Abstract
Cell surface heparan sulfate proteoglycans (HSPGs) serve as primary attachment receptors for human papillomaviruses (HPVs). To demonstrate that a biologically functional HPV-receptor interaction is restricted to a specific subset of HSPGs, we first explored the role of HSPG glucosaminoglycan side chain modifications. We demonstrate that HSPG O sulfation is essential for HPV binding and infection, whereas de-N-sulfated heparin interfered with VLP binding but not with HPV pseudoinfection. This points to differences in VLP-HSPG and pseudovirion-HSPG interactions. Interestingly, internalization kinetics of VLPs and pseudovirions, as measured by fluorescence-activated cell sorting analysis, also differ significantly with approximate half times of 3.5 and 7.5 h, respectively. These data suggest that differences in HSPG binding significantly influence postbinding events. We also present evidence that pseudovirions undergo a conformational change after cell attachment. A monoclonal antibody (H33.J3), which displays negligible effectiveness in preattachment neutralization assays, efficiently neutralizes cell-bound virions. However, no difference in H33.J3 binding to pseudovirions and VLPs was observed in enzyme-linked immunosorbent assay and virus capture assays. In contrast to antibody H33.B6, which displays equal efficiencies in pre- and postattachment neutralization assays, H33.J3 does not block VLP binding to heparin, demonstrating that it interferes with steps subsequent to virus binding. Our data strongly suggest that H33.J3 recognizes a conformation-dependent epitope in capsid protein L1, which undergoes a structural change after cell attachment.
Human papillomaviruses (HPVs) are highly species-specific epitheliotropic DNA viruses. Of the more than 100 different genotypes, HPV type 16 (HPV16), HPV18, HPV31, HPV33, HPV35, HPV45, and HPV58 are most closely associated with cervical epithelial neoplasias and members of the group of HPV imposing a “high risk” for malignant progression to invasive genital carcinomas (30). The nonenveloped papillomavirus is composed of 360 copies of the major capsid protein L1, organized in 72 capsomeres, and probably 12 copies of the minor capsid protein L2 (1, 43). The encapsidated genome is an 8,000-bp circularized double-stranded DNA associated with cellular histones.
Despite their considerable clinical significance, the initial steps leading to infection with these viruses, as well as the mechanisms involved in virus entry into host cells, have not yet been completely elucidated due to the limited growth properties of HPV in cell cultures and the ubiquitous expression of HPV-binding proteins. The use of virus-like particles (VLPs) (20, 25, 36, 46, 49) has helped in the study of the initial interaction of papillomavirus particles with cell surfaces. It was established that VLPs of many HPV types compete for binding to the same highly conserved proteinaceous attachment receptor. In contrast to L1, L2 protein was not essential for binding, since L1 VLPs bound as efficiently as L1L2 VLPs (32, 35, 47). It was then demonstrated that HPV11 VLPs bind to cells via cell surface heparan sulfate (23). Using HPV33 L1L2 pseudovirions encapsidating a green fluorescence protein (GFP) marker plasmid, we identified cell surface heparan sulfate proteoglycans (HSPGs) as the primary attachment receptor required for efficient infection with HPV (18). Meanwhile, interaction with HSPGs has been shown to be essential for infection with various HPV types (11).
HSPGs are complex molecules composed of a core protein with covalently attached glycosaminoglycans (3). These glycosaminoglycans, composed of alternating disaccharide units of uronic acid and amino sugars, are posttranslationally modified by sulfation and acetylation to various degrees, providing a variety of molecules with substantial sequence heterogeneity (14). HSPGs are involved in a wide variety of biological phenomena, including organogenesis, angiogenesis, growth factor/cytokine actions, wound healing, and cell adhesion (for reviews, see references 3, 21, and 33). Moreover, HSPGs are being implicated as primary host cell receptors for many viruses (reviewed in reference 27), although most of these viruses depend on secondary receptor proteins for efficient internalization.
To further investigate the nature of the primary HSPG receptor, heparins esterified with sulfuric acid at different positions were tested for their inhibitory effects on VLP binding and pseudovirus infection. We demonstrate here the requirement of HSPG O sulfation for efficient attachment of HPV particles, as well as specific differences between VLPs and pseudovirions with regard to the quality of binding and kinetics of internalization. Moreover, we used neutralizing monoclonal antibodies in both pre- and postattachment neutralization assays, and our data suggest that cell surface attachment significantly improves the presentation of a neutralizing epitope, a result most likely due to a conformational change in capsid protein L1 of cell-bound pseudovirions.
MATERIALS AND METHODS
Chemicals and antibodies.
Heparins of various molecular weights (H-4784, H-5284, and H-3400) or specific modifications (A-6039, A-8036, and D-4776) were purchased from Sigma. Generation of the HPV33 VLP-specific rabbit polyclonal antiserum K53 and monoclonal anti-L1 antibody 33L1-7 have been described previously (18, 37, 45). Mouse monoclonal antibodies H33.J3, H33.B6, and H33.E12 were generated by immunization of mice with HPV33 VLPs, as described previously (9).
Preparation of VLPs.
VLPs were purified from insect cells infected with recombinant baculoviruses bac33L1 and bac33L1-bac33L2 or from COS7 cells infected with recombinant vaccinia viruses vac33L1 and vac33L1-vac33L2 as described previously (2, 45, 46).
Preparation of HPV33 pseudovirions.
