Abstract
Many adenovirus serotypes enter cells by high-affinity binding to the coxsackievirus-adenovirus receptor (CAR) and integrin-mediated internalization. In the present study, we analyzed the possible receptor function of α3β1 for adenovirus serotype 5 (Ad5). We found that penton base and integrin α3β1 could interact in vitro. In vivo, both Ad5-cell binding and virus-mediated transduction were inhibited in the presence of anti-α3 and anti-β1 function-blocking antibodies, and this occurred in both CAR-positive and CAR-negative cell lines. Peptide library screenings and data from binding experiments with wild-type and mutant penton base proteins suggest that the Arg-Gly-Asp (RGD) in the penton base protein, the best known integrin binding motif, is only part of the binding interface with α3β1, which involved multiple additional contact sites.
Adenovirus (Ad) host cell entry requires an initial attachment to cells which is mediated by the fiber interaction with the coxsackievirus-Ad receptor (CAR) (2). The subsequent association of the capsid protein penton base with integrin molecules promotes Ad entry (31). Integrins are a family of structurally and functionally related cell surface heterodimeric receptors that mediate cell migration and adhesion. The major extracellular ligands for integrins are collagens, laminins, fibronectin, tenascin, vitronectin, von Willebrand factor, and fibrinogen, reflecting the primary function of integrins in cell adhesion to the extracellular matrix. The αvβ1, -3, -5, -6, and -8 integrins, the α5β1 and α8β1 integrins, and the αIIbβ3 integrins form a subgroup that primarily recognizes ligands containing Arg-Gly-Asp (RGD) motifs (see reference 13 and references therein). Many microorganisms utilize integrins to gain entry into cells: the SA11 rotavirus binds to α2β1 and α4β1 (9), αvβ3 and αvβ1 integrins are receptors of the human parechovirus 1 (30), and αvβ5 has been proposed, although not conclusively, as a coreceptor in adeno-associated virus type 2 infection (27, 29). The foot-and-mouth disease virus uses different integrins for cell infection (14, 15, 16). Integrin α3β1 is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (1). Yersinia pseudotuberculosis binds to members of the β1 integrin family in order to enter eukaryotic cells (22).
Several Ad serotypes contain an RGD motif in the penton base protein. This feature, and the Ad cell-detaching property, suggested an interaction of the virus with the integrin receptors. Indeed, αvβ3 and αvβ5 are receptors for human Ad2 and Ad5, and direct binding to isolated αvβ5 was shown for human Ad2, Ad3, Ad4, Ad5, and Ad37 (24, 31). In hematopoietic and melanoma cells, respectively, the αMβ2 and b1 integrins were found to be implicated in human Ad5 infection (3, 12). More recent evidence indicates αvβ1 as an Ad2 and Ad5 coreceptor in the human embryonic kidney (HEK293) cell line (23). Ad interaction with the αvβ1, -3, and -5 integrin subtypes is efficiently competed by RGD-containing peptides (23, 31). A second integrin binding motif is present in the penton base protein of several Ad serotypes, the triplet Leu-Asp-Val (LDV). Its functional role in the interaction with the target cell was demonstrated by Karayan and coworkers, who showed a significant reduced effect of cell detachment of the Ad5 D288K penton base mutant protein (17).
Previous work has shown that a recombinant filamentous phage displaying the human Ad2 penton base protein bound not only to integrins αvβ3 and αvβ5 but also to the subtype α3β1 (4). This integrin is primarily a receptor for laminin, although it recognizes additional ligands, such as collagens, epiligrin, thrombospondin, and fibronectin. α3β1 is widely expressed on nearly all tissue types and is particularly abundant on endothelial and epithelial cells. It is also found on nearly all rapidly growing adherent cell lines (21). α3β1 is an enigmatic integrin subtype, since it can recognize ligands in both RGD-dependent and RGD-independent manners (5, 8).
In this study, we investigated the interaction between human Ad and the integrin subtype α3β1. We present evidence that this surface molecule binds to the capsid protein penton base. In addition, α3β1 seems to be involved in viral infection in both CAR-positive and CAR-negative cells. To identify the amino acid residues implicated in the interaction, we screened a random peptide library for integrin α3β1. The results of this screening and the results of binding and binding competition experiments suggest that the LDV tripeptide is not involved in the penton base-α3β1 interaction and that the RGD motif is only part of multiple binding sites between the penton base and integrin α3β1.
