-encoding sequence was generated by cloning the duplex made with the oligonucleotides Bae.F (ACAACTCGGTGGCGGTACCGGTGTATACGGCGGTCC) and Bae.R (GGACCGCCGTATACACCGGTACCGCCACCGAGTTGT) into EcoRV-digested plasmid pKanΔHI, resulting in the shuttle vector pKanHI-BaeI.
A shuttle vector suitable for modifications of the carboxy terminus of the fiber protein was designed by subcloning an AgeI-MfeI fragment of the previously described pBS.F5LLBamHI (23) into the AgeI-MfeI-digested pKanΔHI. This resulted in plasmid pKanLL-BamHI encoding a modified fiber with a C-terminal peptide linker (G4S)3, followed by a BamHI restriction site. This site was then replaced with the BaeI recognition sequence by inserting a duplex made of two oligonucleotides, LL-Bae-1F (GATCCCGGTGGCGGTACCGGTGTATACGGCGGTTAATAAA) and LL-Bae-1R (GATCTTTATTAACCGCCGTATACACCGGTACCGCCACCGG), thereby generating pKanLL-BaeI.
Plasmid pDV67, which was constructed for the expression of Ad5 fiber and its derivatives in mammalian cells, was obtained from Dan Von Seggern (43). To simplify the transfer of the fiber genes assembled within pDV67 into the pKan3.1-derived fiber shuttle vectors, the MfeI restriction site located upstream from the cytomegalovirus (CMV) promoter was deleted to yield pVSI. A new MfeI site was introduced downstream from the 3′ end of the fiber open reading frame by cloning an MfeI-XbaI linker (CTAGCCAATTGG) into XbaI-digested pVSI, yielding pVSII.
Recombinant genes encoding the Ad5 fiber modified by incorporation of the so-called C domain (Cd) of Staphylococcus aureus protein A within the HI loop and at the carboxy (i.e., C) terminus were assembled in two steps. First, AgeI-MfeI fragments isolated from the plasmids pKanHI-BaeI, pKan-LL-BaeI, pHI.PB10, pHI.PB40, and pHI.PB80 (3) were cloned into AgeI-MfeI-digested pVSII. Next, the nucleotide sequence encoding the Cd of S. aureus protein A was assembled with two pairs of oligonucleotides—(i) T1 (GCGGATAACAAATTCAACAAAGAACAACAAAATGCTTTCTATGAAATCTTACATTTACCTAACTTAAACGAAGAACAACGTAACGGCTTC) and B1 (GTTACGTTGTTCTTCGTTTAAGTTAGGTAAATGTAAGATTTCATAGAAAGCATTTTGTTGTTCTTTGTTGAATTTGTTATCCGCGGATC) and (ii) T2 (ATCCAAAGCCTTAAAGACGATCCTTCAGTGAGCAAAGAAATTTTAGCAGAAGCTAAAAAGCTAAACGATGCTCAAGCACCAAAATAATA) and B2 (TTTTGGTGCTTGAGCATCGTTTAGCTTTTTAGCTTCTGCTAAAATTTCTTTGCTCACTGAAGGATCGTCTTTAAGGCTTTGGATGAAGCC)— and cloned into the BaeI-cleaved derivatives of pVSII described above. The resultant expression plasmids were designated pVS-HI-Cd, pVS-LL-Cd, pVS-PB10-Cd, pVS-PB40-Cd, and pVS-PB80-Cd.
Shuttle vectors containing these modified fiber genes were constructed by replacing the AgeI-MfeI fragment of the shuttle vector pKanΔHI by the AgeI-MfeI fragments of pVS-HI-Cd, pVS-LL-Cd, pVS-PB10-Cd, pVS-PB40-Cd, and pVS-PB80-Cd.
Recombinant Ad genomes incorporating the modified fiber genes were derived by homologous DNA recombination in Escherichia coli BJ5183 with SwaI-linearized plasmid pVL3200 essentially as described previously (8). pVL3200 is a derivative of pTG3602 (8), which contains an Ad5 genome with E1, E3, and the fiber gene deleted. In place of the deleted E1, the genome contains a CMV immediate-early promoter-driven expression cassette comprising the firefly luciferase gene and the green fluorescent protein (GFP) gene linked to an internal ribosome entry site.
To prepare a targeting ligand, the sequence encoding a fusion protein designated Fc-G28.5, comprising the secretory leader sequence, anti-CD40 single-chain antibody (scFv) G28.5 (32) tagged with the Fc domain of human immunoglobulin, and a six-histidine sequence (His6), was assembled within the expression cassette of the AdApt shuttle vector (Crucell, Leiden, The Netherlands). The genome of Ad5.Fc-G28.5 containing this cassette in place of the deleted E1 region was then generated by homologous DNA recombination with the ClaI-linearized pTG3602 rescue vector (8).
Details of all genetic engineering procedures are available upon request.
Viruses.
All Ad vectors were generated by transfection of 293 cells with PacI-digested Ad rescue vectors as described previously (20). The viruses were propagated in 293 cells and purified by equilibrium centrifugation in CsCl gradients according to a standard protocol (15). Protein concentrations in viral preparations were determined by using the Dc protein assay (Bio-Rad, Hercules, Calif.) with purified bovine serum albumin (BSA) as a standard. The virus titers were calculated as follows: 1 μg of protein = 4 × 109 viral particles (vp).
Recombinant proteins.
To express Fc-G28.5, Ad5.Fc-G28.5 was used for infection of 6 × 109 293 cells at a multiplicity of infection (MOI) of 100 vp/cell. The medium from the infected cells was collected at 72 h postinfection and loaded onto a HiTrap rProtein A FF 5-ml column (Amersham) equilibrated with phosphate-buffered saline (PBS). After the column was washed with five column volumes of PBS, bound proteins were eluted with 0.1 M sodium citrate (pH 3.4). To preserve the activity of the scFv, 1-ml fractions were collected into tubes with 200 μl of 1.5 M Tris-HCl (pH 8.8). The collected protein was dialyzed against PBS and loaded onto a 1-ml HiTrap His6 FF column (Amersham). After the column was washed with PBS, the protein was eluted with a linear gradient of imidazole (20 to 500 mM) in PBS. The protein was collected and dialyzed against PBS. The final protein concentration was determined by using the Dc protein assay (Bio-Rad) with BSA as a standard.
