Abstract
C57BL/6J mice infected intravenously with the Sarafend strain of West Nile virus (WNV) develop a characteristic central nervous system (CNS) disease, including an acute inflammatory reaction. Dose response studies indicate two distinct kinetics of mortality. At high doses of infection (108 PFU), direct infection of the brain occurred within 24 h, resulting in 100% mortality with a 6-day mean survival time (MST), and there was minimal destruction of neural tissue. A low dose (103 PFU) of infection resulted in 27% mortality (MST, 11 days), and virus could be detected in the CNS 7 days postinfection (p.i.). Virus was present in the hypogastric lymph nodes and spleens at days 4 to 7 p.i. Histology of the brains revealed neuronal degeneration and inflammation within leptomeninges and brain parenchyma. Inflammatory cell infiltration was detectable in brains from day 4 p.i. onward in the high-dose group and from day 7 p.i. in the low-dose group, with the severity of infiltration increasing over time. The cellular infiltrates in brain consisted predominantly of CD8+, but not CD4+, T cells. CD8+ T cells in the brain and the spleen expressed the activation markers CD69 early and expressed CD25 at later time points. CD8+ T-cell-deficient mice infected with 103 PFU of WNV showed increased mortalities but prolonged MST and early infection of the CNS compared to wild-type mice. Using high doses of virus in CD8-deficient mice leads to increased survival. These results provide evidence that CD8+ T cells are involved in both recovery and immunopathology in WNV infection.
WNV is a member of the Japanese encephalitis virus antigenic group within the family Flaviviridae, which can cause fatal encephalitis associated with damage to the CNS in humans and animals. Initially isolated in 1937, it is now recognized as one of the most widely distributed flaviviruses (11, 24), endemic in Africa, the Middle East, and parts of Asia and Europe. Since 1999, the virus has been recognized in North America by causing an epizootic among birds and horses and an epidemic of meningitis and encephalitis in humans (46). Surveillance of avian mortality showed geographic spread of WNV to the United States as well as to southeastern Canada (5). WNV causes a high frequency of inapparent infection in humans but may also cause fatal encephalitis in the elderly and children (47). There is no available therapy or prophylactic vaccine for WNV infection.
WNV infection has been studied in several animal models, including chickens, geese, rat, mice, hamsters, and monkeys (4, 9, 17, 31, 41, 48). However, the pathophysiology of invasiveness of WNV into the CNS, the mechanism of neurodegeneration, and the immune response after infection are still poorly understood. Mice provide a suitable animal model for flaviviral encephalitis in humans, as WNV-infected mice exhibited similar symptoms to those of humans in natural epidemics (13).
Humoral immunity plays an important role in flavivirus infections (43). The antibody response is predominantly directed against the E protein of flaviviruses and contributes to protection and recovery from disease (3, 7, 29). Mice immunized with the E protein of WNV are protected from infection with WNV, and passive transfer of E protein antisera provided protection against WNV infection in mice (14, 55). B-cell-deficient mice are highly susceptible to WNV infection, with high viral titers in the CNS, and the mice can be protected by passive transfer of WNV immune sera (14).
The precise role of T-cell immunity in WNV infection is still not fully understood (44). The CD8+ Tc cell response is essential for recovery from many primary viral infections (6, 49, 61, 64). As for encephalitic flaviviruses, potent Tc cell responses in the spleen are observed after peripheral infections (15, 23, 25, 37), with unusually extensive cross-reactivities on target cells infected with a wide spectrum of heterologous flaviviruses (15, 23, 26, 50). Virus-immune CD8+ T cells with cytolytic activity in vitro have been isolated from brains of WNV-infected mice (34). This finding, together with the observation that flavivirus infection of mammalian cells leads to an increase in cell surface expression of MHC class I, the recognition elements for CD8+ Tc cells, led to speculations as to possible host-pathogen strategies involving flavivirus-induced Tc cells and associated immunopathologies (22, 38, 39, 44).
In the present study, we investigated the role of CD8+ T cells in recovery and immunopathological processes of WNV encephalitis.
MATERIALS AND METHODS
Abbreviations.
β2-m, β2-microglobulin; CNS, central nervous system; Tc, cytotoxic T (lymphocyte); i.v., intravenous; i.c., intracerebral; LFB, luxol fast blue; MHC, major histocompatibility complex; MST, mean survival time; p.i., postinfection; WNV, West Nile virus; E, envelope; MEM, minimal essential medium; FCS, fetal calf serum; HBSS, Hanks' balanced salt solution; BSA, bovine serum albumin; PBS, phosphate-buffered saline; IHC, immunohistochemical; MAb, monoclonal antibody; IgG, immunoglobulin G; FACS, fluorescence-activated cell sorter; wt, wild type.
Mice.
C57BL/6J (B6, H-2b) mice and β2-m-knockout (β2-m−/−) mice (28, 63) bred onto the B6 background were supplied by the Animal Breeding Facility, The John Curtin School of Medical Research, The Australian National University (Canberra, Australia). All animals were housed in specific-pathogen-free conditions. Mice were used at 6 weeks of age.
Animals were cared for according to the guidelines of the Australian National University Animal Ethics Committee, and experiments conformed to the standards of the National Health and Medical Research Council, Canberra, Australia.
Virus and cells.
WNV, Sarafend strain, was passaged in suckling mouse brains (23, 25). Virus used for experiments was grown in mosquito cell line C6/36 cells to avoid interferon contamination and was titrated on Vero cell monolayers. Working stocks of culture supernatants were stored as aliquots at −70°C.
Vero cells were maintained in monolayer cultures at 37°C in a humidified atmosphere of 5% CO2 in air and were grown in MEM (Gibco-BRL Life Technologies, Inc., New York, N.Y.) supplemented with nonessential amino acids, penicillin-streptomycin-neomycin solution (0.1 g of penicillin G per liter, 0.16 g of streptomycin per liter, 0.16 g of neomycin per liter), and 5% heat-inactivated FCS (Trace Biosciences PTY Ltd., New South Wales, Australia).
Mouse inoculation and tissue processing.
Mice were inoculated through the tail vein with a single i.v. injection of WNV of different doses in 100 μl of HBSS with 0.2% BSA (HBSS-BSA, pH 8). Infected mice were monitored twice a day for signs of illness. Animals injected i.v. with HBSS-BSA only were used as negative controls. At indicated time points, groups of mice were deeply anesthetized with Rhodia Halothane (Merial Australia PTY Ltd., New South Wales, Australia). After cardiac puncture for blood sample collection and exsanguination, animals were perfused with 10 ml of sterile ice-cold PBS to remove leukocytes in blood vessels. PBS was injected into the left ventricle under mild pressure and drained from a cut in the right atrium. The brain was excised intact and then bisected. Half of the brain was homogenized for monocyte isolation. The other half was cut at coronal planes for virus titration and IHC examinations. For virus titration, specimens of brains and other tissues (described below) were quickly removed and frozen in liquid nitrogen. For histology, tissues were immersed in 10% neutral buffered formalin fixative at room temperature overnight and embedded in paraffin. For IHC, specimens of brains and spleens were placed into OCT compound (Sakura Finetek, Inc., Torrance, Calif.) and snap-frozen in liquid nitrogen.
Depletion of CD8+ T cells.
CD8+ T cells in B6 mice were depleted by intraperitoneal injection with 0.5 mg of rat anti-mouse Ly-2 MAb (53-6.7, IgG2a) in 500 μl, using a protocol described elsewhere (57) with modifications. Briefly, MAb was injected 3, 2, and 1 day before and 7 days after virus infection. Efficiency of depletion was assessed by FACS analysis using fluorescein isothiocyanate-conjugated anti-mouse Ly-3.2 MAb (53-5.8, IgG1; Pharmingen, San Diego, Calif.). The extent of depletion was found to be >98% and lasted for the entire experimental period (up to 21 days p.i.).
Virus titration.