The Ad penton base specifically binds to α3β1 in vitro.
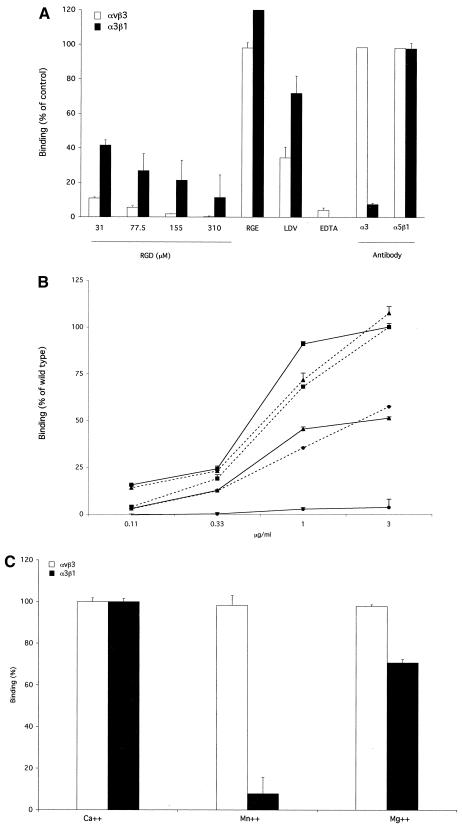
In order to validate the biological significance of the data obtained with α3β1 with recombinant phage (4), we analyzed the integrin binding pattern of purified wild-type (WT) penton base produced as a recombinant protein in insect cells by using recombinant baculovirus. The penton base protein produced in this system keeps its biological properties, i.e., its ability to form pentamers, to bind to fiber, to form penton capsomers, to bind to integrin receptors, to undergo endocytosis, and to migrate to the nuclear pore complex (10). The α3β1 integrin was coated, and plates were incubated with recombinant penton base protein alone or in the presence of different competitors. Bound penton base was detected by the addition of primary anti-Ad antiserum and secondary horseradish peroxidase-conjugated anti-rabbit monoclonal antibody (MAb). For comparison, penton base binding was tested on the immobilized Ad receptor αvβ3. As shown in Fig. 1A, penton base binding to α3β1 was inhibited in the presence of the GRGDSP peptide. However, the competition was substantially weaker than that observed with the αvβ3 receptor. The control peptide GRGESP was not able to significantly affect the binding of the penton base to either of the two integrins tested. The LDV motif has been suggested to act as a possible alternative integrin ligand in Ad penton base, and LDV occurs in the human Ad2 and Ad5 penton bases at positions 287 to 289. In the presence of the peptide PALLDVDA, we observed a 60 to 70% reduction of penton base binding to αvβ3 but only a 25% reduction for α3β1. These data suggest that RGD motifs are involved to a limited extent only in the physical interaction between the penton base and α3β1 and that the LDV motifs have an even more minor role, if any, in this interaction. To check for specificity, binding to both integrins was tested in the presence of anti-α3 antibody. As expected, anti-α3 antibody competed with the penton base for binding to α3β1only. As a negative control, anti-α5β1 antibody was used. The binding of the penton base to αvβ3 and α3β1 was not affected by the addition of this antibody.
FIG. 1.
Binding of purified penton base to immobilized integrins. Ninety-six well plates were coated with purified integrins (0.1 μg/well; Chemicon International, Temecula, Calif.), and the plates were incubated with recombinant, WT, R340E mutant, and D288K mutant penton base proteins (at a concentration of 1 μg/ml in panels A and C and as indicated in panel B [10]) in the presence of different competitors. Followingincubation with anti-Ad anti-serum (10) and secondary incubation with an anti-rabbit horeradish peroxidase-conjugated antibody (Amersham Biosciences, Uppsala, Sweden), binding was analyzed by determining the optical density at 450 nm. Data are average values of results for three different experiments performed with duplicate samples + standard deviations. (A) Results are expressed as percentages of penton base binding values obtained in the absence of competitors. RGD, GRGDSP peptide (Invitrogen Italia SRL, Milan, Italy); RGE, GRGESP peptide at 392 μM (Gibco Invitrogen); LDV, PALLDVDA peptide at 392 μM (Primm, Milan, Italy); EDTA, 20 mM; α3, anti-α3 antibody P1B5 (Chemicon Int.); α5β1, anti-α5β1 antibody JBS5 (Chemicon International). White bars, integrin αvβ3; black bars, integrin α3β1. (B) Results are expressed as percentages of values obtained with 3 μg of the WT penton base protein/ml. Squares, WT penton base protein; circles, R340E penton base protein; triangles, D288K penton base protein. Solid lines, binding to αvβ3; dotted lines, binding to α3β1. (C) Results are expressed as percentages of penton base binding values obtained in the presence of CaCl2 at a concentration of 1 mM. For Ca2+ results, CaCl2 was used at 1 mM; for Mn2+ results, MnCl2 was used at 2 mM; Mg2+ results, MgCl2 was used at 2 mM. White bars, integrin αvβ3; black bars, integrin α3β1.