Pseudovirions were prepared essentially as described previously (19, 45) with slight modifications. Briefly, COS-7 cells were transfected with marker plasmid pEGFPGFP-NLS. Transfected cells were grown for 48 h and were subsequently infected with vaccinia viruses recombinant for HPV33 L1 (vac33L1) and L2 (vac33L2), respectively. Since the L1 and L2 genes were placed under the control of a phage T7 promoter, the helper vaccinia virus VTF7-3 encoding T7 RNA polymerase was used for coinfection. Pseudovirions were extracted from nuclei at 40 h postinfection by sonication in hypotonic buffer supplemented with 0.5% NP-40 and then purified by buoyant cesium chloride density gradients. VLPs and pseudoviruses banded at densities of 1.29 and 1.32 g/cm3 and were separated by at least two fractions, ensuring that only minor cross contamination, if at all, occurred. Only peak fractions were used for further purification via velocity gradient centrifugation, by using sucrose step gradients as described above. This additional purification step removes low-molecular-weight molecules, including L1 capsomeres and monomers.
Enzyme-linked immunosorbent assays.
For all heparin-bovine serum albumin (BSA)-based enzyme-linked immunosorbent assays (ELISAs), microtiter plates (Polysorb obtained from Nunc, Wiesbaden, Germany) were coated overnight with 200 ng of heparin-BSA/well in phosphate-buffered saline (PBS). Plates were subsequently washed three times with 300 μl of PBS-0.1% Tween 20 (PBST). Free binding sites were blocked with 300 μl of BSA (50 μg/ml) in PBST for 1 h at room temperature, and plates were washed again three times with PBST. VLPs or pseudovirions diluted in PBST to the indicated concentrations were added in the absence or presence of various glucosaminoglycans. After 1 h at 37°C, wells were washed three times with 300 μl of PBST/well, and wells were incubated with 100 μl of primary antibody solution for 1 h at 37°C. Then, 100 μl of horseradish peroxidase-coupled secondary antibodies (goat anti-rabbit immunoglobulin G [IgG] or goat anti-mouse IgG; 1:5,000 in PBST), obtained from Jackson Immunochemicals, was added, followed by incubation for additional 30 min at 37°C. After three washes with PBST, the assay was developed with ready-to-use trimethylbenzidine (KPL). The reaction was stopped after 10 min at 37°C with 100 μl of 1 N HCl. Absorbance was measured at 450 nm by using a Multiscan EX (Thermo Life Sciences). To measure the ability of antibodies to interfere with VLP binding to heparin, VLPs were preincubated overnight at 4°C with the indicated antibodies, diluted 1:100 in PBST, and subsequently added to heparin-BSA-coated microtiter plates. Bound VLPs were monitored by using horseradish peroxidase-coupled anti-mouse antibodies as described above.
VLP attachment assay.
For analysis of VLP binding to COS7 cells in the presence of various heparins, 5 × 105 cells were grown in six-well plates and incubated with 250 ng of HPV33 L1L2 VLPs, as described above for virions. After incubation for 60 min at 4°C under constant agitation, cells were washed and resuspended in Laemmli buffer. Lysates containing cell-bound VLPs were separated by standard sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis. Western blots, stained with HPV L1-specific 33L1-7 antibodies and horseradish peroxidase-coupled secondary antibodies, were visualized by enhanced chemiluminescence (Amersham).
Flow cytometry.
To monitor the internalization of pseudovirions and VLPs, cell monolayers of COS7 cells (5 × 105) were exposed to these particles in Dulbecco modified Eagle medium (DMEM) for 45 min at 4°C. Unbound particles were removed by extensive washing. DMEM was than replaced by DMEM-fetal calf serum (FCS), and cells were incubated at 37°C. At the indicated time points, cells were washed again, detached with PBS/EDTA, and fixed with 3.5% formaldehyde in PBS. Cell surface-bound particles were detected by using a FACScan flow cytometer (Becton Dickinson), after sequential incubation of the cells (1 h at 4°C) with the HPV33-specific antiserum K53 (diluted 1:1,000 in PBS-1% FCS) and DTAF (dichlorotriazinylamino-fluorescein)-labeled secondary antibodies.
Infectivity inhibition assay.
COS7 cells (5 × 104 cells/well) were grown in 24-well plates and infected with pseudovirions in a total volume of 250 μl of DMEM in the presence or absence of inhibitors. After 1 h at 4°C under constant agitation the pseudovirions were replaced by 1 ml of supplemented culture medium. Cells were grown for 72 h at 37°C before infectious events were determined by counting the cells with nuclear green fluorescence. For preattachment neutralization assays, pseudovirions were preincubated for 1 h at 4°C with antibodies and then added to the cells. For postattachment neutralization, pseudovirions were bound to cells for 1 h at 4°C. Unbound virions were removed by washing and antibodies were added in a total volume of 250 μl of DMEM-FCS. After 1 h at 37°C, the antibodies were removed, and incubation was continued for 72 h. For depletion of virions prior to pseudoinfection, virions were incubated with monoclonal antibodies coupled to magnetic beads (Dynabeads; anti-mouse IgG) for 1 h at 4°C. Beads were subsequently removed, and residual virions in supernatants were tested for infectivity.
RESULTS
Influence of modified heparins on VLP binding to heparin-BSA and cell surface heparan sulfate.