Brain, serum, and other tissues (retroperitoneal lymph nodes, thymus, muscle, cardiac muscle, salivary gland, lung, liver, spleen, pancreas, kidney, uterus, ovary, and testis) were homogenized in HBSS-BSA (10% [wt/vol]) as a diluent. Serial 10-fold dilutions were inoculated onto Vero cell monolayers grown in six-well plastic plates (tissue culture grade; ICN Biomedicals, Inc., Aurora, Ohio). After 1 h of virus adsorption, monolayers were overlaid with 1% Bacto-Agar (Difco Laboratories, Detroit, Mich.) in MEM with 3% FCS. After 3 days at 37°C in a 5% CO2 atmosphere, monolayers were stained with 0.02% neutral red in HBSS for 16 h and fixed with 5% formaldehyde. The plaques were counted, and the virus titers were expressed as PFU per gram of tissue or PFU per milliliter of serum, as appropriate.
Histology and IHC.
Midsagittal plane-bisected brains and spinal cords were fixed in 10% neutral buffered formalin and embedded in paraffin. For examination of cell morphology and myelin, 6-μm sections were stained with hematoxylin-eosin and LFB.
IHC staining was performed as described elsewhere (56, 57). Briefly, 5-μm-diameter frozen serial sagittal or coronal sections were cut with a cryostat and immediately placed on poly-l-lysine-precoated slides (Sigma, St. Louis, Mo.). After air drying for 24 h, cryostat sections were fixed in cold Zamboni's fixative solution (54) and acetone. An avidin-biotin complex technique was used. CD4+ T lymphocytes were identified by using rat MAb anti-L3T4 (59, 60). Lymphocytes of the CD8+ phenotype were identified by using rat anti-Lyt-2 MAb (32, 45). B cells were identified by using rat anti-CD45R/B220 MAb (10, 21). These reagents were obtained from PharMingen. Rat anti-F4/80 antibody was used to identify macrophages (Serotec, Ltd., Kidlington, Oxford, United Kingdom) (2, 16). The secondary antibody was a biotinylated rabbit anti-rat Ig (DAKO Corporation, Carpinteria, Calif.). For negative controls, primary antibodies were replaced with equivalent concentrations of normal rat Ig (Sigma). All specimens were stained in duplicate. To avoid false-negative staining, a spleen section from a normal mouse was placed and stained on every slide as a positive control. Brown staining of cells was regarded as positive immune reactivity.
Lymphocyte isolation from the brain.
To evaluate the i.c. immune response, the lymphocytes in the brain of wt B6 virus- or mock-infected mice were phenotyped using FACS analysis. After in situ perfusion with PBS (see above), half of the brain was put into ice-cold MEM-10% FCS with 25 mM HEPES (pH 8). The samples were homogenized by gently pressing them through a 100-mesh tissue sieve and digested with 2 mg of collagenase type I (Gibco-Life Technologies, Grand Island, N.Y.) per ml in MEM-5% FCS for 30 min at 37°C with shaking. Homogenates (derived from single animals) were then centrifuged at 400 × g for 10 min, and the pellets were resuspended in 2 ml of 90% Percoll (Sigma) in MEM. The suspension was transferred to a 15-ml test tube and then overlaid gently with 60, 40, and 10% Percoll in MEM. The gradients were centrifuged at 800 × g for 45 min at 22°C. The lymphocytes were collected from the 40 to 60% interface and washed twice with MEM-5% FCS.
Fluorescence staining and flow cytometry analysis.
Frozen brain tissue was cut into 6-μm sections and immediately fixed in acetone and air dried. The slides were incubated with mouse anti-WNV MAb (2B2) (19) for 1 h at room temperature. After washing, sections were incubated with fluorescein isothiocyanate-conjugated sheep anti-mouse IgG (Sigma) for 1 h. The slides were washed twice and counterstained with Harris hematoxylin (Sigma). Negative controls for staining were performed, with normal mouse IgG (Sigma) used as the primary antibodies. The sections were examined under a Zeiss Axiophot fluorescence microscope.
Dual-color analysis was performed to assess the phenotype of splenocytes and the freshly isolated inflammatory cells from brains of B6 virus- or mock-infected mice. Expression of cell surface markers on splenocytes was determined by staining cells with antibodies specific for CD3 (clone 145-2C11, PharMingen), CD4 (GK1.5, PharMingen), CD8 (53-6.7, PharMingen), CD45R/B220 (RA3-6B2, PharMingen), F4/80 (CI:A3-1, Serotec, Inc., Raleigh, N.C.), NK1.1 (PK136, PharMingen), CD25 (PC61-5.3, Caltag Laboratories, Burlingame, Calif.), and CD69 (H1.2F3, PharMingen). The brain-derived lymphocytes were incubated with anti-CD4, -CD8, -CD25, and -CD69 MAbs.
Single-cell suspensions of splenocytes were obtained by gently pressing the organ through a fine steel mash after weighing the spleen. Erythrocytes were lysed with lysing buffer (150 mM ammonium chloride, 10 mM potassium carbonate, 0.1 mM Na4-EDTA [pH 7.5]). Brain infiltrates were prepared as described. A total of 5 × 105 splenocytes or brain-derived lymphocytes from single animals were suspended in 100 μl of cold (4°C) MEM with 5% FCS and incubated with Fc Block (2.4G2; PharMingen) for 15 min at 4°C. After being washed, cells were then incubated with the relevant antibodies at 4°C for 30 min in darkness and then washed three times with FACS washing buffer (2% [vol/vol] FCS and 0.01% [wt/vol] sodium azide [Sigma] in PBS). Cells were fixed with 2% (wt/vol) paraformaldehyde in PBS and stored in darkness at 4°C until analysis with a FACScan (Becton Dickinson, San Jose, Calif.) with CellQuest software.
RESULTS
Effect of WNV dose on disease progression in 6-week-old B6 mice.
To determine the dose of WNV required for CNS involvement to occur, mice were infected with a dose range of 1 to 108 PFU of WNV i.v. Surviving mice were positive in serological tests, indicating active infection at all doses. The cumulative mortalities and the MSTs of groups of mice infected with various doses are shown in Table 1. Using a dosage of 108 PFU caused 100% mortality, with an MST of 5.6 ± 0.7 days. Mortality was first observed at 5 days p.i., and all mice had died by 7 days. Using a dosage of 107 PFU also resulted in 100% death. However, only 3 mice out of 10 died, with an MST similar to that observed with a dose of 108 PFU; the others died after 10 days, with an MST similar to that observed with a dose range of 102 to 106 PFU. No dose response was observed within this dose range. The mortality rates of these groups ranged from 27 to 40% (no significant difference between each group), with an MST of about 11 days. Moribund mice showed clinical signs of wasting, hunching, ruffling of fur, and finally hind-limb paralysis, suggesting that viral encephalitis was the cause of death. At doses of 10 PFU or lower, no death occurred (Table 1). No sex differences in mortality and MST were found (data not shown).
TABLE 1.
Effect of WNV dose on mortality and survival time of B6 mice
| Dosea (PFU/mouse) | Total no. of miceb | No. of dead mice | Survival time (days) | Mortality (%) | MSTc (days) |
|---|---|---|---|---|---|
| 108 | 30 | 15 | 5 | 100 | 5.6 ± 0.7 |
| 11 | 6 | ||||
| 4 | 7 | ||||
| 107 | 3 | 1 | 5 | 100 | 5.7 ± 0.8 |
| 2 | 6 | ||||
| 7 | 1 | 10 | 12.0 ± 1.0 | ||
| 4 | 12 | ||||
| 2 | 13 | ||||
| 106 | 10 | 1 | 10 | 30 | 11.0 ± 1.0 |
| 1 | 11 | ||||
| 1 | 12 | ||||
| 105 | 10 | 1 | 9 | 30 | 10.0 ± 1.0 |
| 1 | 10 | ||||
| 1 | 11 | ||||
| 103 | 30 | 5 | 10 | 27 | 10.5 ± 0.8 |
| 2 | 11 | ||||
| 1 | 12 | ||||
| 102 | 10 | 1 | 9 | 40 | 12.0 ± 3.2 |
| 1 | 10 | ||||
| 1 | 13 | ||||
| 1 | 16 | ||||
| 10 | 10 | 0 | >21 | 0 | |
| 1 | 10 | 0 | >21 | 0 |
Virus dose in 100 μl of HBSS injected via tail vein.
