Abstract
The title compound, C15H10Br2Cl2N2, a 2,8-dibromo-4,10-dichloro Tröger’s base analogue derived from 4-bromo-2-chloroaniline, has a dihedral angle of 110.9 (10)° between the two aryl rings, the largest yet measured for a simple dibenzo analogue.
Related literature
For related literature on the synthesis and crystal structures of dihalogenated Tröger’s base analogues, see: Jensen & Wärnmark (2001 ▶); Faroughi et al. (2006a
▶, 2007a
▶,b
▶). For Tröger’s base analogues substituted with multiple electron-withdrawing groups, see: Faroughi et al. (2006b
▶); Bhuiyan et al. (2006 ▶, 2007 ▶); Vande Velde et al. (2008 ▶). For reactions of halogenated Tröger’s base analogues, see: Jensen et al. (2002 ▶); Hof et al. (2005 ▶). For literature on racemization of Tröger’s base analogues and the effect of substituents ortho to the diazocine N atoms, see: Lenev et al. (2006 ▶).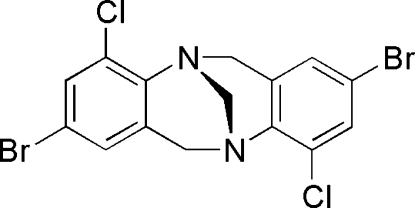
Experimental
Crystal data
C15H10Br2Cl2N2
M r = 449.0
Orthorhombic,
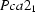
a = 7.910 (2) Å
b = 12.601 (3) Å
c = 15.230 (4) Å
V = 1518.0 (7) Å3
Z = 4
Mo Kα radiation
μ = 5.64 mm−1
T = 294 K
0.30 × 0.12 × 0.07 mm
Data collection
Enraf–Nonius CAD-4 diffractometer
Absorption correction: analytical (de Meulenaer & Tompa, 1965 ▶) T min = 0.52, T max = 0.69
1394 measured reflections
1394 independent reflections
1028 reflections with I > 2σ(I)
1 standard reflection frequency: 30 min intensity decay: none
Refinement
R[F 2 > 2σ(F 2)] = 0.056
wR(F 2) = 0.061
S = 1.61
1394 reflections
189 parameters
H-atom parameters constrained
Δρmax = 0.98 e Å−3
Δρmin = −1.02 e Å−3
Absolute structure: Flack (1983 ▶)
Flack parameter: 0.09 (2)
Data collection: CAD-4 (Schagen et al., 1989 ▶); cell refinement: CAD-4; data reduction: local program; program(s) used to solve structure: SIR92 (Altomare et al., 1994 ▶); program(s) used to refine structure: RAELS (Rae, 1996 ▶); molecular graphics: ORTEPII (Johnson, 1976 ▶); software used to prepare material for publication: local programs.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808026226/tk2290sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808026226/tk2290Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors thank Macquarie University for the award of a Macquarie University Research Development Grant to ACT.
supplementary crystallographic information
Comment
Tröger's base analogues bearing electron-withdrawing groups were long thought to be difficult, if not impossible, to prepare. However, the synthesis of dihalogenated (Jensen & Wärnmark, 2001), octafluoro (Vande Velde et al., 2008) and tetranitro (Bhuiyan et al., 2007) Tröger's base analogues highlight the possiblities that now exist in terms of incorporating electron-withdrawing groups on the starting anilines. The synthetic utility of halogen-substituted Tröger's base analogue has been demonstrated with their conversion to alkyne- (Jensen & Wärnmark, 2001; Jensen et al., 2002) and functionalized phenyl- (Hof et al., 2005) substituted analogues, among others. It is noteworthy that crystal structures of several other 2,4,8,10-tetrasubstituted Tröger's base analogues exhibit large dihedral angles that are close to that in (I). Tröger's base analogues are known to undergo racemization in acidic solution, however the presence of a substituent at the ortho-position, relative to the bridge nitrogen atoms, has been shown to increase the racemization barrier (Lenev et al., 2006).