The design, expression, and purification of the recombinant protein comprising the extracellular domain of human CAR have been reported by Dmitriev et al. (11). The expression of the His6-tagged knob domain of Ad5 fiber in E. coli and its purification by immobilized ion metal affinity chromatography have been described previously (23).
All chromatographic separations were performed by utilizing the ÄKTA purifier system on prepacked columns from Amersham.
The recombinant protein Fc-CD40L, which consists of a genetic fusion of the DNA encoding the human tumor necrosis factor-like domain of human CD40 ligand sequence at its amino terminus to the hinge region of the Fc domain of human IgGγ1, was expressed in murine NS/0 cells and purified as previously described (25).
Preparation of targeted Ad.
Complexes of Ad with Fc-containing targeting ligands were generated during purification of viruses from infected 293 cells. Briefly, 293 cells were infected with Ads at an MOI of 300 vp/cell. Cells were harvested at 55 h postinfection and resuspended in 2% FBS-DMEM. Viruses were released from the cells by three freeze-thaw cycles, and the cell debris was removed by centrifugation. The supernatant was layered onto a preformed step gradient of CsCl and centrifuged for 3 h at 4°C and 25,000 rpm. Banded viruses were collected, mixed with Fc-G28.5 or Fc-CD40L proteins at a concentration of 30 μg/ml, and incubated for 30 min at room temperature. Vector complexes were purified from unbound proteins by equilibrium centrifugation in CsCl gradients, dialyzed (10 mM Tris-HCl [pH 8.0], 50 mM NaCl, 2 mM MgCl2, 10% glycerol), and stored at −80°C until use.
Transient expression of recombinant fiber proteins.
293T/17 cells were transfected with the pVS-derived expression vectors by using the DOTAP liposomal transfection reagent (Roche, Mannheim, Germany) according to the manufacturer's protocol. At 72 h posttransfection, the cells were washed with PBS, harvested, and lysed in cell culture lysis reagent (Promega, Madison, Wis.) at 106 cells/ml. Cell lysates were used for enzyme-linked immunosorbent assay (ELISA) and for immunoblotting.
Western blot.
Samples were incubated in Laemmli sample buffer at 96°C for 5 min and separated on 4 to 20% gradient polyacrylamide gel (Bio-Rad). For “seminative” electrophoresis, samples were not boiled. The proteins were electroblotted onto a polyvinylidene difluoride membrane, and the blots were developed with the WesternBreeze immunodetection system (Invitrogen) according to the manufacturer's protocol with either the 4D2 or Penta-His antibodies as primary probes.
ELISA.
The wells of 96-well Nunc Immuno-Plates (Fisher Scientific, Pittsburgh, Pa.) were coated overnight at 4°C with proteins diluted in 50 mM carbonate buffer (pH 8.6) at a concentration of 5 μg/ml. The unsaturated surface of the wells was then blocked for 1 h at room temperature by the addition of 200 μl of blocking buffer (Tris-buffered saline [TBS] with 0.05% Tween 20 and 0.5% casein) to each well. The blocking buffer was replaced with 100 μl of cell lysates or Ad preparations diluted in binding buffer (TBS with 0.05% Tween 20 and 0.05% casein). Plates were incubated at room temperature for 1 h and then were washed four times with washing buffer (TBS with 0.05% Tween 20). Bound fiber proteins or Ad particles were detected by incubation for 1 h at room temperature with 4D2 MAb or anti-Ad2 polyclonal antibodies, respectively. The wells were washed four times with washing buffer; either goat anti-mouse immunoglobulin G or goat anti-rabbit immunoglobulin antibodies conjugated with horseradish peroxidase (Dako) were then added, and incubation was continued for 1 h. The color was developed with a Sigma FAST o-phenylenediamine dihydrochloride tablet kit as recommended by the manufacturer. The color intensity was measured at 490 nm with an EL800 plate reader (Bio-Tek Instruments, Winooski, Vt.).
Gene transfer assay.
To study Ad-mediated luciferase gene delivery, 5 × 105 cells grown in a 24-well plates were washed once with PBS and preincubated for 10 min at room temperature with 200 μl of either Ad5 knob protein diluted in 2% FBS-DMEM at a concentration of 100 μg/ml or 2% FBS-DMEM alone. Cells were infected at an MOI of 10 vp/cell with Ad vectors diluted in 200 μl of 2% FBS-DMEM and incubated for 30 min at room temperature. The medium containing the unbound viruses was then aspirated, and the cells were washed once with 2% FBS-DMEM. A total of 500 μl of growth medium was then added, and the cells were incubated at 37°C to allow for luciferase expression. After 24 h the cells were lysed in 0.25 ml of luciferase reporter lysis buffer and assayed for luciferase activity by using the luciferase assay system (Promega) according to the manufacturer's protocol. Each datum point was set in triplicate and calculated as the mean of three determinations. Preliminary experiments demonstrated a linear response with the luciferase activity versus the MOI of the input virus over a range of 0.1 to 100 vp/cell.
To target Ad vectors, some experiments included preincubation of the viruses with Fc-containing proteins. Specifically, 1.5 μg of Fc-G28.5 was incubated with 1010 vp of Ad in 10 μl of PBS for 30 min at room temperature. The mixtures were then diluted with 2% FBS-DMEM down to 2.5 × 107 vp/ml, and 200-μl aliquots were added to the cells.
RESULTS
Design and expression of Ad5 fiber proteins modified with the Cd of S. aureus protein A.
To design a versatile mechanism of attachment of targeting ligands to Ad particles, we chose to modify the structure of each of these components with distinct protein moieties capable of forming stable heteroduplexes upon association with each other. To this end, we chose to introduce within the fiber protein of the Ad5 vector the so-called Cd of S. aureus protein A. This domain is known to bind with high selectivity and affinity to the Fc domain of Igs. Therefore, Ad virions incorporating such Cd-modified fibers were expected to bind targeting ligands designed to contain an Fc domain.
A total of five genes coding for different Cd-containing fibers were designed by incorporation of the Cd open reading frame into either the carboxy terminus of the fiber protein (Fb-LL-Cd) or the HI loop of its knob domain. In the latter instance, in addition to direct fusion of the Cd sequence with that of the HI loop (Fb-HI-Cd), we made three other constructs (Fb-HI10-Cd, Fb-HI40-Cd, and Fb-HI80-Cd), in which the Cd was flanked within the loop with flexible linkers derived from the Ad5 penton base protein (3). These additional constructs were designed to avoid potential steric hindrance that could be caused by the proximity of the knob to Cd within the fusion molecule. We sought to avoid such a structural problem by extending Cd away from the knob. The length of the linkers in these constructs was 5, 20, or 40 amino acid residues.