All surviving mice seroconverted; WNV-specific antibodies were detected by enzyme-linked immunosorbent assay at day 21 p.i.
Values represent MST ± standard deviation of mice which died up to 21 days p.i. Differences in mortality were analyzed by χ2 test, and differences in MST were assessed by Student's t test (see Materials and Methods for details).
Statistical analysis of the MST (Student's t test) and percent mortality (χ2 test) between groups infected with different doses found no significant difference between the 102 and 106 PFU virus doses. Significant differences in the MST (P < 0.0001) and mortality (P < 0.0001) were observed when comparing groups infected with 108 and 102 to 106 PFU.
These dose response data are similar to observations made with the related Murray Valley encephalitis virus (33) and indicate that two different mechanisms are involved in mortality caused by encephalitic flavivirus infections. When a low dose (103 PFU) of WNV is inoculated by the i.c. route, death occurs at day 5 to 7 p.i., with 100% mortality and clinical symptoms comparable to those following high-dose (108 PFU) peripheral infection (data not shown). Thus, i.v. inoculation with a high dose of WNV appears to lead to rapid and direct infection of the CNS. Death following lower-dose peripheral infections is delayed by 6 to 7 days compared to that caused by high-dose i.v. or an i.c. injection; this observation suggests that replication in extraneural tissue is required. Doses of 108 and 103 PFU/mouse were chosen as the typical high and low doses, respectively, for further study.
WNV-induced disease and virus replication.
Mice, when infected i.v. with 108 PFU of WNV, became sick rather suddenly 4 days after infection, and they died in the following 3 days. A group infected i.v. with 103 PFU/mouse (low dose) showed a characteristic progression of disease signs. After 6 or 7 days p.i., some of the mice showed slight ruffling of the fur and hunching of the back. Hind-limb weakness progressing to paralysis appeared in the subsequent couple of days, accompanied by marked ataxia. Moribund mice developed clearly recognizable paralysis of tails and hind limbs, with severe hunching and wasting.
The kinetics of viral replication in the brains and peripheral organs of mice inoculated with high or low doses were determined at 1- or 2-day intervals (Table 2 and 3). In the high-dose group (Table 2), WNV could be detected in brains from 24 h p.i. and virus load increased until days 4 to 5, reaching peak titers of 108 to 109 PFU/g; the titers then declined until the animals died. Virus could be isolated from thymus, spleen, lymph node, and liver. Virus titers in these organs decreased steadily and were undetectable after 3 to 5 days. Virus titers in brains of 103 PFU-infected mice are shown in Table 3. Virus was first detected in brain cells on day 7 p.i., in two mice out of six, and the titer increased until the hosts died. In other mice, no virus could be detected in brains over the length of the experimental period of up to 12 days. Virus in hypogastric lymph nodes and spleens was detectable at days 4 and 5 p.i. in some animals but not in others. No virus was found in these tissues from day 7 onward. No virus was detectable in other extraneural tissues, and no demonstrable viremia was observed in any mouse throughout the experiment.
TABLE 2.
Virus titers in B6 mice infected with a high dose (108 PFU) of WNVa
| Days p.i. | Serum | Brain | Liver | Spleen | LN | Thymus |
|---|---|---|---|---|---|---|
| 1 | <102 | 1.0 × 105 | 5.0 × 104 | 1.0 × 104 | 6.0 × 104 | 1.5 × 103 |
| <102 | 2.0 × 104 | 5.0 × 104 | 5.0 × 104 | 7.2 × 104 | 3.2 × 103 | |
| <102 | 3.8 × 104 | 6.8 × 104 | 2.0 × 104 | 9.0 × 103 | 6.8 × 103 | |
| <102 | 7.3 × 103 | 3.2 × 104 | 1.0 × 104 | 4.3 × 104 | 5.2 × 103 | |
| <102 | <103 | 4.7 × 104 | 7.7 × 104 | <103 | <103 | |
| 2 | <102 | 2.0 × 105 | 5.0 × 103 | 1.5 × 103 | 2.2 × 103 | 6.5 × 103 |
| <102 | 1.5 × 104 | 5.0 × 104 | 1.5 × 103 | 8.5 × 103 | 5.4 × 103 | |
| <102 | 9.2 × 104 | 2.9 × 104 | 2.0 × 104 | 6.0 × 104 | 1.3 × 103 | |
| <102 | 6.8 × 105 | 1.0 × 105 | 7.0 × 103 | 1.5 × 104 | <103 | |
| <102 | 3.8 × 105 | 3.2 × 104 | 5.0 × 104 | <103 | <103 | |
| 3 | <102 | 1.7 × 107 | 5.0 × 104 | 5.0 × 104 | 5.0 × 103 | 5.0 × 103 |
| <102 | 1.0 × 107 | 1.2 × 103 | 3.5 × 103 | 3.0 × 104 | 1.8 × 103 | |
| <102 | 7.6 × 107 | 2.0 × 104 | 5.2 × 103 | 9.5 × 103 | 3.0 × 103 | |
| <102 | 3.9 × 105 | <103 | 1.2 × 103 | 4.4 × 103 | <103 | |
| <102 | 5.3 × 107 | <103 | <103 | <103 | <103 | |
| 4 | <102 | 1.2 × 109 | 1.2 × 103 | 6.0 × 104 | 4.5 × 103 | <103 |
| <102 | 3.0 × 108 | 3.2 × 103 | 1.2 × 103 | 2.7 × 103 | <103 | |
| <102 | 7.2 × 108 | <103 | <103 | 1.4 × 103 | <103 | |
| <102 | 5.0 × 108 | <103 | <103 | <103 | <103 | |
| <102 | 1.1 × 109 | <103 | <103 | <103 | <103 | |
| 5 | <102 | 3.2 × 108 | <103 | <103 | 2.0 × 103 | <103 |
| <102 | 2.5 × 108 | <103 | <103 | 2.7 × 103 | <103 | |
| <102 | 1.1 × 109 | <103 | <103 | 4.2 × 103 | <103 | |
| <102 | 7.9 × 108 | <103 | <103 | <103 | <103 | |
| <102 | 2.0 × 108 | <103 | <103 | <103 | <103 | |
| 6 | <102 | 2.0 × 108 | <103 | <103 | <103 | <103 |
| <102 | 3.7 × 107 | <103 | <103 | <103 | <103 | |
| <102 | 6.9 × 107 | <103 | <103 | <103 | <103 |
Values are given as PFU per milliliter of serum, PFU per gram of tissue, and PFU per lymph node (LN). Viral titers were determined by Vero cell plaque assay. The limit of detection was 102 PFU/ml for serum, 103 PFU/g for tissue, or 103 PFU/LN.
TABLE 3.