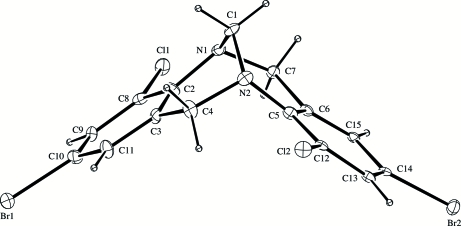
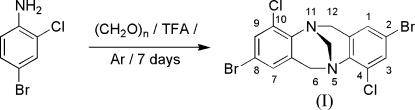
The molecular structure of (I) is shown in Fig. 1 and it was prepared as outlined in Fig. 2.
Experimental
4-Bromo-2-chloroaniline (1 g, 4.84 mmol) and paraformaldehyde (232 mg, 7.74 mmol) were added to an ice-cold solution of trifluoroacetic acid (10 ml). The reaction mixture was then stirred in dark at room temperature for 7 days under an atmosphere of argon. The ice-cold reaction mixture was basified by the dropwise addition of a mixture of ammonia (28%, 20 ml) and water (40 ml), followed by the additon of a saturated sodium hydrogen carbonate solution (20 ml). The resultant mixture was then extracted with dichloromethane (3 x 20 ml) and the combined organic layers were washed with brine (40 ml), dried over anhydrous sodium sulfate, filtered and evaporated to dryness. The crude product was chromatographed (silica gel, dichloromethane:hexane 8:2) to afford 2,8-dibromo-4,10-dichloro-6H,12H-5,11-methanodibenzo [b,f][1,5]diazocine (I) (613 mg, 56%) as a white solid and as a racemic mixture: m.p. 471–472 K; 1H NMR (400 MHz, CDCl3) δ 4.21–4.33 (4H, m), 4.55 (2H, d, J 17.3 Hz), 7.04 (2H, d, J 2.1 Hz), 7.41 (2H, d, J 2.1 Hz); 13C NMR (100 MHz, CDCl3) δ 54.37, 67.32, 117.15, 128.49, 130.17, 131.02, 131.71, 142.33. Analysis found: C 40.46; H 2.22; N 6.46; C15H10Br2Cl2N2 requires C 40.13; H 2.25; N 6.24. Single crystals were obtained from slow evaporation from dichloromethane solution of (I).
Refinement
Hydrogen atoms were included in positions calculated each cycle (C—H = 1.0 Å), and were assigned thermal parameters equal to their bonded atom. The maximum and minimum electron density peaks were located 0.73 and 1.20Å from the Cl2 and Br1 atoms, respectively.
Figures
Fig. 1.
ORTEPII (Johnson, 1976) plot of (I), with ellipsoids at the 10% probability level. H atoms are drawn as spheres of arbitrary radius.
Fig. 2.
Synthetic scheme for the synthesis of (I) showing the numbering system used in naming the compound.