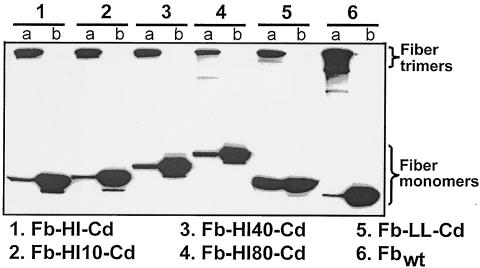
The fiber-Cd genes were assembled in the mammalian expression plasmid pVS2, and the resultant recombinant vectors were then used to direct the expression of these genes in 293T/17 cells. These expression experiments were intended to demonstrate that the designed protein chimeras could be expressed at levels comparable to that of the wild-type (wt) Ad5 fiber (see Fig. 1, Fbwt) and that they possess the structural and functional properties required for both the incorporation of these proteins into Ad virions and for binding to Fc-containing proteins. As seen in Fig. 1, immunoblotting of the lysates of pVS-transfected 293T/17 cells showed that the quantities of the fiber-Cd proteins were similar to the amount of the wt fiber expressed by the control plasmid. A comparison of the mobilities of the chimeras in denatured and nondenatured samples showed clearly that all of the newly designed proteins formed trimers upon self-association. Of note, the substantial amount of the Fb-LL-Cd monomer present in nondenatured sample suggested that the Fb-LL-Cd trimer was less stable than the other designed fibers. Since trimerization of the fiber is a prerequisite of its association with the penton base protein, the results of this assay were indicative of the suitability of the fiber-Cd proteins for Ad capsid modification.
FIG. 1.
Analysis of the transiently expressed fiber-Cd proteins. 293T/17 cells transfected with pVS-derived expression plasmids were lysed, and aliquots of the lysates containing 5 μg of total soluble protein were loaded on a sodium dodecyl sulfate-polyacrylamide electrophoresis gel in sample buffer. The fiber proteins in some of the samples were fully denatured by a 5-min incubation at 96°C (lanes b). These samples were expected to contain the fiber monomers only. In parallel, similarly prepared samples analyzed under seminative conditions were not heat denatured (lanes a) and were supposed to contain the fiber-Cd proteins in a trimeric configuration. Upon separation, the proteins were electroblotted onto a polyvinylidene difluoride membrane and probed with anti-fiber tail MAb 4D2.
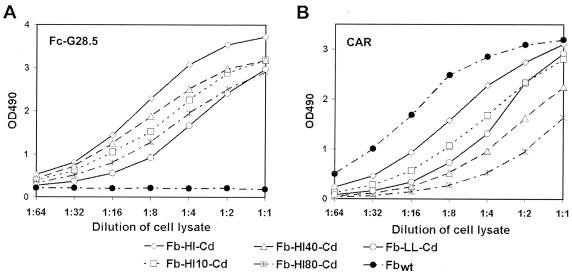
We next examined the Fc-binding capability of the Cd in the context of the fiber-Cd chimeras. This was accomplished by an ELISA that used the lysates of fiber-Cd-expressing 293T/17 cells for a binding assay using the Fc-G28.5 protein (see below) as bait. This assay demonstrated that each of the fiber-Cd chimeras bound to the Fc domain, whereas, predictably, the wt fiber did not bind to Fc-G28.5 even at the highest concentration used (Fig. 2A).
FIG. 2.
Assessment of the Fc- and CAR-binding ability of the transiently expressed fiber-Cd proteins. The bait proteins, Fc-G28.5 (A) and recombinant CAR (B), adsorbed on ELISA plates were probed with serial dilutions of lysates of fiber-Cd-expressing 293T/17 cells. The quantity of the recombinant fibers used in the assay was normalized according to the concentration of total soluble protein in the lysates. The bait-bound fibers were then detected with anti-fiber MAb, followed by horseradish peroxidase-conjugated anti-mouse immunoglobulin G antibodies. OD490, optical density at 490 nm.
In addition, to investigate whether the interaction of the fiber-Cd proteins with CAR was affected by incorporation of the Cd, the capacity of these chimeras to bind CAR was tested. An ELISA with a soluble form of CAR protein, sCAR, as the target showed that, although the receptor-binding site within the modified fibers was affected by incorporation of Cd (Fig. 2B), all modified fibers largely retained the ability to bind CAR.
Therefore, taken together, these experiments made it clear that despite very substantial modifications of the fiber structure, all five fiber-Cd proteins possess key functional properties, which are essential for the realization of this Ad targeting scheme.
Derivation of Ad vectors containing Cd-modified fibers.
To generate Ad vectors containing Cd-modified fibers, the genes encoding these proteins were transferred to the fiber shuttle plasmids and then into an Ad genome from which the early regions E1 and E3 and also the fiber gene had been deleted. This genome, incorporated into the Ad rescue vector pVL3200, was previously modified to contain an expression cassette comprising two reporter genes, the firefly luciferase, and GFP in place of the E1 region. Transcription of these reporters, linked with an internal ribosome entry site, is driven by the hybrid CMV5 promoter, which incorporates functional elements of the immediate-early CMV promoter and the major late promoter of Ad5 (28). The designations of the pVL3200-derived Ad vectors contain the abbreviation “DR,” such as Ad5.DR-LL-Cd, to reflect the presence of a “double reporter” (luciferase and GFP) in their genomes.
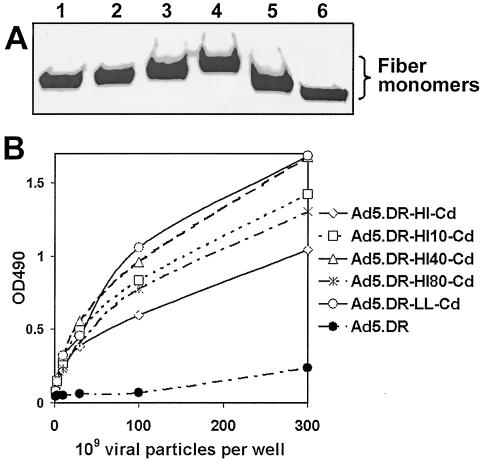
The Ad genomes isolated from the resultant plasmids were used to rescue the Ad vectors of interest by transfection of 293 cells as described in Materials and Methods. Upon rescue and propagation, the viruses were purified, and their titers were determined. The dynamics of the infection by these vectors did not differ from those seen for a control Ad vector, Ad5.DR, incorporating wt fibers. As shown in Table 1, the titers of all six viruses were very similar. Also, as would have been predicted by the trimerization pattern of the transiently expressed fiber-Cd proteins, an immunoblot analysis of purified viruses showed efficient incorporation of these fiber chimeras into Ad capsids (Fig. 3A). In the aggregate, these observations suggested that the modifications of the fiber with Cd did not have any deleterious effect on the assembly of the virions.