Virus titers in B6 mice infected with a low dose (103 PFU) of WNVa
| Days p.i. | Serum | Brain | Liver | Spleen | LNa | Thymus |
|---|---|---|---|---|---|---|
| 1 | <102 | <103 | <103 | <103 | <103 | <103 |
| <102 | <103 | <103 | <103 | <103 | <103 | |
| <102 | <103 | <103 | <103 | <103 | <103 | |
| <102 | <103 | <103 | <103 | <103 | <103 | |
| <102 | <103 | <103 | <103 | <103 | <103 | |
| 2 | <102 | <103 | <103 | <103 | <103 | <103 |
| <102 | <103 | <103 | <103 | <103 | <103 | |
| <102 | <103 | <103 | <103 | <103 | <103 | |
| <102 | <103 | <103 | <103 | <103 | <103 | |
| <102 | <103 | <103 | <103 | <103 | <103 | |
| 3 | <102 | <103 | <103 | <103 | <103 | <103 |
| <102 | <103 | <103 | <103 | <103 | <103 | |
| <102 | <103 | <103 | <103 | <103 | <103 | |
| <102 | <103 | <103 | <103 | <103 | <103 | |
| <102 | <103 | <103 | <103 | <103 | <103 | |
| 4 | <102 | <103 | <103 | 4.5 × 104 | 1.5 × 104 | <103 |
| <102 | <103 | <103 | 6.4 × 103 | 5.3 × 103 | <103 | |
| <102 | <103 | <103 | 1.5 × 104 | 4.7 × 103 | <103 | |
| <102 | <103 | <103 | <103 | <103 | <103 | |
| <102 | <103 | <103 | <103 | <103 | <103 | |
| 5 | <102 | <103 | <103 | 3.6 × 103 | 1.8 × 103 | <103 |
| <102 | <103 | <103 | 2.5 × 103 | 1.1 × 103 | <103 | |
| <102 | <103 | <103 | <103 | <103 | <103 | |
| <102 | <103 | <103 | <103 | <103 | <103 | |
| <102 | <103 | <103 | <103 | <103 | <103 | |
| 7 | <102 | 1.1 × 104 | <103 | <103 | <103 | <103 |
| <102 | 4.5 × 103 | <103 | <103 | <103 | <103 | |
| <102 | <103 | <103 | <103 | <103 | <103 | |
| <102 | <103 | <103 | <103 | <103 | <103 | |
| <102 | <103 | <103 | <103 | <103 | <103 | |
| 9 | <102 | 2.1 × 106 | <103 | <103 | <103 | <103 |
| <102 | 5.5 × 105 | <103 | <103 | <103 | <103 | |
| <102 | 2.5 × 106 | <103 | <103 | <103 | <103 | |
| <102 | <103 | <103 | <103 | <103 | <103 | |
| <102 | <103 | <103 | <103 | <103 | <103 | |
| 11-12 | <102 | 2.0 × 105 | <103 | <103 | <103 | <103 |
| <102 | 2.0 × 106 | <103 | <103 | <103 | <103 | |
| <102 | 2.9 × 106 | <103 | <103 | <103 | <103 | |
| <102 | <103 | <103 | <103 | <103 | <103 | |
| <102 | <103 | <103 | <103 | <103 | <103 |
Units for values and abbreviations are as described in Table 2, footnote a.
Histopathology.
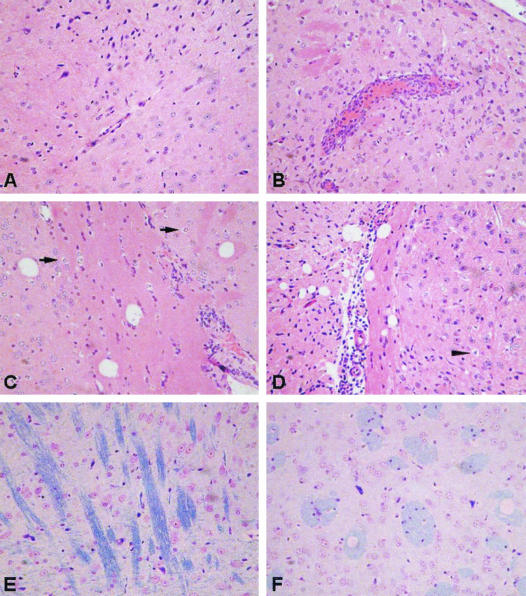
Brains from infected animals were prepared as described in Materials and Methods and examined for pathological changes. The cerebral cortices of mice in the high-dose group showed some vascular congestion by day 4 p.i. Scattered small perivascular foci and perivascular leukocyte infiltration were noted at 4 to 6 days p.i. Parenchymal edema, glial proliferation, and neurodegeneration were also present, but vascular degeneration of myelin and inflammatory cell infiltration within the brain parenchyma were less than that present in low-dose-infected mice (see below), even when mice were moribund (data not shown). In contrast, the cerebral cortices of animals infected with 103 PFU of WNV showed inflammatory changes with leukocytic infiltration that involved both the brain parenchyma and the leptomeninges. Representative histological changes in brains of control and low-dose-infected mice are shown in Fig. 1. Prominent perivascular edema and vascular engorgement could be observed on day 7 p.i., accompanied by polymorphonuclear and mononuclear leukocytic margination and perivascular accumulation (Fig. 1B). Perivascular foci of infection and leukocyte infiltration were found on day 9 p.i. (Fig. 1C). In addition, the cerebral ventricles were dilated, and scattered inflammatory cells were present. Leukocyte infiltration was more prominent, and meningeal involvement increased in the following 2 to 3 days. Scattered individual neurons showed characteristic cytoplasmic rarefaction on day 9. The cytoplasm of these neurons appeared as round, empty spaces with the condensed nucleus at the center due to cytoplasmic condensation and nuclear compaction (Fig. 1C and D). Neuronal degeneration and necrosis were particularly prominent in the perivascular areas where marked leukocytic infiltration was present.
FIG. 1.
Histology of WNV-infected brains. Representative photomicrographs of brain sections from control and 103 PFU WNV-inoculated B6 mice. (A) Uninfected mouse brain; (B) vascular congestion and perivascular leukocyte infiltration in brain parenchyma, 7 days p.i.; (C) mild vacuolization and inflammation in brain parenchyma, 9 days p.i.; (D) severe vacuolization and inflammation in brain parenchyma, 11 days p.i. Neurons show degenerative changes with cytoplasmic rarefaction and rounding (arrows, panel C) and necrosis with nuclear compaction (arrow head, panel D). Panels A through D depict Hematoxylin-eosin stain, ×200 magnification. (E) Normal myelin display from uninfected mouse brain (blue stained); (F) vascular degeneration occurred mainly in the myelin area of the infected mouse brain. LFB stain, ×400 magnification.
Brain inflammation in WNV infection.
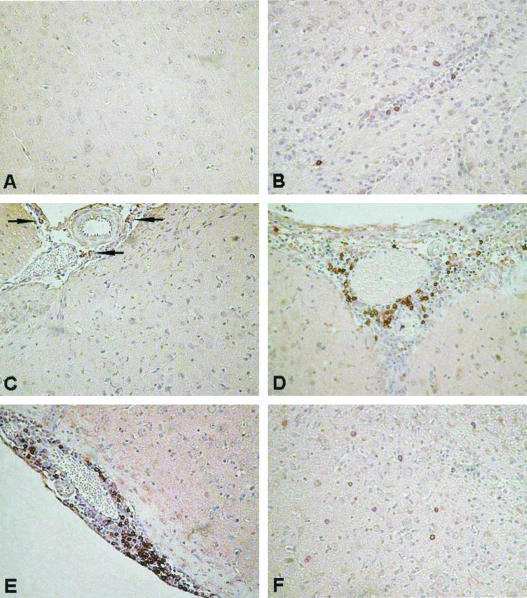
IHC studies of uninfected brains showed small numbers of resident macrophages within brain parenchyma; lymphocytes were not detectable. CD8+ T-cell and B-cell margination and perivascular accumulation, as well as inflammation of leptomeninges, were observed in moribund mice infected with 108 PFU of WNV (data not shown), but infiltration of these cells into the brain parenchyma was far less than that of moribund mice of the low-dose (103 PFU) group. Mice in the low-dose group, with clinical signs and detectable virus in the brain, showed inflammatory cell infiltration from day 7 p.i., and the number of inflammatory cells increased steadily in the brains of sick and moribund mice over time. Most of the infiltrated cells were CD8+ T cells (Fig. 2) and macrophages. Only small numbers of B cells were found, predominantly in perivascular spaces. Virtually no CD4+ cells could be detected at any time point throughout the experiments in the CNS of WNV-infected mice that had been given either a high or low dose of virus.
FIG. 2.
IHC of WNV-infected brains. CD8+ cells localized in the brains of uninfected and 103 PFU WNV-infected B6 wt mice. (A) Undetectable CD8+ cells in the brains of uninfected mice; (B) perivascular CD8+ cell infiltration in brain parenchyma, 7 days p.i.; (C) vascular congestion and CD8+ cell (arrows) infiltration, 7 days p.i.; (D) perivascular CD8+ cell inflammation and infiltration of brain parenchyma, 9 days p.i.; (E) severe vascular congestion, perivascular and leptomeningeal CD8+ cell inflammation, and infiltration of leptomeninges and brain parenchyma, 11 days p.i.; (F) widespread CD8+ cell infiltration of brain parenchyma, 11 days p.i. Magnification, ×400.