Crystal data
| C15H10Br2Cl2N2 | Dx = 1.96 Mg m−3 |
| Mr = 449.0 | Melting point: 471 K |
| Orthorhombic, Pca21 | Mo Kα radiation λ = 0.71073 Å |
| Hall symbol: P 2c -2ac | Cell parameters from 11 reflections |
| a = 7.910 (2) Å | θ = 10–11º |
| b = 12.601 (3) Å | µ = 5.64 mm−1 |
| c = 15.230 (4) Å | T = 294 K |
| V = 1518.0 (7) Å3 | Prism, colourless |
| Z = 4 | 0.30 × 0.12 × 0.07 mm |
| F000 = 872.0 |
Data collection
| Enraf–Nonius CAD-4 diffractometer | θmax = 25º |
| ω–2θ scans | h = 0→9 |
| Absorption correction: analyticalde Meulenaer & Tompa (1965) | k = 0→14 |
| Tmin = 0.52, Tmax = 0.69 | l = −18→0 |
| 1394 measured reflections | 1 standard reflections |
| 1394 independent reflections | every 30 min |
| 1028 reflections with I > 2σ(I) | intensity decay: none |
Refinement
| Refinement on F | w = 1/[σ2(F) + 0.0004F2] |
| R[F2 > 2σ(F2)] = 0.056 | (Δ/σ)max = 0.002 |
| wR(F2) = 0.061 | Δρmax = 0.98 e Å−3 |
| S = 1.61 | Δρmin = −1.02 e Å−3 |
| 1394 reflections | Extinction correction: none |
| 189 parameters | Absolute structure: Flack (1983), 0 Friedel pairs |
| H-atom parameters constrained | Flack parameter: 0.09 (2) |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Br1 | 0.8061 (2) | 0.6867 (1) | 0.3991 (1) | 0.0585 (5) | |
| Br2 | 0.2324 (2) | 0.0800 (1) | 0.0428 (2) | 0.0527 (5) | |
| Cl1 | 0.9770 (5) | 0.5167 (4) | 0.0779 (3) | 0.057 (1) | |
| Cl2 | 0.5654 (5) | 0.0565 (3) | 0.3603 (3) | 0.052 (1) | |
| N1 | 0.9460 (14) | 0.3064 (10) | 0.1658 (8) | 0.041 (3) | |
| N2 | 0.8319 (15) | 0.1842 (9) | 0.2739 (8) | 0.044 (3) | |
| C1 | 0.9798 (19) | 0.2123 (12) | 0.2198 (10) | 0.044 (4) | |
| C2 | 0.8998 (18) | 0.3929 (12) | 0.2193 (10) | 0.043 (4) | |
| C3 | 0.8445 (18) | 0.3790 (11) | 0.3054 (8) | 0.041 (4) | |
| C4 | 0.817 (2) | 0.2693 (12) | 0.3414 (8) | 0.049 (4) | |
| C5 | 0.6842 (19) | 0.1696 (11) | 0.2226 (9) | 0.039 (4) | |
| C6 | 0.6740 (19) | 0.2086 (11) | 0.1360 (9) | 0.036 (4) | |
| C7 | 0.8206 (17) | 0.2753 (12) | 0.0978 (9) | 0.040 (4) | |
| C8 | 0.9155 (17) | 0.4984 (13) | 0.1855 (9) | 0.043 (4) | |
| C9 | 0.8825 (19) | 0.5861 (12) | 0.2397 (11) | 0.048 (4) | |
| C10 | 0.839 (2) | 0.5684 (13) | 0.3247 (11) | 0.050 (4) | |
| C11 | 0.818 (2) | 0.4666 (13) | 0.3570 (10) | 0.056 (4) | |