TABLE 1.
Yields of Ad.DR vectors in 293 cells
| Virus | No. of particles/108 cells |
|---|---|
| Ad5.DR | 1.1 × 1012 |
| Ad5.DR-HI-Cd | 7.5 × 1011 |
| Ad5.DR-HI10-Cd | 6.4 × 1011 |
| Ad5.DR-HI40-Cd | 9.3 × 1011 |
| Ad5.DR-HI80-Cd | 7.6 × 1011 |
| Ad5.DR-LL-Cd | 8.5 × 1011 |
FIG. 3.
Characterization of Ad virions incorporating fiber-Cd proteins. (A) Western blotting of Cd-modified Ad. Aliquots equal to 1010 vp of CsCl-purified Ad vectors were boiled in the sample buffer, and their protein components were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The fibers electrotransferred onto a membrane were identified with anti-fiber tail MAb 4D2. Lane 1, Ad5.DR-HI-Cd; lane 2, Ad5.DR-HI10-Cd; lane 3, Ad5.DR-HI40-Cd; lane 4, Ad5.DR-HI80-Cd; lane 5, Ad5.DR-LL-Cd; lane 6, Ad5.DR. (B) Binding of Cd-containing Ad vectors to Fc-modified targeting ligand. The ligand, Fc-G28.5, was adsorbed on an ELISA plate and incubated with aliquots of the purified Cd-modified Ad virions ranging from 1 × 109 to 3 × 1011 vp. Fc-bound Ad particles were detected by using anti-Ad2 polyclonal antibodies. OD490, optical density at 490 nm.
Production of the targeting Fc single-chain antibody ligand.
Having completed the modification of the Ad vectors, we next sought to design a complementary ligand molecule, which would be capable of targeting the virus via association with its altered capsid. To this end, we used the Fc domain of human Ig as a fusion partner for a targeting single-chain antibody (scFv) to generate a bifunctional “anchor-ligand” molecule. The role of the Fc domain in our targeting scheme is twofold. First, it is used to facilitate the expression and secretion of the targeting ligand; second, it also serves as an anchor, which allows the ligand to associate with the Cd-modified Ad capsids. This concept was realized by designing a recombinant protein comprising the Fc domain of human IgG1 fused at its carboxy terminus with an scFv derived from the anti-human CD40 MAb G28.5. This Fc-G28.5 fusion also contained a carboxy-terminal His6 tag for detection and purification purposes. The Fc-G28.5-encoding gene was placed under the transcriptional control of the CMV5 promoter and incorporated into the genome of a replication-incompetent Ad5 vector, Ad5.Fc-G28.5, where it replaced the E1 region.
Expression of Fc-G28.5 in 293 cells by Ad5.Fc-G28.5 resulted in accumulation of the protein in the culture medium, from which it was purified by affinity chromatography on a protein A column, followed by immobilized ion metal affinity chromatography. A total of 6.8 mg of the fusion was purified in this way upon infection of 6 × 109 293 cells. Analytical gel filtration chromatography of Fc-G28.5 showed that it was present in the sample in the form of a dimer, which is typical of Fc-containing proteins. Electrophoresis of the resultant preparation showed that the Fc-G28.5 ligand was >95% pure (data not shown) and thus suitable for subsequent vector targeting experiments.
At this point we sought to confirm that both components of the newly designed gene delivery system, the viral vector and the targeting ligand, were able to associate with each other. This was addressed by an ELISA in which Fc-G28.5, used as the bait, was probed with purified Ad particles. As expected, this assay showed strong binding of each of the Cd-modified vectors to the ligand, whereas virtually no binding was observed with the control Ad lacking Cd in the capsid (Fig. 3B). Thus, these findings proved the feasibility of the formation of targeting vector complexes and therefore rationalized subsequent cell transduction studies.
Preliminary assessment of gene transfer properties of Ad-ligand targeting complexes.
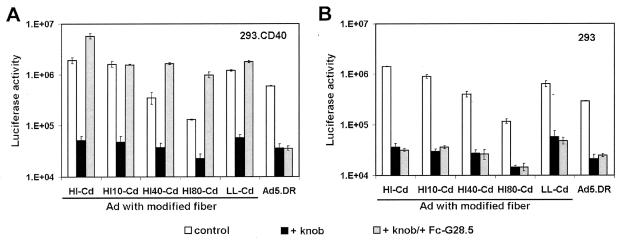
A comparison of the gene delivery characteristics of the Ad::Fc-G28.5 complexes was done by means of a transduction experiment with 293.CD40 cells as the target. Prior to infection with the modified Ad vectors, the cells were preincubated with either medium alone, medium containing recombinant Ad5 fiber knob protein, or medium containing the knob and Fc-G28.5 ligand. Since all of the Ad vectors used in these studies contained fibers with functional CAR-binding sites, we sought to discriminate between the gene transfer which could occur via CAR-mediated cell entry versus that which was expected to result from the attachment of the targeting complexes to CD40. This was accomplished by blocking CAR on the surface of the target cells with the knob protein (23). Ad vectors incorporating wt fibers, as well as parental 293 cells, which do not express any detectable CD40, were used as negative controls.
This experiment showed that all Cd-modified Ads were able to use the Fc-G28.5 ligand for CD40-mediated infection, with no significant variations between the vectors (Fig. 4). These data obviated the need to study all five modified vectors. Therefore, we chose to proceed with Ad5.DR-HI10-Cd, Ad5.DR-HI40-Cd, and Ad5.DR-LL-Cd, since these constructs represented two different Ad fiber modification approaches: the redesign of the HI loop and the carboxy terminus of the protein.
FIG. 4.
Ligand-mediated transduction of CD40+ cell targets. 293.CD40 (A) or 293 (B) cells preincubated with either Ad5 fiber knob protein, fiber knob plus Fc-G28.5 protein, or plain medium were infected with each of the Cd-modified vectors at an MOI of 10 vp/cell. Ad5DR vector incorporating wt Ad5 fibers was used as a control. The bars correspond to the luciferase activity in relative light units detected in transduced cells at 24 h postinfection (i.e., the average activity obtained in three replicates). The error bars show standard deviations.