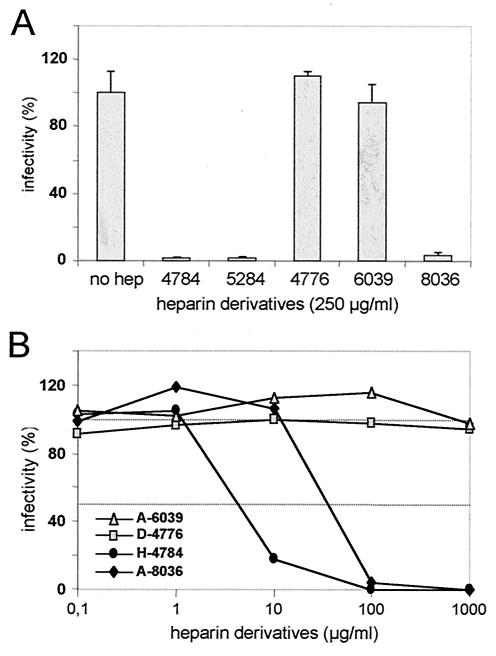
Heparin and cell surface heparan sulfate, but not chondroitin sulfate or dermatan sulfate containing similar amounts of negatively charged residues, are potent inhibitors of HPV binding and infection, suggesting that the HPV-receptor interaction depends on defined structural features of HSPG molecules rather than nonspecific electrostatic interactions with positively charged clusters of amino acids in the viral capsid. Heparins of different molecular weights, as well as defined contents on N-linked and O-linked sulfate groups, were therefore tested for their capacity to interfere with HPV VLP binding to heparin-BSA-coated microtiter plates (Fig. 1A). A 50% inhibition of binding was achieved with heparin with a molecular mass of 16,000 Da (H-4784) at a concentration of ca. 20 μg/ml, whereas >500 μg/ml was needed with heparin with a molecular mass of 6,000 Da (H-5284). Heparin H-3400 with a molecular mass of 3,000 Da did not block VLP binding to heparin-BSA. In contrast to unmodified heparin, neither de-N-sulfated (D-4776) nor de-O-sulfated/acetylated (A-6039) or acetylated heparin (A-8036) displayed any inhibitory effect, confirming an important role of N- and O-linked sulfates in VLP binding. The molecular masses of these derivatives were confirmed to be similar to that of unmodified heparin by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and alcian blue staining (data not shown). We next measured VLP binding to HSPGs expressed on COS7 cells (Fig. 1B). Surprisingly, with the exception of heparin A-6039, all tested heparin derivatives affected VLP binding when present at a concentration of 250 μg/ml. This may be best explained by the higher degree of sulfation of heparin than of cell surface heparan sulfate (28), resulting in a stronger VLP binding to heparin-BSA-coated plates than to cell surface HSPGs.
FIG. 1.
VLP interaction with HSPGs. (A) VLPs were bound to heparin-BSA-coated microtiter plates in the presence of soluble heparins of different molecular masses (H-4784 [16 kDa], H-5284 [6 kDa], and H-3400 [3 kDa]) or de-N-sulfated (D-4776), de-O-sulfated/acetylated (A-6039), or acetylated heparin (A-8036). Bound VLPs were determined. Binding in the absence of glucosaminoglycan was set as 100%. (B) VLP binding to COS7 cells in the presence of heparin derivatives. Bound VLPs were monitored by Western blot with L1-specific antibody 33L1-7.
Inhibition of HPV pseudovirus infection requires both N and O sulfation of glucosaminoglycans.
In order to investigate whether the addition of heparin derivatives also affects virus infection, we performed a pseudovirus infection assay in the presence of these compounds. As shown in Fig. 2A, heparin H-4784, as well as heparins H-5284 and A-8036, prevented infection almost completely when present at a concentration of 250 μg/ml. As expected, de-O-sulfated heparin A-6039 did not inhibit pseudoinfection. Interestingly, completely de-N-sulfated heparin D-4776, which still efficiently interferes with VLP binding to COS7 cells, had no measurable inhibitory effect on infections, even at concentrations up to 1 mg of the modified glucosaminoglycan/ml (Fig. 2B). Reduction of the level of infection by 50% was achieved in the presence of only 5 to 7 μg of unmodified heparin H-4784/ml, whereas for heparin A-8036, in which N sulfation is partially replaced by acetylation, ∼10-fold-higher concentrations were required. These data demonstrate that, in addition to O-linked sulfates, N sulfation of heparan sulfate is not required for cell binding of the majority of VLPs but is an essential prerequisite for HPV pseudovirus infection.
FIG. 2.
Pseudoinfection in the presence of heparin derivatives. COS7 cells were incubated with pseudovirions in the presence of heparin derivatives at the indicated concentrations. Infectious events were monitored for 72 h postinfection. The number of green fluorescent cells in the absence of inhibitors was set as 100%. Mean values of three independent experiments are shown. (A) Effect of heparin derivatives (H-4776, A-6039, and A-8036) compared to unmodified heparins (H-4784 and H-5284) of various molecular weights. (B) Concentration dependence of the inhibitory effect of heparin derivatives. A value of 100% infectivity corresponds to 370 infected cells.
VLP uptake occurs faster than virus internalization.