In order to confirm that the RGD and LDV motifs in the penton base do not play crucial roles in the interaction with the α3β1 integrin, we analyzed the binding to αvβ3 and α3β1 of penton base protein mutants modified in the RGD or LDV peptides. As shown in Fig. 1B, the R340E mutation only partially impaired the penton base-α3β1 interaction, whereas the D288K mutation had no detectable effect. Both mutants bound to αvβ3 with lower affinity than did the WT penton base, as previously reported (17).
It has been suggested that α3β1 may have multiple binding sites, or at least multiple ligand binding mechanisms. In particular, it has been demonstrated that binding to laminin or collagen depends on Ca2+. Conversely, the interaction with fibronectin is favored in the presence of Mn2+ (5, 8). We found that the binding of the penton base to integrin α3β1, as well as to αvβ3, was completely abolished by the chelating agent EDTA (20 mM), indicating that both interactions are ion dependent (Fig. 1A). However, the pattern of binding in the presence of different ions was different for the two receptors. For α3β1, the binding that was observed in the presence of Ca2+ was better than that in Mg2+, while Mn2+ was unable to support binding (Fig. 1C). On αvβ3, Ca2+, Mn2+, and Mg2+ all allowed penton base binding. Taken together, these results suggest that there is a specific, ion-dependent interaction between Ad2 penton base and integrin α3β1.
Peptide ligands of integrin α3β1 searched by phage biopanning.
A previous study showed that a filamentous phage displaying the full-length Ad2 penton base bound more efficiently to α3β1 than a phage displaying only the region spanning residues 286 to 392 (4). Considering that both RGD and LDV motifs are included in the residue 286-to-392 fragment, this result suggests that other amino acid residues may be implicated in the interaction between Ad and the α3β1 integrin. This is in accordance with the data of competition experiments depicted in Fig. 1. First, GRGDSP and PALLDVDA peptides are inefficient competitors of the penton base-α3β1 interaction. Second, Ca2+ favors the binding to the penton base, a condition that, for natural α3β1 ligands, is related to RGD-independent reactions (5, 8). Third, the R340E mutation only slightly impaired penton base binding to α3β1 integrin, and the D288K mutation showed no effect.
To gain insight into the molecular nature of the α3β1 binding reaction, we biopanned a phage-displayed hexapeptide library for immobilized α3β1 integrin (28). Several rounds of panning were performed on α3β1, and elution of bound phages was performed under different conditions. It was first performed with acidic buffer to identify the major peptide ligand(s) of this integrin (28), and then it was performed with the penton base protein used as a competitor, a method which provides mimotopes of interacting sites in partner proteins (11). No RGD- or LDV-containing phagotopes were isolated by elution with the acidic buffer or the penton base protein. This suggests that if the RGD motif of the penton base is involved in its binding to α3β1 integrin, as suggested by our binding data and peptide competition experiments, this motif does not play a major role in this interaction. It is noteworthy to emphasize that RGD-containing peptides have been predominantly isolated from phage libraries selected for purified αvβ3, α5β1, and αIIbβ3 integrin subtypes (7, 19, 20).
Out of 22 independent phages recovered by acid elution and sequenced, 14 carried the phagotope sequence NNAGFL and one carried the sequence NGGVKS (Table 1, left column). This suggests that NNAGFL, and more generally the pentapeptide motif NNAG(F/V), is one of the preferred ligands of the α3β1 integrin. Scanning of the Ad2 or Ad5 peptide sequence showed that three regions shared some homology with this α3β1 ligand: NNAIV and NNSG are found at positions 185 to 189 and 311 to 314, respectively, and, considering the high homology between N and Q, the motif QNGVL is present at positions 198 to 202.