Preparation and characterization of preformed Ad-ligand complexes.
Although the preliminary vector validation experiments showed the ability of Cd-modified viruses to use an Fc-fused scFv ligand for targeting, we sought to further examine the properties of these targeting complexes. This time, the Ad-ligand complexes were made at a preparative scale and purified from unbound ligand, which otherwise would competitively inhibit the infectivity of the vectors. This was done to test whether the vector complexes could be made in bulk and, upon purification, stored until use without losing their infectivity. By mixing the components of the complex at a high ligand/virus ratio (1,800:1), we sought to create conditions under which all of the Cd anchoring sites within the virions would be occupied by the ligand.
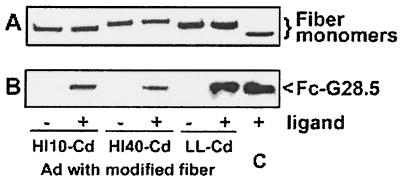
Each of the three viruses—Ad5.DR-HI10-Cd, Ad5.DR-HI40-Cd, and Ad5.DR-LL-Cd—was mixed and incubated with the targeting Fc-scFv ligand as described in Materials and Methods and subsequently purified in CsCl gradients. Next, we assessed the efficiency of association of the ligand with each of the viruses in an immunoblot assaywith a Penta-His MAb, which binds to the His6 tag present in the ligand molecule. This analysis showed that Fc-G28.5 protein bound most efficiently to Ad5.DR-LL-Cd, while the amounts of the ligand found in preparations of Ad5.DR-HI10-Cd and Ad5.DR-HI40-Cd were smaller (Fig. 5).
FIG. 5.
Incorporation of Fc-G28.5 fusion protein into targeting vector complexes. Targeting complexes formed by association of the Fc-G28.5 ligand with either Ad5.DR-HI10-Cd, Ad5.DR-HI40-Cd, or Ad5.DR-LL-Cd were purified from unincorporated ligands on CsCl gradients, and aliquots of each preparation corresponding to 1.5 × 109 vp were analyzed by immunoblotting alongside samples of Ad vectors that were not incubated with Fc-G28.5. (A) Membrane probed with anti-fiber MAb; (B) result of the ligand detection done with Penta-His MAb. +, samples preincubated with ligand; −, samples containing Ad vectors only. Lane C shows a mixture of 1.5 × 109 vp of Ad5.DR with 12 ng of Fc-G28.5.
Transduction properties of the preformed Ad-ligand complexes on established cell lines.
The receptor specificity of the resultant vector complexes was assessed by using them to infect Namalwa human lymphoblastoid cells, which are CAR positive and naturally express CD40. As seen in Fig. 6, the vector complexes clearly outperformed the relevant untargeted Ad, with the difference in the infection efficiencies being in the range of an order of magnitude for each vector. Importantly, this augmentation of infectivity was entirely due to the targeting of the vectors to CD40, since the addition of the fiber knob protein had no effect on the gene transfer. Of special note, Ad5.DR-HI10-Cd demonstrated an infection profile that was very similar to that of Ad5.DR-HI40-Cd (not shown).
FIG. 6.
Transduction of cells by the preformed targeted vector complexes. CD40+ Namalwa cells were infected with either Ad5.DR-HI40-Cd or Ad5.DR-LL-Cd at MOIs of 10 or 500 vp/cell, respectively. Each of the Cd-modified vectors was used either alone (−) or in association with the Fc-G28.5 ligand (+). Ad5.DR was used as an unmodified vector control. The infection was done with or without recombinant Ad fiber knob protein being added to the incubation mixture. The luciferase activity in the transduced cells is shown in relative light units. The standard deviations are represented by the error bars.
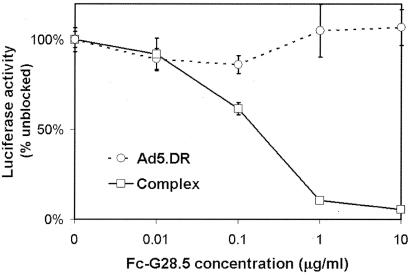
The CD40 dependence of the infection by the targeted complexes was further confirmed by transducing Namalwa cells with Ad5.DR-LL-Cd::Fc-G28.5 in the presence of various concentrations of free ligand. This resulted in the inhibition of transduction in an Fc-G28.5 concentration-dependent manner, which unambiguously demonstrated the direct involvement of CD40 in the cell entry pathway used by the ligand-containing vector complex (Fig. 7). As expected, the infectivity of the Ad5.DR vector, which contains wt fibers and is thus unable to associate with Fc-G28.5, was not affected by the addition of the free ligand.
FIG. 7.
Ligand-mediated inhibition of gene transfer by Ad5.DR-LL-Cd::Fc-G28.5 vector complex. CD40+ Namalwa cells preincubated with medium alone or with increasing concentrations of the Fc-G28.5 ligand were transduced with the preformed Ad5.DR-LL-Cd::Fc-G28.5 vector at an MOI of 100 vp/cell. Ad5.DR vector containing unmodified fiber was used as a negative control. The luciferase activity detected in the lysates of cells transduced with the viruses in the presence of competing ligand protein was normalized to that in the cells infected in the absence of free Fc-G28.5. The datum points represent the results of three independent determinations with the error bars corresponding to standard deviations.
In vitro transduction of primary human DCs with the CD40-targeted vectors.
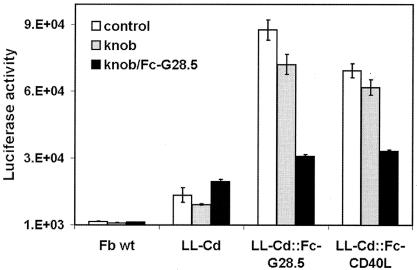
An additional test of the cell transduction ability of the Ad5.DR-LL-Cd::Fc-G28.5 vector was done with human DCs as targets. These DCs were derived from CD14+ monocytes isolated from human peripheral blood as described in Materials and Methods. For the purpose of comparison, a similarly prepared vector complex containing the CD40-binding domain of human CD40 ligand, CD40L, fused with Fc was also used. This experiment demonstrated that the Cd-modified vector, when complexed with either of the two targeting ligands, was able to deliver the reporter gene to DCs 28- to 35-fold more efficiently than the control unmodified vector, Ad5.DR (Fig. 8). In line with previous reports of the poor expression of CAR (2, 37, 38) and elevated levels of CD40 in DC (6, 7), the use of the Ad5 fiber knob and scFvG28.5 as inhibitors of infection revealed that the CD40-mediated component of overall gene transfer by the targeted vectors was higher than that involving CAR, which was observed for untargeted Ad. On another note, the scFvG28.5 constituent of the targeting protein was more efficient in directing the vector complex to DCs than was the natural ligand of CD40, CD40L, thus further supporting the choice of scFvs as targeting moieties for Ad.