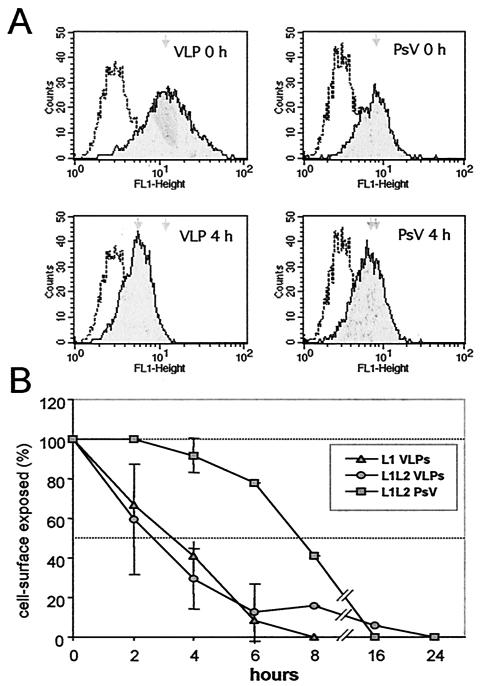
The results presented above show that VLPs and virions differ in their quality of cell binding. To investigate whether this has any consequences for postbinding events, we measured the kinetics of VLP and pseudovirus internalization by fluorescence-activated cell sorting analysis. At 4 h postbinding to COS7 cells, ca. 50% of VLPs were no longer detectable on the cell surface (Fig. 3A). To ensure that the particles were internalized rather than shed from the cell surface, internalization was blocked by cytochalasin D (1 μg/ml). Under these conditions, no signal reduction was observed by 6 h postbinding (data not shown), suggesting that the reduction of cell surface exposed VLPs was due to particle uptake. In contrast to the results obtained with VLPs, no obvious uptake of pseudovirions was observed 4 h postbinding (Fig. 3A). A more detailed analysis (Fig. 3B) revealed a half time of VLP uptake of ca. 3 h. The presence of L2 did not significantly change the VLP uptake kinetic, since similar results were obtained with L1- and L1L2-VLPs. In contrast, internalization of highly purified pseudoviruses significantly differed from the uptake of VLPs and occurred at a much slower rate, with a half time of 7.5 h. This suggests that differences in the quality of binding of VLPs and pseudovirions may strongly affect postbinding events.
FIG. 3.
Fluorescence-activated cell sorting analysis of VLP and pseudovirus (PsV) internalization. (A) Histograms of cell surface-exposed particles after binding at 4°C (0 h) and further incubation at 37°C (4 h). (B) Time course of internalization of L1 VLPs, L1L2 VLPs, and L1L2 pseudovirions. Mean peak values of seven experiments (± the standard deviations) are shown. The half time of VLP and pseudovirus internalization differs by several hours.
Monoclonal antibody H33.J3 efficiently binds HPV33 VLPs and pseudovirions.
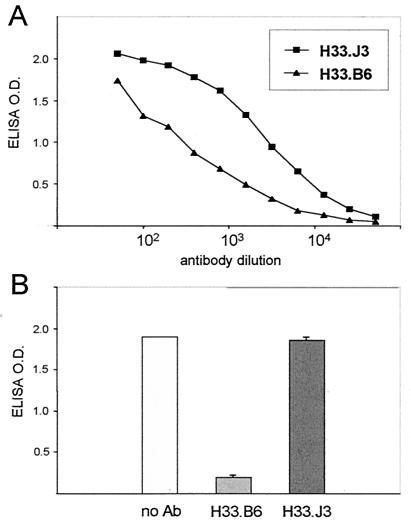
The requirement of a conformational change of virions prior to internalization may account for the observed delay in virus uptake. To follow this up, we tested HPV33-specific neutralizing antibodies in pre- and postattachment neutralization assays. Two monoclonal antibodies, H33.B6 and H33.J3, were chosen for further analysis because they behaved differently in our assay systems. These antibodies were raised in mice immunized with HPV33 L1 VLPs and do not require the presence of L2 for bindingVLPs. Both antibodies specifically recognize HPV33 VLPs (Fig. 4A) and capsomeres, but not denatured L1 protein (data not shown) attached to microtiter plates, either directly or via heparin-BSA. The reactivity of H33.J3 with VLPs was consistently stronger than that observed with H33.B6. To test their ability to interfere with VLP binding to glucosaminoglycans, VLPs were preincubated with H33.B6 and H33.J3, respectively, and exposed to heparin-BSA-coated microtiter plates (Fig. 4B). Whereas H33.B6 strongly reduced VLP binding, H33.J3 did not impair VLP-heparin interactions despite its better reactivity with VLPs. These data prove that the mode of binding of H33.B6 and H33.J3 to the HPV33 capsid differs.
FIG. 4.
Interference of monoclonal antibodies with VLP binding to heparin. (A) Titration of two HPV33 VLP-specific monoclonal antibodies (H33.J3 and H33.B6) with HPV33 L1L2 VLPs bound to heparin-BSA-coated microtiter plates. (B) VLPs were incubated with monoclonal antibodies H33.B6 and H33.J3 prior to addition to heparin-BSA-coated plates. The amounts of bound VLPs were determined by using horseradish peroxidase-labeled antibodies. Antibody H33.B6 but not H33.J3 interferes with binding to glucosaminoglycans.
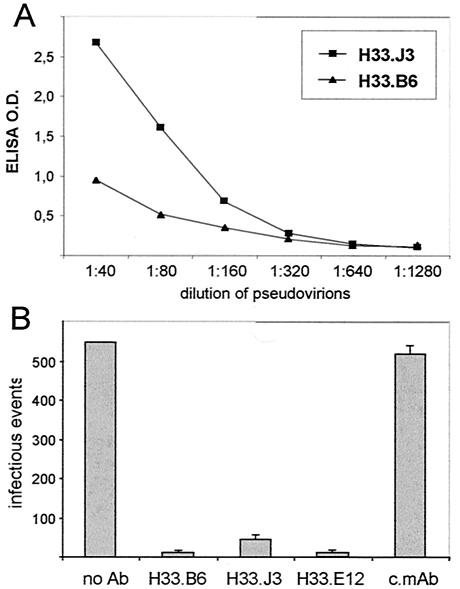
Both antibodies also reacted with highly purified HPV33 L1L2 pseudovirions, as measured by two means. We again observed a higher reactivity of H33.J3 with pseudovirions attached to heparin-coated ELISA plates than of H33.B6, even though the titers of both antibody preparations were similar (Fig. 5A). The binding of these antibodies to virions was further tested by using a pseudovirus depletion assay (Fig. 5B). Antibodies were attached to magnetic beads, added to pseudovirions, and subsequently removed. The supernatants, containing unbound virions, were added to COS7 cells to determine residual infectivity. Supernatants obtained after incubation with magnetic beads containing either no or a control antibody yielded >500 infectious units. In contrast, incubation with beads loaded with H33.B6 and H33.J3 or with H33.E12, which was used as positive control, strongly reduced the number of infectious events. These results implicate that both antibodies not only bind to DNA-free VLPs but also to DNA-harboring pseudovirions.