TABLE 1.
Phage biopanning of α3β1 integrin
| Phagotopes from indicated elutiona
| ||
|---|---|---|
| [H+] (×4) | [H+]+Pb(×2) | [H+]+Pb(×3) |
| NNAGFLb | SVNMDK | PELRIHc |
| NNAGFL | SVNMDK | PELRIH |
| NNAGFL | SVNMDK | PELRIH |
| NNAGFL | SVNMDK | PELRIH |
| NNAGFL | SVNMDK | PELRIH |
| NNAGFL | SSLSLS | PELRIH |
| NNAGFL | SSLSLS | PELRIH |
| NNAGFL | SMHNLV | |
| NNAGFL | QSHSLV | ARGHLR |
| NNAGFL | PSASIF | LRLQLR |
| NNAGFL | AGSRPS | |
| NNAGFL | IASGRA | PIGRGE |
| NNAGFL | PIGRGE | |
| NNAGFL | PELRIHc | PARVAS |
| NGGVKS | PELRIH | |
| PELRIH | LHEAHA | |
| ATGPTF | LHEAHA | |
| GILTRR | LGTLLN* | LHEAHA |
| KLLSYI | LGTLLN* | LHEAHA |
| PVLSRM | ASLLGR* | FLLKHR |
| AMLWIW | FGMLLN* | |
| YWDLIT | VVYSLR | |
| LTWLVL | NNAGFLb | VVYLSR |
| AVSWTY | ||
| DKPKQH | SVAQMY | |
| DKPKQH | QYGKTW | |
| DIMTHN | ||
| ADLFPG | ||
| LRDDHG | ||
| WNPTRK | ||
| FEQWGN | ||
| VWRYWY | ||
| RCQSGT | ||
| TTGITA | ||
Hexapeptides were eluted by acidic buffer alone ([H+]×4, four cycles), or by acidic buffer (one cycle) followed by two (×2) or three (×3) cycles of ligand displacement using recombinant penton base protein (Pb). Phagotopes are arranged according to their respective frequency, recurrent peptide motifs, and homology. Asterisks indicate phagotopes which share some homology with penton base sequence 253SRLSNLLGIRKR264 in forward or reverse orientation.
Phagotopes which are common to two columns are marked by matching footnotes.
The NNAFGL phagotope was also recovered among the α3β1-bound phages eluted by displacement with the penton base protein (Table 1, middle column), but NNAFGL was found only once out of 30 phages. Likewise, some homology could be observed between penton base-eluted phagotopes ASLLG, LGTLLN, and FGMLLN and the 253SRLSNLLGIRKR264 motif present in the Ad2 or Ad5 penton base sequence. The conserved sequence within residues 253 to 264 has been identified as the fiber-binding region in the penton base (11). This would suggest that one of the α3β1-binding sites in the penton base would neighbor or overlap the fiber-binding domain. However, none of these sequences was enriched by an extra cycle of adsorption and penton base elution (Table 1, right column), suggesting that they all represent minor binding sites with low affinity for α3β1.
Only one type of phagotope, PELRIH, was significantly enriched between the second and third cycle of phage adsorption and elution with the penton base protein (Table 1, compare the middle and right columns). Interestingly, the PELRIH motif shows significant homology with the pentapeptide motif DLRLK found in the α3β1-binding peptide GEFYFDLRLKGDKY of basement membrane collagen (25) and with hexapeptide NLRLSR found in the laminin-derived KQNCLSSRASFRGCVRNLRLSR peptide, identified as an α3β1 binding sequence (6). As no homologous sequence was found in the Ad2 or Ad5 penton base, this implies that the PELRIH motif does not occur in the linear sequence but is constituted of discontinuous amino acid residues.
Integrin α3β1 plays a role in Ad binding to mammalian cells.
The next step of this work was aimed at defining whether a specific interaction takes place between human Ad particles and α3β1 receptors displayed on the cell surface. For this purpose, we analyzed the binding of adenovirions and recombinant penton base to HeLa and SCC-25 human cell lines, which are known to express β1 integrins. HeLa cells also display CAR receptors but are poor in αvβ3 and αvβ5 integrin subtypes (4, 18). The tongue squamous-cell carcinoma-derived cell line SCC-25 is characterized by high levels of α2β1 and α3β1 integrins but displays low levels of CAR, αvβ3, and αvβ5 receptors (18).