FIG. 8.
Targeted transduction of human monocyte-derived DCs. DCs derived from human monocytes as described in Materials and Methods were transduced with either Ad5.DR (Fb wt) or Cd-modified Ad5.DR-LL-Cd vector. In the latter instance, the vector was used in either the untargeted form or precomplexed with one of the targeting ligands, Fc-G28.5 or Fc-CD40L. Recombinant Ad5 fiber knob and/or Fc-G28.5 proteins were added to some samples to block the interaction between the virus and the CAR or CD40, respectively. Each datum point is an average of two measurements. The error bars show standard deviations.
DISCUSSION
We describe here an attempt to develop an Ad vector targeting approach that would combine the advantages of the previously established protein bridge-mediated and genetic modification of virus tropism.
This approach was dictated by the major limitation to the genetic targeting of Ad, which otherwise remains the most straightforward and efficient way to modify Ad tropism. This limitation is the structural and biosynthetic incompatibility of the protein components of Ad capsid, including the receptor-binding fiber, with certain types of protein molecules, which could be attractive candidates as Ad targeting ligands. These candidate proteins include a number of naturally existing molecules, both secretory and anchored within the cell membrane, whose functional structure requires extensive posttranslational modifications, which are not available to the Ad proteins localized within the nucleus of infected cells. The major structural feature that limits the use of these proteins as Ad ligands is the presence of the disulfide bonds in their molecules. These bonds can only be formed in the oxidative environment of the endoplasmic reticulum (ER) by disulfide isomerases, which are residents of the ER. Soon after translation, the fiber and other proteins constituting the Ad capsid traffic to the nucleus whose reducing environment prevents the formation of disulfide bonds. Obviously, the same would hold true for any extraneous protein genetically fused with the fiber. The redirecting of the fiber to the ER, although technically feasible, does not solve the problem, since the fiber is then excluded from the assembly of the progeny Ad virions, which takes place in the nucleus. Being only theoretical until recently, these considerations have been proved lately by the experiments done by Magnusson et al. (27), who showed that two types of ligands containing disulfide bonds, the epidermal growth factor and scFv, cannot be genetically fused with the functional fiber.
This problem of the incompatibility of Ad proteins with desired targeting ligands was resolved in the present study by allowing the virus and the ligand to follow their natural biosynthetic pathways in a nonconflictual manner and, upon proper folding and assembly, associate in a functional vector complex. The study presented here establishes the feasibility of this concept by showing that individual components of such a binary system may be engineered and then put together to form a targeted vector. The molecular constituents of the mechanism of self-assembly used in our study are the Fc domain of human immunoglobulin and the Fc-binding domain of S. aureus protein A, which are used to modify the ligand and the virus, respectively. The natural affinity of the protein A for Fc thus underpins the targeted complex formation.
This vector targeting approach has been previously used to modify the tropism of Sindbis virus (30), retrovirus (29), and adenoassociated virus (33). Perhaps the major reason that precluded the use of this strategy to target Ad was the need to incorporate into the virions a substantial portion of protein A, whose size, even if minimized, exceeded that of the peptide ligands previously used for Ad targeting. The perception of the ligand-accommodating capacity of Ad virion has changed recently as a result of a study done in our laboratory (3) and also a study by Parrott and Barry (31); these studies demonstrated that relatively large polypeptide ligands may fit into the framework of the receptor-binding fiber knob domain without affecting the overall structure of the fiber. We chose to capitalize on these recent findings to incorporate a 59-amino-acid long domain C of protein A into either the HI loop or carboxy terminus of the Ad5 fiber to create a docking site for an Fc-modified targeting ligand. We demonstrated that none of the modifications affected the yield or the growth dynamics of the resultant Ad vectors and that the engineered fibers could be incorporated into mature Ad virions very efficiently. Apparently, none of these modifications caused any significant changes in the folding of the fiber, since its binding to natural Ad receptor, CAR, which requires the involvement of amino acid residues localized on two knob subunits, was not affected.
In our experimental scheme, the Fc domain of Ig fused with the ligand served a double duty: in addition to being a facilitator of the expression and secretion of the ligand, it also functioned as an element of the two-component mechanism mediating the association of the ligand with the virus. The Fc domain of Ig has long been used for the purposes of recombinant protein expression (14, 16, 19, 25). Its incorporation into the protein of interest normally results in a substantial increase in the yield of the protein and also greatly simplifies the purification of the fusion on protein A-containing matrixes. The use of this domain in our study fully met our expectations since it allowed us to produce the secretory form of the targeting ligand in substantial amounts and easily purify it by affinity chromatography. We then showed that, when mixed, the virus and the ligand undergo self-assembly into a targeting complex, which can be purified from unincorporated ligand and then stored as a ready-to-use reagent while retaining its gene delivery properties.
When tested in an in vitro gene transfer to cells of established lines, the preformed complexes of Ad with Fc-tagged anti-CD40 scFv or CD40L showed selective gene transfer to target cells via the CD40-mediated pathway. Importantly, these experiments demonstrated that association with the targeting ligand results in structural interference with the CAR binding site within the knob, thereby rendering the vector complexes truly targeted.
The subsequent use of these CD40-targeted vectors to infect human monocyte-derived DCs allowed us to demonstrate an augmentation of overall gene transfer, which was 30-fold higher than that achieved with an isogenic control Ad incorporating unmodified, wt fibers, thereby suggesting that the vectors designed in the present study may be a more efficient means of delivery of antigen-encoding genes to DCs for genetic immunization.