FIG. 5.
Reactivity of monoclonal antibodies with HPV33 L1L2 pseudovirions. (A) Interaction of H33.J3 and H33.B6 with heparin-bound pseudovirions. (B) Interaction of monoclonal antibodies with pseudovirions in solution. Pseudovirions were incubated with magnetic beads loaded with the indicated monoclonal antibody. After removal of beads, supernatants were tested for residual infectivity. All HPV33-specific conformation-dependent monoclonal antibodies, but not a control monoclonal antibody (c.mAb), efficiently depleted pseudovirions.
H33.J3 differs in its efficacy in pre- and postattachment neutralization of HPV33 pseudovirions.
The ability of H33.B6 and H33.J3 to recognize conformational surface-exposed epitopes, as well as their capacity to deplete virions from solution, are characteristic features of HPV-neutralizing antibodies. To our surprise, H33.B6 and H33.J3 strongly differed in their neutralization activity. Upon preincubation of pseudovirions with serial dilutions of H33.B6, complete neutralization was observed up to a 500-fold dilution (Fig. 6A), and partial inhibition was even obtained with a 10,000-fold-diluted antibody. In contrast, partial neutralization of only 60 and 30% was observed at 2- and 20-fold dilutions of H33.J3, respectively. Prolonged preincubation of H33.J3 with pseudovirions did not improve neutralization (data not shown). Therefore, H33.B6 which, compared to H33.J3, reacted significantly more weakly with HPV33 particles in ELISAs, much better neutralizes pseudovirions in this preincubation assay.
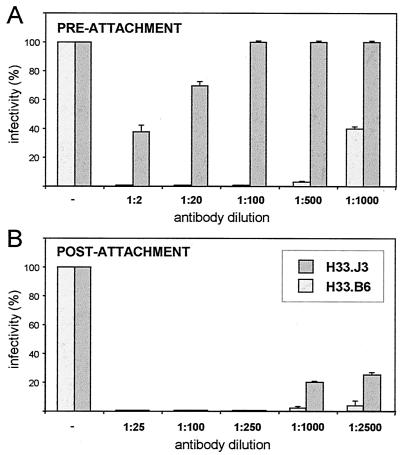
FIG. 6.
Pre- and postattachment neutralization assays. (A) For preattachment neutralization, pseudovirions were incubated with the indicated dilutions of monoclonal antibodies for 1 h at 4°C and subsequently added to COS7 cells. Infection was monitored for 72 h after the pseudovirions were replaced by supplemented DMEM. (B) For postattachment neutralization, pseudovirions were bound to COS7 cells for 1 h at 4°C, and cell-bound virions were exposed to antibodies at the indicated dilutions. Antibodies were removed after 1 h at 37°C. A value of 100% infectivity corresponds to 72 infected cells. Antibody H33.J3, but not antibody H33.B6, displays different capacities in pre- and postattachment neutralization assays.
A completely different result was obtained in a postattachment neutralization assay (Fig. 6B). Here, pseudovirions were first added to cells, unbound virions were removed, and antibodies were added to cells prior to the shift to 37°C. Under these conditions, H33.J3 neutralized pseudovirus infection almost as efficiently as H33.B6. A 70% reduction in infectivity was still obtained with a 1,000-fold dilution of H33.J3. Obviously, cell-binding renders virions more sensitive to H33.J3 neutralization, even though the antibody binds free pseudovirions very effectively. The neutralization efficacy of H33.B6, however, was only marginally increased.
DISCUSSION
We have shown here that pseudovirions are more demanding with respect to interaction with heparan sulfates than VLPs. Whereas O sulfation of HSPGs is sufficient for VLP binding, pseudoviruses also require N sulfation. Nevertheless, VLPs and virions must bind to the same primary receptor, since VLPs are able to block infection in competition assays (35). The differences in the quality of binding may be due to the structural changes observed after DNA encapsidation, leading to a tighter packaging in trypsin-resistant particles (15). The higher trypsin sensitivity of VLPs compared to virions suggest an increased surface exposure of positively charged lysine and arginine residues found in the C-terminal arm of L1, which may contribute to the higher promiscuity of VLP binding to HSPGs (7, 15, 31). In contrast to pseudovirus and VLP interactions with HSPG, no difference was observed for VLPs from various sources (baculovirus versus vaccinia virus produced), confirming that DNA encapsidation rather than the production system is responsible for the observed structural and functional differences.