| C12 | 0.5565 (17) | 0.1082 (10) | 0.2538 (8) | 0.031 (3) | |
| C13 | 0.4158 (17) | 0.0854 (10) | 0.2036 (8) | 0.035 (3) | |
| C14 | 0.4117 (17) | 0.1221 (11) | 0.1168 (9) | 0.040 (4) | |
| C15 | 0.5433 (19) | 0.1819 (10) | 0.0853 (9) | 0.034 (3) | |
| H1C1 | 1.0780 | 0.2273 | 0.2593 | 0.044 | |
| H2C1 | 1.0078 | 0.1513 | 0.1804 | 0.044 | |
| H1C4 | 0.7008 | 0.2660 | 0.3674 | 0.049 | |
| H2C4 | 0.9027 | 0.2559 | 0.3883 | 0.049 | |
| H1C7 | 0.7726 | 0.3410 | 0.0708 | 0.040 | |
| H2C7 | 0.8794 | 0.2326 | 0.0517 | 0.040 | |
| HC9 | 0.8906 | 0.6600 | 0.2162 | 0.048 | |
| HC11 | 0.7825 | 0.4567 | 0.4195 | 0.056 | |
| HC13 | 0.3194 | 0.0438 | 0.2286 | 0.035 | |
| HC15 | 0.5411 | 0.2061 | 0.0228 | 0.034 |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Br1 | 0.060 (1) | 0.060 (1) | 0.0549 (9) | −0.0011 (8) | −0.001 (1) | −0.0138 (9) |
| Br2 | 0.0470 (9) | 0.0650 (9) | 0.0461 (8) | −0.0118 (8) | −0.0026 (9) | −0.0091 (9) |
| Cl1 | 0.061 (3) | 0.066 (3) | 0.044 (2) | −0.019 (2) | 0.010 (2) | 0.000 (2) |
| Cl2 | 0.063 (3) | 0.056 (2) | 0.037 (2) | 0.000 (2) | 0.006 (2) | 0.018 (2) |
| N1 | 0.029 (7) | 0.058 (8) | 0.037 (7) | 0.001 (6) | 0.002 (6) | −0.003 (6) |
| N2 | 0.046 (7) | 0.048 (7) | 0.038 (7) | 0.004 (6) | −0.009 (6) | 0.020 (6) |
| C1 | 0.029 (9) | 0.067 (9) | 0.036 (8) | 0.005 (8) | −0.016 (7) | 0.012 (8) |
| C2 | 0.051 (9) | 0.043 (9) | 0.034 (8) | 0.009 (8) | 0.020 (8) | −0.006 (7) |
| C3 | 0.045 (9) | 0.059 (9) | 0.019 (8) | −0.012 (8) | −0.004 (7) | −0.005 (7) |
| C4 | 0.083 (9) | 0.053 (8) | 0.013 (7) | 0.004 (9) | −0.006 (7) | 0.007 (7) |
| C5 | 0.048 (9) | 0.043 (9) | 0.026 (7) | 0.000 (7) | 0.013 (7) | 0.007 (7) |
| C6 | 0.039 (8) | 0.042 (8) | 0.026 (8) | 0.010 (8) | 0.002 (6) | −0.006 (7) |
| C7 | 0.037 (8) | 0.052 (9) | 0.030 (8) | −0.007 (8) | 0.007 (7) | −0.002 (7) |
| C8 | 0.029 (8) | 0.062 (9) | 0.039 (9) | −0.002 (8) | −0.002 (7) | 0.004 (9) |
| C9 | 0.047 (9) | 0.038 (9) | 0.059 (9) | −0.010 (8) | −0.001 (8) | −0.007 (8) |
| C10 | 0.047 (9) | 0.057 (9) | 0.046 (9) | −0.005 (8) | 0.017 (8) | −0.007 (8) |
| C11 | 0.085 (9) | 0.052 (9) | 0.029 (8) | −0.011 (9) | 0.009 (9) | −0.007 (8) |
| C12 | 0.037 (8) | 0.032 (8) | 0.024 (7) | 0.009 (7) | 0.010 (6) | −0.002 (6) |
| C13 | 0.046 (9) | 0.043 (9) | 0.016 (6) | 0.014 (8) | 0.001 (6) | 0.000 (7) |