From the standpoint of technology development, the strategy of Ad targeting described here may be viewed as a new version of the protein bridge-based approach, which offers significant advantages over previously described methods. For instance, by providing a universal solution for the expression of secretory targeting ligands, our approach compares favorably to a previously used strategy using chemical cross-linking of antibodies to form a targeting conjugate: generation of those conjugates proved to be inefficient and thus requires large amounts of starting components. Reproducibility in the yields is also an issue. If compared to the approach using targeting fusion proteins incorporating extracellular component of CAR and a targeting moiety, the strategy presented here is more versatile since it should be applicable to Ad serotypes that do not recognize CAR and whose receptors are either unknown or not of protein nature. The high degree of the structural similarity of Ad fiber knob domains from different serotypes (12, 41, 47) predicts the compatibility of the protein A domain C with the frameworks of fiber knobs other than that of Ad5.
Although the Cd-modified Ad vectors described here were designed to be targeted with the Fc-ligand fusions, they should be fully suitable for vector targeting utilizing full size antibodies as well. A recent report by Volpers et al. (42), which described the construction and characterization of a similarly designed Ad vector, clearly showed the feasibility of such a targeting approach.
Acknowledgments
We are grateful to Dan Von Seggern for providing pDV67. We thank Jeffrey Engler for making the anti-fiber antibodies available for this study. We also thank Joanne T. Douglas for critical reading of the manuscript.
This study was supported by the U.S. Army Medical Research and Materiel Command under contract no. DAMD17-02-C-0006 and by grants P50 CA89019, 1R41CA 91608-01, and R01 CA86881.
V.K., J.T.D., and D.T.C. are equity holders in VectorLogics, Inc.
REFERENCES
- 1.Arnberg, N., K. Edlund, A. H. Kidd, and G. Wadell. 2000. Adenovirus type 37 uses sialic acid as a cellular receptor. J. Virol. 74:42-48. [PMC free article] [PubMed] [Google Scholar]
- 2.Asada-Mikami, R., Y. Heike, S. Kanai, M. Azuma, K. Shirakawa, Y. Takaue, V. Krasnykh, D. T. Curiel, M. Terada, T. Abe, and H. Wakasugi. 2001. Efficient gene transduction by RGD-fiber modified recombinant adenovirus into dendritic cells. Jpn. J. Cancer Res. 92:321-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belousova, N., V. Krendelchtchikova, D. T. Curiel, and V. Krasnykh. 2002. Modulation of adenovirus vector tropism via incorporation of polypeptide ligands into the fiber protein. J. Virol. 76:8621-8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 5.Bewley, M. C., K. Springer, Y. B. Zhang, P. Freimuth, and J. M. Flanagan. 1999. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science 286:1579-1583. [DOI] [PubMed] [Google Scholar]
- 6.Caux, C., N. Burdin, L. Galibert, P. Hermann, N. Renard, C. Servet-Delprat, and J. Banchereau. 1994. Functional CD40 on B lymphocytes and dendritic cells. Res. Immunol. 145:235-239. [DOI] [PubMed] [Google Scholar]
- 7.Caux, C., C. Massacrier, B. Vanbervliet, B. Dubois, C. Van Kooten, I. Durand, and J. Banchereau. 1994. Activation of human dendritic cells through CD40 cross-linking. J. Exp. Med. 180:1263-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chartier, C., E. Degryse, M. Gantzer, A. Dieterle, A. Pavirani, and M. Mehtali. 1996. Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J. Virol. 70:4805-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chroboczek, J., R. W. Ruigrok, and S. Cusack. 1995. Adenovirus fiber. Curr. Top. Microbiol. Immunol. 199:163-200. [DOI] [PubMed] [Google Scholar]
- 10.Defer, C., M. T. Belin, M. L. Caillet-Boudin, and P. Boulanger. 1990. Human adenovirus-host cell interactions: comparative study with members of subgroups B and C. J. Virol. 64:3661-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dmitriev, I., E. Kashentseva, B. E. Rogers, V. Krasnykh, and D. T. Curiel. 2000. Ectodomain of coxsackievirus and adenovirus receptor genetically fused to epidermal growth factor mediates adenovirus targeting to epidermal growth factor receptor-positive cells. J. Virol. 74:6875-6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durmort, C., C. Stehlin, G. Schoehn, A. Mitraki, E. Drouet, S. Cusack, and W. P. Burmeister. 2001. Structure of the fiber head of Ad3, a non-CAR-binding serotype of adenovirus. Virology 285:302-312. [DOI] [PubMed] [Google Scholar]
- 13.Freimuth, P., K. Springer, C. Berard, J. Hainfeld, M. Bewley, and J. Flanagan. 1999. Coxsackievirus and adenovirus receptor amino-terminal immunoglobulin V-related domain binds adenovirus type 2 and fiber knob from adenovirus type 12. J. Virol. 73:1392-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillies, S. D., Y. Lan, B. Brunkhorst, W. K. Wong, Y. Li, and K. M. Lo. 2002. Bi-functional cytokine fusion proteins for gene therapy and antibody-targeted treatment of cancer. Cancer Immunol. Immunother. 51:449-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham, F. L., and L. Prevec. 1995. Methods for construction of adenovirus vectors. Mol. Biotechnol. 3:207-220. [DOI] [PubMed] [Google Scholar]
- 16.Haspel, J., C. Blanco, J. Jacob, and M. Grumet. 2001. System for cleavable Fc fusion proteins using tobacco etch virus (TEV) protease. BioTechniques 30:60-66. [DOI] [PubMed] [Google Scholar]
- 17.Henry, L. J., D. Xia, M. E. Wilke, J. Deisenhofer, and R. D. Gerard. 1994. Characterization of the knob domain of the adenovirus type 5 fiber protein expressed in Escherichia coli. J. Virol. 68:5239-5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong, J. S., and J. A. Engler. 1996. Domains required for assembly of adenovirus type 2 fiber trimers. J. Virol. 70:7071-7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard, M. R., A. P. Lodge, J. E. Reed, C. J. McNamee, and D. J. Moss. 2002. High-level expression of recombinant Fc chimeric proteins in suspension cultures of stably transfected J558L cells. BioTechniques 32:1282-1286, 1288. [DOI] [PubMed] [Google Scholar]
- 20.Krasnykh, V., I. Dmitriev, G. Mikheeva, C. R. Miller, N. Belousova, and D. T. Curiel. 1998. Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J. Virol. 72:1844-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krasnykh, V., and J. T. Douglas. 2002. Targeted adenoviral vectors I: transductional targeting, p. 205-245. In D. T. Curiel and and J. T. Douglas (ed.), Adenoviral vectors for gene therapy. Academic Press, Inc., San Diego, Calif.