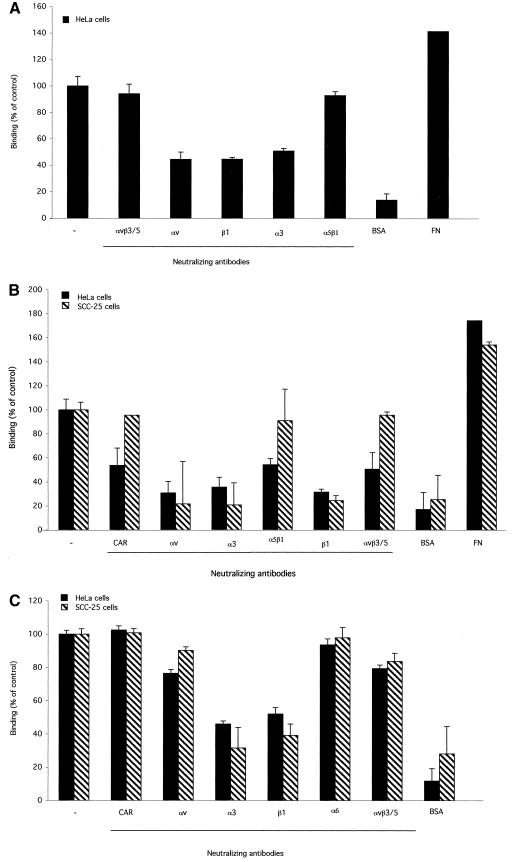
As shown in Fig. 2, when 96-well plates were coated with soluble penton base or purified Ad5 particles, specific adhesion of HeLa cells was observed. Binding to the penton base and to Ad5 was not substantially competed by preincubation with function-blocking antibodies directed towards α5β1, αvβ3, and αvβ5 integrin subtypes (Fig. 2A and B). However, significant competition was observed when HeLa cell adhesion to Ad5 or the penton base was tested after preincubation with anti-αv, anti-β1, or anti-α3 antibodies. SCC-25 adhesion to Ad5-coated plates, following preincubation with different anti-integrin antibodies, resulted in a binding pattern similar to that observed with HeLa cells, and no inhibition of binding was observed in the presence of anti-αvβ3 and anti-αvβ5 or anti-α5β1 antibodies (Fig. 2B). However, binding to coated plates was significantly inhibited in the presence of anti-α3, anti-β1, and anti-αv antibodies (Fig. 2B). In contrast to HeLa cells and consistent with the low level of CAR receptors on SCC-25 cells, binding was not altered by the addition of anti-CAR antibodies (Fig. 2B). For comparison, we analyzed HeLa and SCC-25 binding to the α3β1 natural ligand fibronectin. This protein also interacts with other integrin subtypes, including αvβ1/3 and αvβ5. As shown in Fig. 2C, the anti-α3 and anti-β1 antibodies inhibited the interaction between the cells and fibronectin to an extent comparable to that observed for Ad (Fig. 2B). By contrast, cell binding to fibronectin was only slightly impaired by the addition of anti-αv and anti-αvβ3/5 antibodies. These data validate the significance of competition studies performed with the penton base and with Ad but also indicate that, under the conditions tested, viral affinity for the different integrin receptors does not completely overlap that of fibronectin. For a negative control, we used an antibody directed to the anti-α6 integrin chain, which is not an α3β1 ligand and, as shown in Fig. 2C, which did not inhibit cell binding to fibronectin.
FIG.2.