| C14 | 0.029 (8) | 0.039 (8) | 0.052 (9) | 0.006 (7) | 0.010 (7) | −0.015 (8) |
| C15 | 0.034 (8) | 0.039 (8) | 0.027 (7) | 0.004 (7) | 0.009 (7) | −0.004 (7) |
Geometric parameters (Å, °)
| Br1—C10 | 1.890 (15) | C4—H2C4 | 1.000 |
| Br2—C14 | 1.888 (14) | C5—C6 | 1.410 (18) |
| Cl1—C8 | 1.724 (14) | C5—C12 | 1.358 (18) |
| Cl2—C12 | 1.750 (13) | C6—C7 | 1.546 (20) |
| N1—C1 | 1.468 (18) | C6—C15 | 1.333 (19) |
| N1—C2 | 1.409 (17) | C7—H1C7 | 1.000 |
| N1—C7 | 1.487 (18) | C7—H2C7 | 1.000 |
| N2—C1 | 1.474 (19) | C8—C9 | 1.404 (20) |
| N2—C4 | 1.491 (18) | C9—C10 | 1.357 (19) |
| N2—C5 | 1.417 (18) | C9—HC9 | 1.000 |
| C1—H1C1 | 1.000 | C10—C11 | 1.385 (21) |
| C1—H2C1 | 1.000 | C11—HC11 | 1.000 |
| C2—C3 | 1.394 (18) | C12—C13 | 1.381 (17) |
| C2—C8 | 1.431 (20) | C13—C14 | 1.402 (18) |
| C3—C4 | 1.503 (20) | C13—HC13 | 1.000 |
| C3—C11 | 1.371 (20) | C14—C15 | 1.371 (19) |
| C4—H1C4 | 1.000 | C15—HC15 | 1.000 |
| C1—N1—C2 | 110.4 (12) | N1—C7—C6 | 112.5 (11) |
| C1—N1—C7 | 107.4 (11) | N1—C7—H1C7 | 108.7 |
| C2—N1—C7 | 115.7 (11) | N1—C7—H2C7 | 108.7 |
| C1—N2—C4 | 106.0 (11) | C6—C7—H1C7 | 108.7 |
| C1—N2—C5 | 112.2 (11) | C6—C7—H2C7 | 108.7 |
| C4—N2—C5 | 114.1 (12) | H1C7—C7—H2C7 | 109.5 |
| N1—C1—N2 | 111.3 (11) | Cl1—C8—C2 | 119.3 (11) |
| N1—C1—H1C1 | 109.0 | Cl1—C8—C9 | 120.4 (12) |
| N1—C1—H2C1 | 109.0 | C2—C8—C9 | 120.2 (12) |
| N2—C1—H1C1 | 109.0 | C8—C9—C10 | 118.6 (15) |
| N2—C1—H2C1 | 109.0 | C8—C9—HC9 | 120.7 |
| H1C1—C1—H2C1 | 109.5 | C10—C9—HC9 | 120.7 |
| N1—C2—C3 | 121.9 (14) | Br1—C10—C9 | 118.5 (13) |
| N1—C2—C8 | 119.2 (12) | Br1—C10—C11 | 120.0 (11) |
| C3—C2—C8 | 118.8 (13) | C9—C10—C11 | 121.4 (15) |
| C2—C3—C4 | 120.3 (13) | C3—C11—C10 | 121.6 (14) |
| C2—C3—C11 | 119.1 (14) | C3—C11—HC11 | 119.2 |
| C4—C3—C11 | 120.6 (12) | C10—C11—HC11 | 119.2 |
| N2—C4—C3 | 113.5 (10) | Cl2—C12—C5 | 120.5 (12) |
| N2—C4—H1C4 | 108.4 | Cl2—C12—C13 | 117.9 (10) |
| N2—C4—H2C4 | 108.4 | C5—C12—C13 | 121.6 (13) |
| C3—C4—H1C4 | 108.4 | C12—C13—C14 | 118.2 (13) |
| C3—C4—H2C4 | 108.4 | C12—C13—HC13 | 120.9 |
| H1C4—C4—H2C4 | 109.5 | C14—C13—HC13 | 120.9 |
| N2—C5—C6 | 121.2 (13) | Br2—C14—C13 | 119.2 (11) |
| N2—C5—C12 | 119.6 (13) | Br2—C14—C15 | 121.0 (11) |