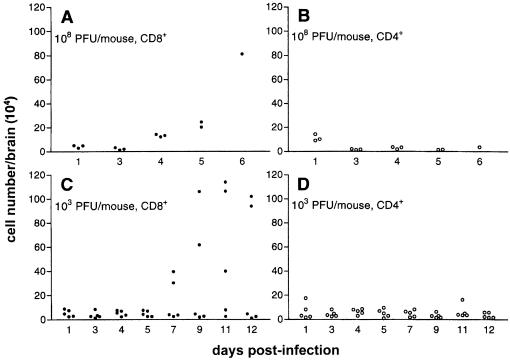
Inflammatory cells were isolated from brains of infected mice by density gradient centrifugation. Virtually no lymphocytes were found in the brains of uninfected mice. Although rarely detected by IHC, we found that there was recruitment of CD8+ T cells from day 4 onward in the brains of mice infected with a high dose of WNV (Fig. 3A). The number of CD8+ T cells in brains of two or three out of groups of five mice given a low dose of WNV significantly increased from day 7, to more than 30-fold by day 12 (Fig. 3C), a result that is compatible with our IHC observations. No change due to WNV infection in the number of CD4+ cells was found (Fig. 3B and D).
FIG. 3.
Leukocyte isolation from WNV-infected mouse brains. Yields of inflammatory T cells in brains of WNV-infected mice. Mice were infected i.v. with a high dose (108 PFU) (A and B) or a low dose (103 PFU) (C and D) of virus. The infiltrated lymphocytes were isolated from the brains of individual mice by density gradient isolation at each time point and were analyzed using flow cytometry as described in Materials and Methods. T cells were undetectable in the brains of uninfected mice.
Phenotypic analysis of splenocytes from WNV-infected mice.
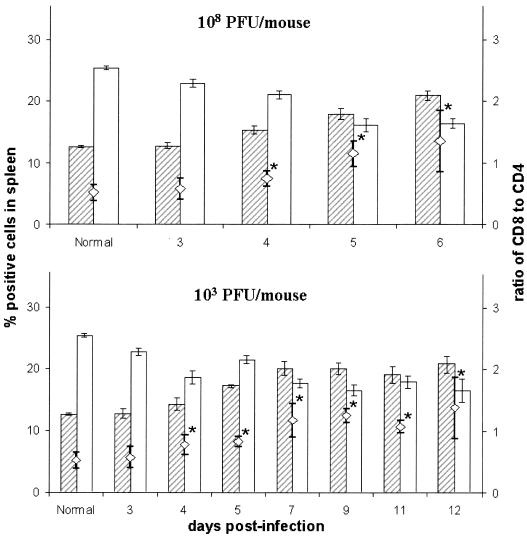
To evaluate the systemic immune response after WNV infection, the composition of leukocyte populations in the spleen was enumerated by FACS phenotyping. No significant difference was observed in weight and total cell number of spleens from uninfected and high- and low-dose WNV-infected mice over the entire experimental period of 6 and 12 days, respectively (data not shown). The mean percentage of T cells (CD3+) (∼40%) did not significantly differ as a result of WNV infection from the mean value of uninfected mice. Similarly, the percentages of B cells (CD45R/B220+) and NK cells (NK1.1+) in the spleens of either 108 PFU- or 103 PFU WNV-infected or uninfected mice were not significantly different (data not shown). The percentages of macrophages in the spleen significantly increased to a peak at day 5 p.i. in both high- and low-dose groups compared with those in uninfected mice (17.9% ± 4.7% and 14.4% ± 2.0% versus 11.8% ± 3.7%; P < 0.01 and P < 0.05, respectively). WNV infection caused significant changes in the ratio of CD8+ to CD4+ T cells in the spleens, due to the increase in the number of CD8+ T cells, in both high- and low-dose groups, with a simultaneous decrease in CD4+ T cells (Fig. 4).
FIG. 4.
T-cell composition of spleens after WNV infection. Percentages of CD8+ (shaded bars) and CD4+ (empty bars) cells in spleens of mice infected with 108 PFU (upper panel) and 103 PFU (lower panel) of WNV. Values shown are the mean values ± standard errors of the means of three to five mice per group. The CD8/CD4 ratios are indicated by open diamonds (◊). *, significant difference (P < 0.05) between infected and control mice.
Activation state of CD8+ T cells in spleen and brain after WNV infection.
The expansion of the peripheral CD8+ T-cell pool in the spleen as a consequence of WNV infection and subsequent infiltration into the CNS suggests an important role of this immune cell subpopulation in the control of WNV and possible immunopathological consequences. To further investigate the activation state of the CD8+ T-cell population within splenocyte and brain infiltrates following WNV infections, we studied the expression of markers that are usually associated with an activated phenotype (40, 62). CD8+ T cells from mice infected with 103 PFU of WNV were analyzed by flow cytometry for expression of the early activation marker CD69, which is expressed rapidly after lymphocyte activation (62), and the α-chain of the interleukin 2 receptor (CD25), which is expressed on activated T and B lymphocytes (40). Control mice were injected with the same volume of diluted, uninfected C6/36 cell culture supernatant, mimicking the composition of the viral inoculum. Cells were stained with anti-CD8 and either anti-CD69 or anti-CD25 MAb. Double-positive cells (CD8+-CD69+ or CD8+-CD25+) were enumerated relative to the total number of CD8+ cells (Table 4). The number of CD8+-CD69+ T cells in the spleen, as well as the expression level of CD69, increased at 1 and 2 days p.i. No significant changes were found at later time points. The percentage of CD8+-CD69+ cells in brain infiltrates decreased from a peak of 30% at 1 day p.i. to 6% (P < 0.001) on day 3, and the level decreased further to <1% by day 9. The number of CD8+ T cells in the brains of uninfected animals was too small to allow us to evaluate their activation state. WNV infection resulted in a significant increase of the percentage of CD8+ T cells expressing CD25 from day 4 onward in the spleen and day 5 among brain infiltrates (Table 4).
TABLE 4.
Percentage of activated CD8+ T cells in spleen and brain cells after infection of mice with 103 PFU of WNVa
| Days p.i. | % CD8+ T cells expressing activation markers (SD)
|
|||
|---|---|---|---|---|
| Spleen
|
Brain
|
|||
| CD69 | CD25 | CD69 | CD25 | |
| 0 (Control) | 1.6 (1.1) | 1.5 (1.0) | NDb | ND |
| 1 | 5.7 (1.1)c | 1.7 (0.5) | 28.8 (7.7) | 1.6 (1.2) |
| 2 | 4.7 (2.2)c | 1.4 (0.4) | 31.1 (5.3) | 1.0 (0.6) |
| 3 | 2.3 (0.5) | 1.8 (0.4) | 6.3 (3.3)d | 1.6 (0.8) |
| 4 | 2.4 (0.6) | 6.7 (0.8)c | 3.7 (1.6)d | 4.5 (5.0) |
| 5 | 2.5 (1.3) | 3.8 (3.0)c | 3.9 (1.9)d | 6.2 (7.7) |
| 7 | 2.0 (1.1) | 4.2 (1.4)c | 3.6 (2.8)d | 9.6 (5.2)e |
| 9 | 1.8 (0.9) | 3.4 (0.9)c | 0.8 (0.5)d | 9.7 (3.6)e |
| 11 | 1.5 (0.8) | 2.8 (1.5) | 0.9 (0.8)d | 10.9 (9.2)e |
| 12 | 1.7 (1.2) | 3.1 (1.7)c | 0.8 (1.1)d | 9.8 (9.2)e |
B6 mice were injected i.v. with 103 PFU of WNV (n = 5).
ND, not detectable.
P < 0.05 compared with mock-treated mice (control).
P < 0.05 compared with days 1 and 2.
P < 0.05 compared with days 1, 2, and 3.
Mortality due to WNV infection of CD8+ T-cell-deficient (β2-m−/−) mice.