Mammalian cell adhesion to purified penton base, Ad, or fibronectin in the presence of anti-integrin antibodies. Ninety-six well plates were coated with purified penton base at 0.5 μg/well (10), Ad5 particles at 1.15 × 1010 particles/well (kindly provided by N. La Monica, IRBM, Pomezia [Rome], Italy), or fibronectin at 0.1 μg/well (Sigma Aldrich). A total of 106 HeLa (ATCC CCL-2) or SCC-25 (ATCC CRL-1328) cells were added to the wells in the presence or absence of the different antibodies (20 μg/ml). Attached cells were quantitated by crystal violet staining and optical density determinations at 540 nm. Data are presented as average values of results of duplicate samples + standard deviations. Antibodies: anti-αvβ3, LM609; anti-αvβ5, P1F6; anti-αv, I3C2; anti-α3, P1B5; anti-β1, JB1; anti-α5β1, JBS5; anti-α6, MAb 1378; anti-CAR, RmcB. Except for RmcB and I3C2 (kindly provided by J. Bergelson and R. Klein,respectively), all the antibodies were purchased from Chemicon International. Competition with anti-integrin antibodies of HeLa binding to Ad was performed in the presence of the anti-CAR antibody. FN, coated fibronectin (0.1 μg/well; Sigma Aldrich); BSA, coated bovine serum albumin 3% (Sigma Aldrich).(A) Binding of HeLa to the penton base; (B) cell binding to Ad; (C) cell binding to fibronectin.
Taken together, these results suggest a role for the combination of α3 and β1 integrin subunits in Ad interaction with human cells, in both the presence and absence of the adenoviral primary receptor CAR. In addition, our data are in accordance with those from Li and collaborators, suggesting that αvβ1 may act as a coreceptor for Ad (23).
Integrin α3β1 plays a role in Ad infection of mammalian cells.
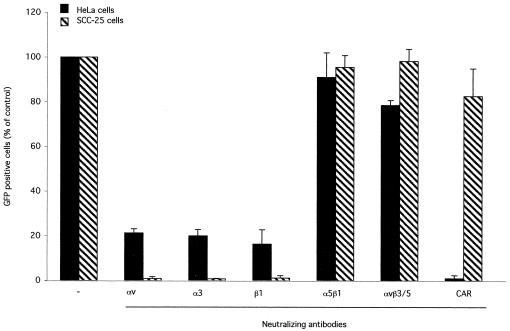
The results obtained in in vitro binding experiments involving the binding of Ad to living cells and to α3β1 integrin suggested that α3β1 may play a role in Ad infection. To verify this hypothesis, HeLa and SCC-25 cells were infected at a multiplicity of infection of 50 particles/cell with a recombinant Ad5 carrying genes encoding the green fluorescent protein (Ad5-GFP). Infection was performed in the presence or absence of function-blocking anti-integrin antibodies, and GFP-positive cells were analyzed by flow cytometry at 42 h after infection. As expected from binding competition experiments with both cell lines, gene transduction was inhibited in the presence of anti-αv, anti-β1, and anti-α3 antibodies, whereas it was not significantly altered in the presence of anti-αvβ3, anti-αvβ5, and anti-α5β1 antibodies (Fig. 3). It should be noted that transduction was inhibited in a more dramatic way in the presence of competitive antibodies than was binding. These results suggest, as previously described (26), that integrin receptors play key roles in viral internalization rather than in particle binding to cell membranes. The anti-CAR antibody completely inhibited GFP transduction of HeLa cells but had no effect on SCC-25 cells, a result which was consistent with the receptor patterns.
FIG. 3.
Transduction of mammalian cells in the presence of anti-integrin antibodies. A total of 106 HeLa or SCC-25 cells were preincubated with anti-integrin antibodies (30 μg/ml) and then infected at a multiplicity of infection of 50 particles/cell with Adeno-GFP (kindly provided by N. La Monica, IRBM). Competition with anti-integrin antibodies of HeLa infection was performed in the presence of the anti-CAR antibody. GFP expression was determined by fluorescence-activated cell sorter analysis at 42 h postinfection. Data presented are mean values of results of duplicate samples + standard deviations. Antibodies: anti-αvβ3, LM609; anti-αvβ5, P1F6; anti-αv, I3C2; anti-α3, P1B5; anti-β1, JB1a; anti-α5β1, JBS5; anti-CAR, RmcB.
Conclusions.