| C6—C5—C12 | 118.9 (15) | C13—C14—C15 | 119.6 (13) |
| C5—C6—C7 | 119.8 (13) | C6—C15—C14 | 121.6 (13) |
| C5—C6—C15 | 119.9 (14) | C6—C15—HC15 | 119.2 |
| C7—C6—C15 | 120.1 (12) | C14—C15—HC15 | 119.2 |
| C2—N1—C1—N2 | 57.5 (15) | C2—C3—C11—C10 | 2.3 (24) |
| C2—N1—C1—H1C1 | −62.8 | C2—C3—C11—HC11 | −177.7 |
| C2—N1—C1—H2C1 | 177.8 | C4—C3—C11—C10 | −178.6 (16) |
| C7—N1—C1—N2 | −69.4 (15) | C4—C3—C11—HC11 | 1.4 |
| C7—N1—C1—H1C1 | 170.3 | N2—C5—C6—C7 | −4.4 (20) |
| C7—N1—C1—H2C1 | 50.8 | N2—C5—C6—C15 | 171.2 (13) |
| C1—N1—C2—C3 | −18.2 (18) | C12—C5—C6—C7 | −177.9 (12) |
| C1—N1—C2—C8 | 159.5 (13) | C12—C5—C6—C15 | −2.3 (20) |
| C7—N1—C2—C3 | 103.9 (16) | N2—C5—C12—Cl2 | 5.4 (18) |
| C7—N1—C2—C8 | −78.3 (17) | N2—C5—C12—C13 | −175.2 (12) |
| C1—N1—C7—C6 | 44.6 (15) | C6—C5—C12—Cl2 | 179.1 (10) |
| C1—N1—C7—H1C7 | 165.1 | C6—C5—C12—C13 | −1.6 (20) |
| C1—N1—C7—H2C7 | −75.8 | C5—C6—C7—N1 | −10.2 (17) |
| C2—N1—C7—C6 | −79.1 (15) | C5—C6—C7—H1C7 | −130.7 |
| C2—N1—C7—H1C7 | 41.3 | C5—C6—C7—H2C7 | 110.2 |
| C2—N1—C7—H2C7 | 160.4 | C15—C6—C7—N1 | 174.2 (13) |
| C4—N2—C1—N1 | −69.8 (13) | C15—C6—C7—H1C7 | 53.8 |
| C4—N2—C1—H1C1 | 50.5 | C15—C6—C7—H2C7 | −65.3 |
| C4—N2—C1—H2C1 | 169.9 | C5—C6—C15—C14 | 3.9 (21) |
| C5—N2—C1—N1 | 55.3 (16) | C5—C6—C15—HC15 | −176.1 |
| C5—N2—C1—H1C1 | 175.6 | C7—C6—C15—C14 | 179.5 (12) |
| C5—N2—C1—H2C1 | −65.0 | C7—C6—C15—HC15 | −0.5 |
| C1—N2—C4—C3 | 42.4 (15) | Cl1—C8—C9—C10 | −178.1 (12) |
| C1—N2—C4—H1C4 | 163.0 | Cl1—C8—C9—HC9 | 1.9 |
| C1—N2—C4—H2C4 | −78.2 | C2—C8—C9—C10 | 1.4 (23) |
| C5—N2—C4—C3 | −81.5 (16) | C2—C8—C9—HC9 | −178.6 |
| C5—N2—C4—H1C4 | 39.1 | C8—C9—C10—Br1 | 176.8 (11) |
| C5—N2—C4—H2C4 | 157.9 | C8—C9—C10—C11 | −3.7 (25) |
| C1—N2—C5—C6 | −17.3 (19) | HC9—C9—C10—Br1 | −3.2 |
| C1—N2—C5—C12 | 156.2 (13) | HC9—C9—C10—C11 | 176.3 |
| C4—N2—C5—C6 | 103.2 (14) | Br1—C10—C11—C3 | −178.6 (12) |
| C4—N2—C5—C12 | −83.3 (16) | Br1—C10—C11—HC11 | 1.4 |
| N1—C2—C3—C4 | −5.9 (21) | C9—C10—C11—C3 | 1.9 (26) |
| N1—C2—C3—C11 | 173.3 (14) | C9—C10—C11—HC11 | −178.1 |
| C8—C2—C3—C4 | 176.4 (14) | Cl2—C12—C13—C14 | −176.9 (10) |
| C8—C2—C3—C11 | −4.4 (21) | Cl2—C12—C13—HC13 | 3.1 |
| N1—C2—C8—Cl1 | 4.3 (19) | C5—C12—C13—C14 | 3.7 (19) |
| N1—C2—C8—C9 | −175.2 (13) | C5—C12—C13—HC13 | −176.3 |