Due to the variability of the core proteins and the modifications in the attached glucosaminoglycans, HSPGs have the potential for almost unlimited heterogeneity. Of these numerous molecules, only a few may serve as primary attachment receptors for virions. It has been shown for HSV-1 that only the 3-O-sulfated HSPG, which is a rarely found modification of HSPG, serves as a functional uptake receptor (40). Moreover, the specific virus-HSPG interaction may differ between closely related viruses, as shown for HSV-1 and HSV-2 (44). It is therefore conceivable that HPV VLPs may use various forms of HSPGs for attachment, based on less-stringent requirements for binding compared to pseudovirions. This notion is supported by the observed differences in the uptake kinetics of pseudovirions and VLPs. The exceptionally long presence of pseudovirions on the cell surface was also demonstrated previously by the sensitivity of virions toward neutralizing agents, such as antibodies and heparin (8, 10, 18). The prolonged exposure on the cell surface was partially explained by the putative involvement of a secondary receptor protein responsible for the uptake of virions (18, 39). Indeed, recent reports suggest that the minor capsid protein L2 may bind to a cell surface protein(s) other than HSPGs (24, 48). In line with this, the classical receptor- and clathrin-dependent endocytic pathway has been demonstrated to be essential for papillomavirus infection (6, 13, 39). The fast uptake of VLPs compared to pseudovirions may not necessarily occur via the same mechanism. VLPs may only require the primary HSPG receptor for internalization. Two routes of particle uptake and differences in the uptake mechanisms of virions and VLPs were also observed for polyomaviruses, resulting either in nuclear targeting or, as for the bulk of empty VLPs, in lysosomal degradation (26).
The fate of VLPs and pseudovirions in postattachment events is not only determined by their structural differences; pseudovirions themselves also seem to undergo a conformational change after binding to the cell surface. Binding of H33.J3 resulted in the neutralization of cell-bound but not free virions. This antibody efficiently binds to pseudovirions in dilution, suggesting that the mode of binding to free and cell-bound virions is different. Aggregate formation between pseudovirus particles and contaminating VLPs as an explanation for the ability of H33.J3 to deplete virions from solution can be excluded, since complexed particles were not found in electron microscopic analyses of pseudovirus preparations (data not shown). In addition, VLPs had been effectively removed by our purification scheme. H33.J3, which does not block VLP binding to heparin, has characteristics similar to the BPV1-specific antibody 5B6 (5). This antibody was shown to bind both monovalently and bivalently to the sides of hexavalent capsomeres and efficiently neutralizes infection without significantly impairing virus binding to the cell surface. It was therefore suggested to interfere with viral uncoating due to cross-linking of capsomeres. It is also conceivable that H33.J3 primarily binds monovalently to free pseudovirions. Cell attachment may induce a conformational change, resulting in bivalent binding of the antibody and thus cross-linking of capsomeres, which subsequently interferes with virus uncoating. Such antibodies, which perform poorly in preattachment neutralization assays but neutralize effectively in postattachment neutralization assays, were recently detected in human immunodeficiency virus-positive human sera in significant amounts (4) and may also play a role during natural infection with HPV.
Conformational changes of outer surface structures after cell binding are common phenomena that have been described for both enveloped and nonenveloped viruses (16, 17, 29, 38). For example, human immunodeficiency virus type 1 enters susceptible cells via a complex cascade of receptor-mediated events. Primary interaction of the viral protein gp120 with the CD4 receptor induces conformational changes in gp120 and gp41 necessary for subsequent internalization events. Similarly, after binding to its receptor, poliovirus undergoes a structural rearrangement and exposes a membrane-reactive hydrophobic stretch of amino acids of capsid protein VP1, normally hidden inside the capsid (16). Corresponding observations have been made for other enteroviruses (12, 22, 34). For DNA tumor viruses of the Polyomaviridae family, which are structurally closely related to papillomaviruses (7, 31, 41), conformational changes after interaction with their receptors have not been observed (42). However, evidence for papillomavirus capsid reorganization is accumulating. Yang et al. (48) have recently identified an HPV16 L2 peptide, which binds to cells, resulting in its internalization. Since mutation of this peptide significantly impaired infectivity of pseudotyped virions, interaction of this peptide with its cellular binding partner may be important for productive infection. Interestingly, in virions this peptide is not accessible to antibodies. Moreover, removal of the primary glucosaminoglycan receptor by treatment with heparinase eliminates binding of virions but not the interaction of the L2 peptide with cell surface molecules (48). In line with our observation, this is also best explained by a conformational change in order to expose the peptide on the capsid surface after cell attachment. Therefore, the data taken together suggest that both capsid proteins may be affected by the proposed structural rearrangement of the virus surface.
Based on these and published data, we propose that papillomavirus virions may exist in two forms. The closed form is the predominant species of genome-containing virions in solution. Binding of cell surface receptors cause a transition in the virion from the closed to a VLP-like open form, which may initiate internalization and uncoating. Further investigations will determine whether this transition is the time-limiting step of papillomavirus uptake and responsible for the different uptake rates of VLPs and pseudovirions.
Acknowledgments
We are grateful to C. Sapp for expert technical assistance and R. E. Streeck and S. Bhakdi for helpful suggestions.