β2-m-deficient mice are phenotypically CD8+ T cell deficient due to the lack of the MHC class I CD8+ T-cell-selecting molecules (63). The β2-m−/− mice were infected i.v. with either 108 or 103 PFU of WNV (Table 5). In the 108 PFU group, 20% of the β2-m−/− mice survived for more than 6 weeks. This contrasts with the 100% mortality rate for the B6 wt mice. In addition, the 80% of β2-m−/− mice which died showed a significant increase in survival time (MST, 9.7 ± 2.9 days, P < 0.001) relative to the MST of wt animals of 5.6 ± 0.7 days (Table 1). In the low-dose group, i.e., β2-m−/− mice infected with 103 PFU of virus, the absence of CD8+ T cells led to an increase in the mortality rate (80.0%) when compared with wt mice (26.7%, Table 1). However, these mice showed a significant (P = 0.0012) increase in MST, from 10.5 ± 0.8 B6 mice (Table 1) to 13.0 ± 2.0 β2-m−/− mice.
TABLE 5.
Mortality of β2-m−/− mice due to WNV infection
| Dose (PFU/mouse) | Total no. of mice | No. of dead mice | Survival time (days) | Mortality (%) | MSTa (days) |
|---|---|---|---|---|---|
| 108 | 28 | 2 | 5 | 82 | 9.7 ± 2.9b |
| 2 | 6 | ||||
| 3 | 7 | ||||
| 3 | 8 | ||||
| 5 | 11 | ||||
| 3 | 12 | ||||
| 4 | 13 | ||||
| 1 | 14 | ||||
| 103 | 30 | 1 | 9 | 80 | 13.0 ± 2.0b |
| 2 | 10 | ||||
| 3 | 11 | ||||
| 5 | 12 | ||||
| 3 | 13 | ||||
| 2 | 14 | ||||
| 6 | 15 | ||||
| 2 | 16 |
Values represent MST ± standard deviation of mice which died up to 21 days after infection.
Statistically significant (P < 0.05) compared to B6 mice (see Materials and Methods for details).
Virus growth and pathology in β2-m−/− mice.
The β2-m−/− mice were infected with either 108 or 103 PFU of WNV, and viral titers were estimated in blood, brain, liver and spleen (Table 6 and 7). With a dosage of 108 PFU of WNV, we detected virus in the blood of only two animals with low titers. From brain cells, virus was isolated after 1 day p.i., increased, and reached plateau levels by day 5 to 6, similar to that seen in B6 mice. Unlike wt mice, β2-m−/− mice survived up to 16 days p.i. (Table 5) and with high viral loads (Table 6). In β2-m−/− mice, in contrast to B6 wt mice, no virus was detectable in liver and spleen over the first 4 days p.i. At later time points (between days 5 and 10 p.i.), low titers were found in some, but not all, β2-m−/− mice.
TABLE 6.
Virus replication in β2-m−/− mice infected with a high dose (108 PFU) of WNVa
| Days p.i. | Serum | Brain | Liver | Spleen |
|---|---|---|---|---|
| 1 | <102 | 4.0 × 104 | <103 | <103 |
| <102 | 7.3 × 103 | <103 | <103 | |
| <102 | 2.0 × 104 | <103 | <103 | |
| 2 | <102 | 1.5 × 106 | <103 | <103 |
| <102 | 2.0 × 105 | <103 | <103 | |
| <102 | 3.0 × 106 | <103 | <103 | |
| 3 | <102 | 7.0 × 103 | <103 | <103 |
| <102 | 5.1 × 109 | <103 | <103 | |
| 5.0 × 103 | 6.6 × 106 | 6.0 × 104 | <103 | |
| 4 | <102 | 1.6 × 108 | <103 | <103 |
| <102 | 6.2 × 107 | <103 | <103 | |
| <102 | 5.8 × 108 | <103 | <103 | |
| 5 | <102 | 3.4 × 109b | <103 | <103 |
| 7.2 × 104 | 4.4 × 107 | <103 | <103 | |
| <102 | 6.5 × 108 | 5.0 × 103 | 1.0 × 103 | |
| 6 | <102 | 4.9 × 106 | <103 | <103 |
| <102 | 7.2 × 109 | <103 | <103 | |
| <102 | 1.0 × 109b | 4.0 × 103 | 2.0 × 103 | |
| 7 | <102 | 5.0 × 107 | <103 | <103 |
| <102 | 2.0 × 108b | 4.0 × 105 | 6.1 × 103 | |
| <102 | 6.4 × 109b | <103 | <103 | |
| 9 | <102 | 3.7 × 109 | <103 | <103 |
| <102 | 2.6 × 109b | 4.0 × 104 | 2.5 × 104 | |
| <102 | 1.5 × 109b | 5.0 × 103 | 2.5 × 103 | |
| 11 | <102 | 9.3 × 106 | <103 | <103 |
| <102 | 4.8 × 106 | <103 | <103 | |
| <102 | 2.9 × 109b | <103 | <103 | |
| 13 | <102 | 7.0 × 109b | <103 | <103 |
| <102 | 3.6 × 108 | <103 | <103 | |
| <102 | 1.7 × 109b | <103 | <103 |
Units for values are as described in Table 2, footnote a. The limit of detection was 102 PFU/ml for serum or 103 PFU/g for tissue. Mice were randomly selected from a pool of experimental mice early after infection. Table contains data from four independent experiments.
Values for moribund mice, which were used at later time points.
TABLE 7.
Virus replication in β2-m−/− mice infected with a low dose (103 PFU) of WNVa
| Days p.i. | Serum | Brain | Liver | Spleen |
|---|---|---|---|---|
| 1 | <102 | <103 | <103 | <103 |
| <102 | <103 | <103 | <103 | |
| <102 | <103 | <103 | <103 | |
| 2 | <102 | 1.0 × 103 | <103 | <103 |
| <102 | <103 | <103 | <103 | |
| <102 | <103 | <103 | <103 | |
| 3 | <102 | <103 | <103 | <103 |
| <102 | <103 | <103 | <103 | |
| <102 | <103 | <103 | <103 | |
| 4 | <102 | <103 | <103 | <103 |
| <102 | 4.8 × 103 | <103 | <103 | |
| 5.0 × 104 | 6.6 × 103 | 7.2 × 103 | 2.0 × 103 | |
| 5 | <102 | 8.3 × 106 | 1.5 × 103 | 2.0 × 103 |
| <102 | 6.2 × 104 | 2.3 × 104 | 1.5 × 103 | |
| <102 | 9.2 × 103 | <103 | <103 | |
| 6 | <102 | 4.8 × 106 | <103 | <103 |
| <102 | 3.5 × 104 | <103 | <103 | |
| <102 | 6.9 × 106 | <103 | <103 | |
| 7 | <102 | 1.6 × 106 | 1.0 × 103 | <103 |
| <102 | 6.3 × 109 | <103 | <103 | |
| <102 | 3.6 × 105 | 4.0 × 104 | 1.0 × 103 | |
| 9 | <102 | 5.0 × 108 | <103 | <103 |
| <102 | 6.0 × 109b | <103 | <103 | |
| <102 | 2.2 × 105 | <103 | <103 | |
| 11 | <102 | 4.9 × 109b | <103 | <103 |
| <102 | 3.2 × 109b | <103 | <103 | |
| <102 | 5.6 × 106 | <103 | <103 | |
| 13 | <102 | 8.4 × 108 | <103 | <103 |
| <102 | 9.2 × 109b | <103 | <103 | |
| <102 | 7.7 × 107 | <103 | <103 | |
| 15 | <102 | 2.1 × 109b | <103 | <103 |
| <102 | 4.6 × 108 | <103 | <103 | |
| <102 | 7.5 × 109b | <103 | <103 |
Units for values are as described in Table 2, footnote a. The limit of detection is 102 PFU/ml for serum or 103 PFU/g for tissue. Mice were randomly selected from a pool of experimental mice early after infection. Table contains data from four independent experiments.
Values for moribund mice, which were used at later time points.
With a low dose of WNV, no virus was found in the blood. Virus was detectable in the brain 3 days earlier than in B6 mice and reached substantially higher titers (Table 7). The peak of WNV replication in β2-m−/− mice occurred earlier than in wt mice (days 7 and 11 p.i., respectively), and as a cohort, the mice survived longer with a high concentration (∼109 PFU/g tissue) of virus in the brain. In the liver and the spleen, virus was isolated from one or both organs of some of the animals between days 4 and 7 p.i.