The results presented here suggest that a direct interaction occurs between the Ad penton base protein and the integrin receptor α3β1 in vitro and in vivo. We also provide data indicating that this integrin subtype plays a role in Ad infection. Furthermore, we present several independent evidences, i.e., binding experiments with penton base mutants, peptide binding competition, ion dependency, and peptide selection, showing that the interaction of the penton base with α3β1 is not dependent upon RGD motifs but is rather mediated by multiple discrete binding sites, some of them probably being formed from discontinuous amino acid residues. To our knowledge, this is the first study reporting the involvement of α3β1 in Ad infection and suggesting the peptide ligand preferences of this integrin receptor. Moreover, our results suggest that Ad exploits the integrin α3β1 not only as a primary receptor for anchoring its virions at the cell membrane but also to enter the cell and transduce its DNA, thus acting as a secondary, endocytosis receptor. These findings, together with those already published on other integrin subtypes (23, 31), support the hypothesis of a multiple receptor choice for Ad-cell interaction. This creates a biological advantage for the virus, since different integrin receptors are expressed on different tissues. Even though it is difficult to predict the specific role of the various integrins in the virus infection process, the basolateral membrane of epithelial and endothelial cells, where α3β1 is highly expressed (21), may be a secondary infection site relative to those tissues where CAR and αv receptors are major players of viral entry. Our findings are also important in view of the use of Ad vectors for gene therapy applications. Although a current opinion in the field assumes that RGD-deleted Ads would be incapable of interaction with integrins, our studies on α3β1 suggest that this assumption should be more cautiously evaluated.
Acknowledgments
Barbara Salone and Yuri Martina contributed equally to this work.
We are grateful to Saw See Hong for providing us with anti-Ad antibodies and the hexapeptide library.
This work was supported by the contributions of CNR, Progetto Finalizzato Biotecnologie, Istituto Pasteur Cenci Bolognetti, Università di Roma La Sapienza, and of Consorzio Interuniversitario Biotecnologie. Part of this study was financed by the French Centre National de la Recherche Scientifique (CNRS-SDV, Chercheur Associé 2002).
REFERENCES
- 1.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. [DOI] [PubMed] [Google Scholar]
- 2.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 3.Davison, E., R. M. Diaz, I. R. Hart, G. Santis, and J. F. Marshall. 1997. Integrin α5β1-mediated adenovirus infection is enhanced by the integrin-activating antibody TS2/16. J. Virol. 71:6204-6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Giovine, M., B. Salone, Y. Martina, V. Amati, G. Zambruno, E. Cundari, C. M. Failla, and I. Saggio. 2001. Binding properties, cell delivery and gene transfer of adenoviral penton base displaying bacteriophage. Virology 282:102-112. [DOI] [PubMed] [Google Scholar]
- 5.Elices, M., L. Urry, and M. Hemler. 1991. Receptor functions for the integrin VLA-3: fibronectin, collagen, and laminin binding are differentially influenced by Arg-Gly-Asp peptide and by divalent cations. J. Cell Biol. 112:169-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gehlsen, K., P. Sriramarao, L. Furcht, and A. Skubitz. 1992. A synthetic peptide derived from the carboxy terminus of the laminin A chain represents a binding site for the alpha 3 beta 1 integrin. J. Cell Biol. 117:449-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Healy, J., O. Murayama, T. Maeda, K. Yoshino, K. Sekiguchi, and M. Kikuchi. 1995. Peptide ligands for integrin αvβ3 selected from random phage display libraries. Biochemistry 34:3948-3955. [DOI] [PubMed] [Google Scholar]
- 8.Hemler, M., M. Elices, B. Chan, B. Zetter, N. Matsuura, and Y. Takada. 1990. Multiple ligand binding functions for VLA-2 (alpha 2 beta 1) and VLA-3 (alpha 3 beta 1) in the integrin family. Cell Differ. Develop. 32:229-238. [DOI] [PubMed] [Google Scholar]
- 9.Hewish, M. J., Y. Takada, and B. S. Coulson. 2000. Integrins α2β1 and α4β1 can mediate SA11 rotavirus attachment and entry into cells. J. Virol. 74:228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong, S., B. Gay, L. Karayan, M. Dabauvalle, and P. Boulanger. 1999. Cellular uptake and nuclear delivery of recombinant adenovirus penton base. Virology 262:163-177. [DOI] [PubMed] [Google Scholar]