- 22.Krasnykh, V. N., J. T. Douglas, and V. W. van Beusechem. 2000. Genetic targeting of adenoviral vectors. Mol. Ther. 1:391-405. [DOI] [PubMed] [Google Scholar]
- 23.Krasnykh, V. N., G. V. Mikheeva, J. T. Douglas, and D. T. Curiel. 1996. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J. Virol. 70:6839-6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Law, L. K., and B. L. Davidson. 2002. Adenovirus serotype 30 fiber does not mediate transduction via the coxsackie-adenovirus receptor. J. Virol. 76:656-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo, K. M., Y. Sudo, J. Chen, Y. Li, Y. Lan, S. M. Kong, L. Chen, Q. An, and S. D. Gillies. 1998. High level expression and secretion of Fc-X fusion proteins in mammalian cells. Protein Eng. 11:495-500. [DOI] [PubMed] [Google Scholar]
- 26.Louis, N., P. Fender, A. Barge, P. Kitts, and J. Chroboczek. 1994. Cell-binding domain of adenovirus serotype 2 fiber. J. Virol. 68:4104-4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magnusson, M. K., S. See Hong, P. Henning, P. Boulanger, and L. Lindholm. 2002. Genetic retargeting of adenovirus vectors: functionality of targeting ligands and their influence on virus viability. J. Gene Med. 4:356-370. [DOI] [PubMed] [Google Scholar]
- 28.Massie, B., J. Dionne, N. Lamarche, J. Fleurent, and Y. Langelier. 1995. Improved adenovirus vector provides herpes simplex virus ribonucleotide reductase R1 and R2 subunits very efficiently. Bio/Technology 13:602-608. [DOI] [PubMed] [Google Scholar]
- 29.Ohno, K., and D. Meruelo. 1997. Retrovirus vectors displaying the IgG-binding domain of protein A. Biochem. Mol. Med. 62:123-127. [DOI] [PubMed] [Google Scholar]
- 30.Ohno, K., K. Sawai, Y. Iijima, B. Levin, and D. Meruelo. 1997. Cell-specific targeting of Sindbis virus vectors displaying IgG-binding domains of protein A. Nat. Biotechnol. 15:763-767. [DOI] [PubMed] [Google Scholar]
- 31.Parrot, M. B., and M. A. Barry. 2002. Metabolically biotinylated adenovirus vectors for targeted gene therapy. Mol. Ther. 5:S432. [DOI] [PubMed] [Google Scholar]
- 32.Pereboev, A. V., C. K. Asiedu, Y. Kawakami, S. S. Dong, J. L. Blackwell, E. A. Kashentseva, P. L. Triozzi, W. A. Aldrich, D. T. Curiel, J. M. Thomas, and I. P. Dmitriev. 2002. Coxsackievirus-adenovirus receptor genetically fused to anti-human CD40 scFv enhances adenoviral transduction of dendritic cells. Gene Ther. 9:1189-1193. [DOI] [PubMed] [Google Scholar]
- 33.Ried, M. U., A. Girod, K. Leike, H. Buning, and M. Hallek. 2002. Adeno-associated virus capsids displaying immunoglobulin-binding domains permit antibody-mediated vector retargeting to specific cell surface receptors. J. Virol. 76:4559-4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roelvink, P. W., A. Lizonova, J. G. Lee, Y. Li, J. M. Bergelson, R. W. Finberg, D. E. Brough, I. Kovesdi, and T. J. Wickham. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 72:7909-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shayakhmetov, D. M., T. Papayannopoulou, G. Stamatoyannopoulos, and A. Lieber. 2000. Efficient gene transfer into human CD34+ cells by a retargeted adenovirus vector. J. Virol. 74:2567-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shenk, T. 1995. Adenoviridae: the viruses and their replication, vol. 2. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 37.Stockwin, L. H., T. Matzow, N. T. Georgopoulos, L. J. Stanbridge, S. V. Jones, I. G. Martin, M. E. Blair-Zajdel, and G. E. Blair. 2002. Engineered expression of the coxsackie B and adenovirus receptor (CAR) in human dendritic cells enhances recombinant adenovirus-mediated gene transfer. J. Immunol. Methods 259:205-215. [DOI] [PubMed] [Google Scholar]
- 38.Tillman, B. W., T. D. de Gruijl, S. A. Luykx-de Bakker, R. J. Scheper, H. M. Pinedo, T. J. Curiel, W. R. Gerritsen, and D. T. Curiel. 1999. Maturation of dendritic cells accompanies high-efficiency gene transfer by a CD40-targeted adenoviral vector. J. Immunol. 162:6378-6383. [PubMed] [Google Scholar]
- 39.Tomko, R. P., R. Xu, and L. Philipson. 1997. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 94:3352-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Triozzi, P. L., R. Khurram, W. A. Aldrich, M. J. Walker, J. A. Kim, and S. Jaynes. 2000. Intratumoral injection of dendritic cells derived in vitro in patients with metastatic cancer. Cancer 89:2646-2654. [DOI] [PubMed] [Google Scholar]
- 41.van Raaij, M. J., N. Louis, J. Chroboczek, and S. Cusack. 1999. Structure of the human adenovirus serotype 2 fiber head domain at 1.5 Å resolution. Virology 262:333-343. [DOI] [PubMed] [Google Scholar]
- 42.Volpers, C., C. Thirion, V. Biermann, S. Hussmann, H. Kewes, P. Dunant, H. von der Mark, A. Herrmann, S. Kochanek, and H. Lochmuller. 2003. Antibody-mediated targeting of an adenovirus vector modified to contain a synthetic immunoglobulin G-binding domain in the capsid. J. Virol. 77:2093-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Von Seggern, D. J., S. Huang, S. K. Fleck, S. C. Stevenson, and G. R. Nemerow. 2000. Adenovirus vector pseudotyping in fiber-expressing cell lines: improved transduction of Epstein-Barr virus-transformed B cells. J. Virol. 74:354-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, K., S. Huang, A. Kapoor-Munshi, and G. Nemerow. 1998. Adenovirus internalization and infection require dynamin. J. Virol. 72:3455-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wickham, T. J., E. J. Filardo, D. A. Cheresh, and G. R. Nemerow. 1994. Integrin αvβ5 selectively promotes adenovirus mediated cell membrane permeabilization. J. Cell Biol. 127:257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]
- 47.Xia, D., L. J. Henry, R. D. Gerard, and J. Deisenhofer. 1994. Crystal structure of the receptor-binding domain of adenovirus type 5 fiber protein at 1.7 Å resolution. Structure 2:1259-1270. [DOI] [PubMed] [Google Scholar]