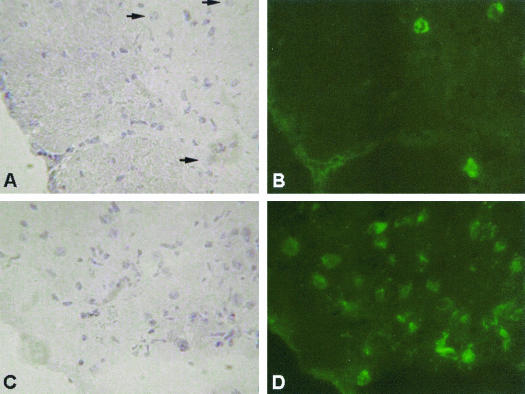
To investigate virus location and spread within the CNS, brains from β2-m−/− mice were investigated for the presence of WNV by immunofluorescence staining after infection with 103 PFU of WNV. Virus was found widespread in the cortex, and single neurons were shown to be infected. Compared to B6 mice, more neurons were infected in β2-m−/− mice (Fig. 5); this finding is consistent with the increased virus concentrations in the brains of these mice. Histological examinations showed greatly reduced mononuclear cell infiltration in β2-m−/− compared to wt mice. In the high-dose group, vascular congestion and parenchymal edema was found in the brains of moribund animals early after infection, with mild perivascular cuffs and leukocyte infiltration. Neurodegeneration and vascular degeneration of myelin were present, but pyknosis and necrosis of neurons was not markedly increased in β2-m−/− mice compared to that in wt mice. Brain tissue from β2-m−/− mice that survived a high dose of WNV infection for more than 21 days was similar in appearance to brains from either wt or β2-m−/− mice infected with a low dose, with the exception that scattered neuron degeneration and vacuolization of parenchyma, especially in the myelin area, was present.
FIG. 5.
Comparison of virus spread in brain tissue from B6 and β2-m−/− mice on day 9 after 103 PFU of WNV infection i.v. Brain sections from B6 (A and B) and β2-m−/− (C and D) mice were stained for the WNV (right panel) and counterstained with hematoxylin (left panel) as described in Materials and Methods. Arrows denote individual infected neurons. Magnification, ×630.
Mortality and virus replication in B6 mice with CD8+ T-cell depletion.
The increased susceptibility of the β2-m−/− mice after low-dose WNV infection suggested that CD8+ T cells are critical in recovery from the disease. To confirm this supposition, we compared β2-m−/− mice and B6 mice with CD8+ cell depletion in our low-dose WNV infection model. After in vivo CD8+ cell depletion, mice were increasingly susceptible to WNV encephalitis. Seven out of 10 mice died after a low dose of WNV inoculation (Table 8). The MST in this group was significantly prolonged when compared to that of B6 mice but was comparable to that of β2-m−/− mice. The virus titers in the brains of moribund mice at each time point was also comparable to that observed in β2-m−/− mice. No virus was detectable in serum, liver, and spleen in moribund mice at any time point.
TABLE 8.
Mortality and virus replication in the brain of CD8+ T- cell-depleted B6 mice after 103 PFU of WNV infection
| Total no. of mice | No. of dead mice | Survival time (days) | Mortality (%) | MST (days) | Brain virus titer (PFU/g) |
|---|---|---|---|---|---|
| 10 | 1 | 8 | 70.00 | 12.29 ± 2.56a | 4.6 × 107 |
| 1 | 10 | 7.0 × 109 | |||
| 1 | 12 | 2.0 × 109 | |||
| 2 | 13 | 2.7 × 108b | |||
| 2 | 15 | 3.2 × 109b |
Values represent MST ± standard deviation of seven mice. Values are statistically significant (P < 0.05) compared to B6 mice but not significant compared to β2-m−/− mice infected with 103 PFU of WNV.
Mean of two mice at each time point.
DISCUSSION
Many strains of WNV are neuroinvasive and can induce fatal encephalitis in mice and humans (18, 52, 58). In the present study, we investigated the mouse-virulent Sarafend strain of WNV as a model of WNV-induced encephalitis.
The dose response of B6 mice to an intravenous infection with WNV revealed an unusual pattern. At very low doses, i.e., 1 to 10 PFU of WNV, no clinical signs of disease or mortality were observed; however, all animals seroconverted. This result provides evidence that the PFU counting method underestimates infectious dose by at least 1 log. At a high dose of infection (108 PFU), 100% of the animals died within a period of 6 days. This contrasts with the results for mice infected with doses of 102 to 106 PFU, a range which was fatal for approximately 30% of mice regardless of dose, with an MST of about 11 days. At a dose of 107 PFU, 100% mortality occurred just as with 108 PFU; however, two cohorts based on MST could be identified: one resembling the high-dose (108 PFU) group for which the MST was 6 days, and the other resembling the low-dose (102 to 106 PFU) group, for which the MST was 11 days. The lack of a dose response over a 4-log difference in virus inoculum is puzzling and difficult to explain, especially the invariance of the MST over this dose range. It is noteworthy that a similar dose response pattern was observed with a closely related encephalitic flavivirus, Murray Valley encephalitis (33).
The two distinctly different MSTs observed in mice given either a high or low viral dose i.v. suggest that different pathological processes are involved. In the high-dose group, virus could be recovered from brain cells less than 24 h after infection and was present in liver and lymphoid organs in most animals in the early stage. This result suggests that breach of the blood brain barrier occurred without the necessity of prior viral replication in the periphery. This finding contrasts with a low dose (103 PFU) of infection, which resembles a dose range more akin to natural infections via an arthropod vector. In this group, virus could first be isolated from brain tissue at 7 days p.i., and transient detection of virus in lymphoid tissue occurred at 4 to 5 days p.i. This may suggest that viral replication in extraneural tissue prior to virus spread into the CNS is required. However, the low virus concentrations found in the selected tissues we analyzed suggest that the primary site for peripheral virus replication has yet to be identified. Virus could not be isolated from blood in any of the immunocompetent animals at any time point p.i. This finding is consistent with our understanding that primarily birds, but not mice or humans, are part of the natural replication cycle of encephalitic flaviviruses (53). Only birds, but not mice or humans, develop a viremia which enables bloodsucking arthropods to become infected and thereby continue the life cycle.
Our histological examinations confirm and extend our data on mortality, morbidity, and virus titers. In the high-dose group, comparatively mild leptomeningeal inflammation and rare parenchymal infiltration by inflammatory cells were observed. Death was most likely due to direct neuronal apoptosis as a result of viral replication in neuronal tissue, which has been previously documented to occur with flaviviruses (12, 20, 42). In addition, the early onset of mortality in the high-dose group is uncharacteristic, given the involvement of T-cell-mediated immunopathology. On the other hand, mice in the low-dose group developed encephalitis associated with inflammatory cell infiltration. Marked levels of infiltrates were observed in meningeal vessels at times when virus was found in brain and within the CNS parenchyma 1 to 2 days later. The quantity of infiltrates steadily increased, with CD8+ T cells being the predominant lymphocyte subpopulation. The inflammatory cells were distributed in perivascular regions and in neuron-degenerated regions of the brain parenchyma. Paralysis and death occurred in the final stage due to the structural damage of CNS tissue.