| C3—C2—C8—Cl1 | −177.9 (11) | C12—C13—C14—Br2 | 173.5 (9) |
| C3—C2—C8—C9 | 2.6 (21) | C12—C13—C14—C15 | −2.1 (18) |
| C2—C3—C4—N2 | −7.5 (20) | HC13—C13—C14—Br2 | −6.5 |
| C2—C3—C4—H1C4 | −128.1 | HC13—C13—C14—C15 | 177.9 |
| C2—C3—C4—H2C4 | 113.1 | Br2—C14—C15—C6 | −177.3 (11) |
| C11—C3—C4—N2 | 173.3 (14) | Br2—C14—C15—HC15 | 2.7 |
| C11—C3—C4—H1C4 | 52.7 | C13—C14—C15—C6 | −1.7 (20) |
| C11—C3—C4—H2C4 | −66.1 | C13—C14—C15—HC15 | 178.3 |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: TK2290).
References
- Altomare, A., Cascarano, G., Giacovazzo, C., Guagliardi, A., Burla, M. C., Polidori, G. & Camalli, M. (1994). J. Appl. Cryst.27, 435.
- Bhuiyan, M. D. H., Jensen, P. & Try, A. C. (2007). Acta Cryst. E63, o4393.
- Bhuiyan, M. D. H., Try, A. C., Klepetko, J. & Turner, P. (2006). Acta Cryst. E62, o4887–o4888.
- Faroughi, M., Try, A. C., Klepetko, J. & Turner, P. (2007a). Tetrahedron Lett.48, 6548–6551.
- Faroughi, M., Try, A. C. & Turner, P. (2006a). Acta Cryst. E62, o3674–o3675.
- Faroughi, M., Try, A. C. & Turner, P. (2006b). Acta Cryst. E62, o3893–o3894.
- Faroughi, M., Try, A. C. & Turner, P. (2007b). Acta Cryst. E63, o2695.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Hof, F., Schar, M., Scofield, D. M., Fischer, F., Diederich, F. & Sergeyev, S. (2005). Helv. Chim. Acta, 88, 2333–2344.
- Jensen, J., Strozyk, M. & Wärnmark, K. (2002). Synthesis, pp. 2761–2765.
- Jensen, J. & Wärnmark, K. (2001). Synthesis, pp. 1873–1877.
- Johnson, C. K. (1976). ORTEPII Report ORNL-5138. Oak Ridge National Laboratory, Tennessee, USA.
- Lenev, D. A., Lyssenko, K. A., Golovanov, D. G., Buss, V. & Kostyanovsky, R. G. (2006). Chem. Eur. J.12, 6412–6418. [DOI] [PubMed]
- Meulenaer, J. de & Tompa, H. (1965). Acta Cryst.19, 1014–1018.
- Rae, A. D. (1996). RAELS University of New South Wales, Australia.
- Schagen, J. D., Straver, L., van Meurs, F. & Williams, G. (1989). CAD-4 Manual Enraf–Nonius, Delft, The Netherlands.
- Vande Velde, C. M. L., Didier, D., Blockhuys, F. & Sergeyev, S. (2008). Acta Cryst. E64, o538. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808026226/tk2290sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808026226/tk2290Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report