This study was funded by a grant (SFB490.B5) to M.S. from Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Baker, T. S., W. W. Newcomb, N. H. Olson, L. M. Cowsert, C. Olson, and J. C. Brown. 1991. Structures of bovine and human papillomaviruses: analysis by cryoelectron microscopy and three-dimensional image reconstruction. Biophys. J. 60:1445-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergsdorf, C., C. Beyer, V. Umansky, M. Werr, and M. Sapp. 2003. Highly efficient transport of carboxyfluorescein diacetate succinimidyl ester into COS7 cells using human papillomavirus-like particles. FEBS Lett. 536:120-124. [DOI] [PubMed] [Google Scholar]
- 3.Bernfield, M., M. Götte, P. W. Park, O. Reizes, M. L. Fitzgerald, J. Lincecum, and M. Zako. 1999. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68:729-777. [DOI] [PubMed] [Google Scholar]
- 4.Binley, J. M., C. S. Cayanan, C. Wiley, N. Schulke, W. C. Olson, and D. R. Burton. 2003. Redox-triggered infection by disulfide-shackled human immunodeficiency virus type 1 pseudovirions. J. Virol. 77:5678-5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booy, F. P., R. B. Roden, H. L. Greenstone, J. T. Schiller, and B. L. Trus. 1998. Two antibodies that neutralize papillomavirus by different mechanisms show distinct binding patterns at 13 Å resolution. J. Mol. Biol. 281:95-106. [DOI] [PubMed] [Google Scholar]
- 6.Bousarghin, L., A. Touze, P. Y. Sizaret, and P. Coursaget. 2003. Human papillomavirus types 16, 31, and 58 use different endocytosis pathways to enter cells. J. Virol. 77:3846-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, X. S., R. L. Garcea, I. Goldberg, G. Casini, and S. C. Harrison. 2000. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol. Cell 5:557-567. [DOI] [PubMed] [Google Scholar]
- 8.Christensen, N. D., N. M. Cladel, and C. A. Reed. 1995. Postattachment neutralization of papillomaviruses by monoclonal and polyclonal antibodies. Virology 207:136-142. [DOI] [PubMed] [Google Scholar]
- 9.Christensen, N. D., J. Dillner, C. Eklund, J. J. Carter, G. C. Wipf, C. A. Reed, N. M. Cladel, and D. A. Galloway. 1996. Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology 223:174-184. [DOI] [PubMed] [Google Scholar]
- 10.Christensen, N. D., C. A. Reed, T. D. Culp, P. L. Hermonat, M. K. Howett, R. A. Anderson, and L. J. Zaneveld. 2001. Papillomavirus microbicidal activities of high-molecular-weight cellulose sulfate, dextran sulfate, and polystyrene sulfonate. Antimicrob. Agents Chemother. 45:3427-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Combita, A. L., A. Touze, L. Bousarghin, P. Y. Sizaret, N. Munoz, and P. Coursaget. 2001. Gene transfer using human papillomavirus pseudovirions varies according to virus genotype and requires cell surface heparan sulfate. FEMS Microbiol. Lett. 204:183-188. [DOI] [PubMed] [Google Scholar]
- 12.Crowell, R. L., and L. Philipson. 1971. Specific alterations of coxsackievirus B3 eluted from HeLa cells. J. Virol. 8:509-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Day, P. M., D. R. Lowy, and J. T. Schiller. 2003. Papillomaviruses infect cells via a clathrin-dependent pathway. Virology 307:1-11. [DOI] [PubMed] [Google Scholar]
- 14.Esko, J. D., and U. Lindahl. 2001. Molecular diversity of heparan sulfate. J. Clin. Investig. 108:169-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fligge, C., F. Schäfer, H. C. Selinka, C. Sapp, and M. Sapp. 2001. DNA-induced structural changes in the papillomavirus capsid. J. Virol. 75:7727-7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fricks, C. E., and J. M. Hogle. 1990. Cell-induced conformational change in poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J. Virol. 64:1934-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furuta, R. A., C. T. Wild, Y. Weng, and C. D. Weiss. 1998. Capture of an early fusion-active conformation of HIV-1 gp41. Nat. Struct. Biol. 5:276-279. [DOI] [PubMed] [Google Scholar]
- 18.Giroglou, T., L. Florin, F. Schäfer, R. E. Streeck, and M. Sapp. 2001. Human papillomavirus infection requires cell surface heparan sulfate. J. Virol. 75:1565-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giroglou, T., M. Sapp, C. Lane, C. Fligge, N. D. Christensen, R. E. Streeck, and R. C. Rose. 2001. Immunological analyses of human papillomavirus capsids. Vaccine 19:1783-1793. [DOI] [PubMed] [Google Scholar]
- 20.Hagensee, M. E., N. Yaegashi, and D. A. Galloway. 1993. Self-assembly of human papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J. Virol. 67:315-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iozzo, R. V. 2001. Heparan sulfate proteoglycans: intricate molecules with intriguing functions. J. Clin. Investig. 108:165-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jimenez-Clavero, M. A., E. Escribano-Romero, A. J. Douglas, and V. Ley. 2001. The N-terminal region of the VP1 protein of swine vesicular disease virus contains a neutralization site that arises upon cell attachment and is involved in viral entry. J. Virol. 75:1044-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joyce, J. G., J.-S. Tung, C. T. Przysiecki, J. C. Cook, E. D. Lehman, J. A. Sands, K. U. Jansen, and P. M. Keller. 1999. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J. Biol. Chem. 274:5810-5822. [DOI] [PubMed] [Google Scholar]