- 11.Hong, S., and P. Boulanger. 1995. Protein ligands of human adenovirus type 2 outer capsid identified by biopanning of a phage displayed peptide library on separate domains of WT and mutant penton capsomers. EMBO J. 14:4714-4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang, S., R. I. Endo, and G. R. Nemerow. 1995. Upregulation of integrins αvβ3 and αvβ5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J. Virol. 69:2257-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphries, M. 2000. Integrin cell adhesion receptors and the concept of agonism. Trends Pharmacol. Sci. 21:29-32. [DOI] [PubMed] [Google Scholar]
- 14.Jackson, T., D. Sheppard, M. Denyer, W. Blakemore, and A. M. Q. King. 2000. The epithelial integrin αvβ6 is a receptor for foot-and-mouth disease virus. J. Virol. 74:4949-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson, T., A. P. Mould, D. Sheppard, and A. M. Q. King. 2002. Integrin αvβ1 is a receptor for foot-and-mouth disease virus. J. Virol. 76:935-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson, T., A. Sharma, R. A. Ghazaleh, W. E. Blakemore, F. M. Ellard, D. L. Simmons, J. W. I. Newman, D. I. Stuart, and A. M. Q. King. 1997. Arginine-glycine-aspartic acid-specific binding by foot-and-mouth-disease viruses to the purified integrin αvβ3 in vitro. J. Virol. 71:8357-8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karayan, L., S. S. Hong, B. Gay, J. Tournier, A. D. d'Angeac, and P. Boulanger. 1997. Structural and functional determinants in adenovirus type 2 penton base recombinant protein. J. Virol. 71:8678-8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasono, K., J. Blackwell, J. Douglas, I. Dmitriev, T. Strong, P. Reynolds, D. Kropf, W. Carroll, G. Peters, P. Bucy, D. Curiel, and V. Krasnykh. 1999. Selective gene delivery to head and neck cancer cells via an integrin targeted adenoviral vector. Clin. Cancer Res. 5:2571-2579. [PubMed] [Google Scholar]
- 19.Koivunen, E., D. Gay, and E. Ruoslahti. 1993. Selection of peptides binding to the alpha5beta1 integrin from phage display library. J. Biol. Chem. 268:20205-20210. [PubMed] [Google Scholar]
- 20.Koivunen, E., B. Wang, and E. Ruoslahti. 1995. Phage libraries displaying cyclic peptides with different ring sizes: ligand specificities of the RGD directed integrins. Bio/Technology 13:265-270. [DOI] [PubMed] [Google Scholar]
- 21.Kreidberg, J. 2000. Functions of α3β1 integrin. Curr. Opin. Cell Biol. 12:548-553. [DOI] [PubMed] [Google Scholar]
- 22.Krukonis, E., P. Dersch, J. Eble, and R. Isberg. 1998. Differential effects of integrin α chain mutations on invasin and natural ligand interaction. J. Biol. Chem. 273:31837-31843. [DOI] [PubMed] [Google Scholar]
- 23.Li, E., S. L. Brown, D. G. Stupack, X. S. Puente, D. A. Cheresh, and G. R. Nemerow. 2001. Integrin αvβ1 is an adenovirus coreceptor. J. Virol. 75:5405-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathias, P., M. Galleno, and G. R. Nemerow. 1998. Interactions of soluble recombinant integrin αvβ5 with human adenoviruses. J. Virol. 72:8669-8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miles, A., J. Knutson, A. Skubitz, L. Furcht, J. McCarthy, and G. Fields. 1995. A peptide model of basement membrane collagen alpha1 (IV) 531-543 binds to alpha3beta1 integrin. J. Biol. Chem. 270:29047-29050. [DOI] [PubMed] [Google Scholar]
- 26.Nemerow, G., and P. Stewart. 1999. Role of αv integrins in adenovirus cell entry and gene delivery. Microbiol. Mol. Biol. Rev. 63:725-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu, J., and K. Brown. 1999. Integrin αvβ5 is not involved in adeno-associated virus type 2 (AAV2) infection. Virology 264:436-440. [DOI] [PubMed] [Google Scholar]
- 28.Smith, G. P., and J. K. Scott. 1993. Libraries of peptides and proteins displayed on filamentous phage. Methods Enzymol. 217:228-257. [DOI] [PubMed] [Google Scholar]
- 29.Summerford, C., J. Bartlett, and R. Samulski. 1999. AlphaVbeta5 integrin: a co-receptor for adeno-associated virus type 2. Nat. Med. 5:78-82. [DOI] [PubMed] [Google Scholar]
- 30.Triantafilou, K., M. Triantafilou, Y. Takada, and N. Fernandez. 2000. Human parechovirus 1 utilizes integrin αvβ3 and αvβ1 as receptors. J. Virol. 74:5856-5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]