- 24.Kawana, Y., K. Kawana, H. Yoshikawa, Y. Taketani, K. Yoshiike, and T. Kanda. 2001. Human papillomavirus type 16 minor capsid protein L2 N-terminal region containing a common neutralization epitope binds to the cell surface and enters the cytoplasm. J. Virol. 75:2331-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirnbauer, R., F. Booy, N. Cheng, D. R. Lowy, and J. T. Schiller. 1992. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 89:12180-12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krauzewicz, N., J. Stokrova, C. Jenkins, M. Elliott, C. F. Higgins, and B. E. Griffin. 2000. Virus-like gene transfer into cells mediated by polyoma virus pseudocapsids. Gene Ther. 7:2122-2131. [DOI] [PubMed] [Google Scholar]
- 27.Liu, J., and S. C. Thorp. 2002. Cell surface heparan sulfate and its roles in assisting viral infections. Med. Res. Rev. 22:1-25. [DOI] [PubMed] [Google Scholar]
- 28.Marks, R. M., H. Lu, R. Sundaresan, T. Toida, A. Suzuki, T. Imanari, M. J. Hernaiz, and R. J. Linhardt. 2001. Probing the interaction of dengue virus envelope protein with heparin: assessment of glycosaminoglycan-derived inhibitors. J. Med. Chem. 44:2178-2187. [DOI] [PubMed] [Google Scholar]
- 29.Matsuyama, S., and F. Taguchi. 2002. Receptor-induced conformational changes of murine coronavirus spike protein. J. Virol. 76:11819-11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMurray, H. R., D. Nguyen, T. F. Westbrook, and D. J. McAnce. 2001. Biology of human papillomaviruses. Int. J. Exp. Pathol. 82:15-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Modis, Y., B. L. Trus, and S. C. Harrison. 2002. Atomic model of the papillomavirus capsid. EMBO J. 21:4754-4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Müller, M., L. Gissmann, R. J. Cristiano, X. Y. Sun, I. H. Frazer, A. B. Jenson, A. Alonso, H. Zentgraf, and J. Zhou. 1995. Papillomavirus capsid binding and uptake by cells from different tissues and species. J. Virol. 69:948-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park, P. W., O. Reizes, and M. Bernfield. 2000. Cell surface heparan sulfate proteoglycans: selective regulators of ligand-receptor encounters. J. Biol. Chem. 275:29923-29926. [DOI] [PubMed] [Google Scholar]
- 34.Pasch, A., J. H. Kupper, A. Wolde, R. Kandolf, and H. C. Selinka. 1999. Comparative analysis of virus-host cell interactions of haemagglutinating and non-haemagglutinating strains of coxsackievirus B3. J. Gen. Virol. 80:3153-3158. [DOI] [PubMed] [Google Scholar]
- 35.Roden, R. B., R. Kirnbauer, A. B. Jenson, D. R. Lowy, and J. T. Schiller. 1994. Interaction of papillomaviruses with the cell surface. J. Virol. 68:7260-7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rose, R. C., W. Bonnez, R. C. Reichman, and R. L. Garcea. 1993. Expression of human papillomavirus type 11 L1 protein in insect cells: in vivo and in vitro assembly of viruslike particles. J. Virol. 67:1936-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sapp, M., U. Kraus, C. Volpers, P. J. Snijders, J. M. Walboomers, and R. E. Streeck. 1994. Analysis of type-restricted and cross-reactive epitopes on virus-like particles of human papillomavirus type 33 and in infected tissues using monoclonal antibodies to the major capsid protein. J. Gen. Virol. 75:3375-3383. [DOI] [PubMed] [Google Scholar]
- 38.Sattentau, Q. J., and J. P. Moore. 1991. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J. Exp. Med. 174:407-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selinka, H. C., T. Giroglou, and M. Sapp. 2002. Analysis of the infectious entry pathway of human papillomavirus type 33 pseudovirions. Virology 299:279-287. [DOI] [PubMed] [Google Scholar]
- 40.Shukla, D., J. Liu, P. Blaiklock, N. W. Shworak, X. Bai, J. D. Esko, G. H. Cohen, R. J. Eisenberg, R. D. Rosenberg, and P. G. Spear. 1999. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99:13-22. [DOI] [PubMed] [Google Scholar]
- 41.Stehle, T., S. J. Gamblin, Y. Yan, and S. C. Harrison. 1996. The structure of simian virus 40 refined at 3.1 Å resolution. Structure 4:165-182. [DOI] [PubMed] [Google Scholar]
- 42.Stehle, T., and S. C. Harrison. 1997. High-resolution structure of a polyomavirus VP1-oligosaccharide complex: implications for assembly and receptor binding. EMBO J. 16:5139-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trus, B. L., R. B. Roden, H. L. Greenstone, M. Vrhel, J. T. Schiller, and F. P. Booy. 1997. Novel structural features of bovine papillomavirus capsid revealed by a three-dimensional reconstruction to 9 Å resolution. Nat. Struct. Biol. 4:413-420. [DOI] [PubMed] [Google Scholar]
- 44.Trybala, E., J. A. Liljeqvist, B. Svennerholm, and T. Bergstrom. 2000. Herpes simplex virus types 1 and 2 differ in their interaction with heparan sulfate. J. Virol. 74:9106-9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Unckell, F., R. E. Streeck, and M. Sapp. 1997. Generation and neutralization of pseudovirions of human papillomavirus type 33. J. Virol. 71:2934-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Volpers, C., P. Schirmacher, R. E. Streeck, and M. Sapp. 1994. Assembly of the major and the minor capsid protein of human papillomavirus type 33 into virus-like particles and tubular structures in insect cells. Virology 200:504-512. [DOI] [PubMed] [Google Scholar]
- 47.Volpers, C., F. Unckell, P. Schirmacher, R. E. Streeck, and M. Sapp. 1995. Binding and internalization of human papillomavirus type 33 virus-like particles by eukaryotic cells. J. Virol. 69:3258-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang, R., P. M. Day, W. H. T. Yutzy, K. Y. Lin, C. F. Hung, and R. B. Roden. 2003. Cell surface-binding motifs of L2 that facilitate papillomavirus infection. J. Virol. 77:3531-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou, J., X. Y. Sun, D. J. Stenzel, and I. H. Frazer. 1991. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology 185:251-257. [DOI] [PubMed] [Google Scholar]