The lack of CD4+ and predominance of CD8+ T cells in CNS infiltrates has been observed previously in flavivirus infections (34) and in other viral infections of the CNS, such as lymphocytic choriomeningitis virus infection (8), but no satisfactory explanation has been advanced so far which is able to explain the prevalence of one type of T effector cells over the other. B6 mice are genetically fully competent to respond to WNV antigens, with a vigorous CD4+ T-cell response in the periphery (30). For two reasons, it also seems improbable that the lack of ligand for CD4+ T cells (MHC class II) is responsible for this CD8+ T-cell predominance, as has been proposed by Liu et al. (34). First, flavivirus infection upregulates class I but also class II MHC (35, 36) on cells of the CNS. Furthermore, predominant CD4+ T-cell infiltrates rather than CD8+ T-cell infiltrates are observed in other encephalitic etiologies, such as experimental allergic encephalomyelitis (1, 27). A slight but significant shift in the ratio of CD4+ to CD8+ T cells occurs in the periphery (spleen) when mice are infected with either the high or low doses of virus (Fig. 4). The dynamics and reasons for these changes in the CD8+/CD4+ ratio are unknown and may be the result of selective recruitment of CD8+ T cells or their proliferation or of selective depletion of CD4+ cells, possibly as a result of viral infection. We do not know if these rather small changes in ratio in the spleen are deterministic or if they influence the pattern observed in the CNS. The increase in the percentages of CD8+ T cells expressing early (CD69) and late (CD25) cell activation markers in both periphery and CNS do strongly suggest that these cells are exerting effector functions. This is dramatically highlighted by the use of CD8+ T-cell-deficient β2-m−/− mice exhibiting the differing pattern of mortality and MST in response to high and low doses of WNV compared to that of wt B6 mice. In the former, mortality is decreased to 82%, and mice that succumbed had a significantly increased MST compared to that of B6. Furthermore, these β2-m−/− mice had high virus titers in brain cells after infection and throughout the experimental period. This provides clear evidence of an immunopathological process exerted by CD8+ T cells being involved in wt mice after WNV infection. However, in the low-dose group, mortality was significantly increased (80 versus 27%), and virus could be isolated from β2-m−/− brains 3 days before it could be detected from B6 brains. This implies that CD8+ T cells play an important role in recovery from low-dose WNV infections of mice.
β2-m−/− mice, besides exhibiting a CD8+ T-cell deficiency, are primarily deficient in MHC class I antigen expression, which may potentially affect WNV replication and/or tissue tropism and neuroinvasion. To verify the results obtained from β2-m−/− mice, we generated B6 mice that were deficient in CD8+ T cells by in vivo MAb treatments. Such mice exhibited a disease pattern identical to that obtained with β2-m−/− mice that had been given 103 PFU of WNV, namely increased mortality and increased MST. We are in the process of investigating whether these two outcomes, protection or immunopathology, are the result of one and the same or differing effector functions of CD8+ T cells, i.e., cytokine release (gamma interferon) and/or cytolytic activity. It has been shown previously that these two effector functions are not necessarily triggered simultaneously in the same effector cells in flavivirus-immune CD8+ T cells (51). Identification of CD8+ T cells differing in effector functions leading to recovery versus immunopathology would constitute a major advance and might potentially lead to therapeutic advances for treatment of this increasingly important emerging viral disease.
Acknowledgments
We thank Ron Tha Hla and Megan Pavy for technical assistance, David O. Willenborg (Neurosciences Research Unit, Canberra Hospital) for reviewing the pathology, and Ann Cowling (Statistical Consulting Unit, The Australian National University) for advice in data analysis. We also thank Roy A. Hall for provision of MAb 2B2.
REFERENCES
- 1.Allen, S. J., D. Baker, J. K. O'Neill, A. N. Davison, and J. L. Turk. 1993. Isolation and characterization of cells infiltrating the spinal cord during the course of chronic relapsing experimental allergic encephalomyelitis in the Biozzi AB/H mouse. Cell. Immunol. 146:335-350. [DOI] [PubMed] [Google Scholar]
- 2.Austyn, J. M., and S. Gordon. 1981. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 11:805-815. [DOI] [PubMed] [Google Scholar]
- 3.Barrett, A. D. 1997. Japanese encephalitis and dengue vaccines. Biologicals 25:27-34. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Nathan, D., I. Huitinga, S. Lustig, N. van Rooijen, and D. Kobiler. 1996. West Nile virus neuroinvasion and encephalitis induced by macrophage depletion in mice. Arch. Virol. 141:459-469. [DOI] [PubMed] [Google Scholar]
- 5.Biggerstaff, B. J., and L. R. Petersen. 2002. Estimated risk of West Nile virus transmission through blood transfusion during an epidemic in Queens, New York City. Transfusion 42:1019-1026. [DOI] [PubMed] [Google Scholar]
- 6.Blanden, R. V. 1974. T cell response to viral and bacterial infection. Transplant Rev. 19:56-88. [DOI] [PubMed] [Google Scholar]
- 7.Cecilia, D., D. A. Gadkari, N. Kedarnath, and S. N. Ghosh. 1988. Epitope mapping of Japanese encephalitis virus envelope protein using monoclonal antibodies against an Indian strain. J. Gen. Virol. 69:2741-2747. [DOI] [PubMed] [Google Scholar]
- 8.Ceredig, R., J. E. Allan, Z. Tabi, F. Lynch, and P. C. Doherty. 1987. Phenotypic analysis of the inflammatory exudate in murine lymphocytic choriomeningitis. J. Exp. Med. 165:1539-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers, T. J., M. Halevy, A. Nestorowicz, C. M. Rice, and S. Lustig. 1998. West Nile virus envelope proteins: nucleotide sequence analysis of strains differing in mouse neuroinvasiveness. J. Gen. Virol. 79:2375-2380. [DOI] [PubMed] [Google Scholar]
- 10.Coffman, R. L. 1982. Surface antigen expression and immunoglobulin gene rearrangement during mouse pre-B cell development. Immunol. Rev. 69:5-23. [DOI] [PubMed] [Google Scholar]
- 11.Davis, B. S., G. J. Chang, B. Cropp, J. T. Roehrig, D. A. Martin, C. J. Mitchell, R. Bowen, and M. L. Bunning. 2001. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J. Virol. 75:4040-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Despres, P., M. P. Frenkiel, P. E. Ceccaldi, C. Duarte Dos Santos, and V. Deubel. 1998. Apoptosis in the mouse central nervous system in response to infection with mouse-neurovirulent dengue viruses. J. Virol. 72:823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deubel, V., L. Fiette, P. Gounon, M. T. Drouet, H. Khun, M. Huerre, C. Banet, M. Malkinson, and P. Despres. 2001. Variations in biological features of West Nile viruses. Ann. N. Y. Acad. Sci. 951:195-206. [DOI] [PubMed] [Google Scholar]
- 14.Diamond, M. S., B. Shrestha, A. Marri, D. Mahan, and M. Engle. 2003. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J. Virol. 77:2578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gajdosova, E., C. Oravec, and V. Mayer. 1981. Cell-mediated immunity in flavivirus infections. I. Induction of cytotoxic T lymphocytes in mice by an attenuated virus from the tick-borne encephalitis complex and its group-reactive character. Acta Virol. 25:10-18. [PubMed] [Google Scholar]
- 16.Gordon, S., L. Lawson, S. Rabinowitz, P. R. Crocker, L. Morris, and V. H. Perry. 1992. Antigen markers of macrophage differentiation in murine tissues. Curr. Top. Microbiol. Immunol. 181:1-37. [DOI] [PubMed] [Google Scholar]
- 17.Goverdhan, M. K., A. B. Kulkarni, A. K. Gupta, C. D. Tupe, and J. J. Rodrigues. 1992. Two-way cross-protection between West Nile and Japanese encephalitis viruses in bonnet macaques. Acta Virol. 36:277-283. [PubMed] [Google Scholar]
- 18.Halevy, M., Y. Akov, D. Ben-Nathan, D. Kobiler, B. Lachmi, and S. Lustig. 1994. Loss of active neuroinvasiveness in attenuated strains of West Nile virus: pathogenicity in immunocompetent and SCID mice. Arch. Virol. 137:355-370. [DOI] [PubMed] [Google Scholar]
- 19.Hall, R. A., G. W. Burgess, B. H. Kay, and P. Clancy. 1991. Monoclonal antibodies to Kunjin and Kokobera viruses. Immunol. Cell Biol. 69(Pt. 1):47-49. [DOI] [PubMed] [Google Scholar]
- 20.Hase, T., P. L. Summers, and D. R. Dubois. 1990. Ultrastructural changes of mouse brain neurons infected with Japanese encephalitis virus. Int. J. Exp. Pathol. 71:493-505. [PMC free article] [PubMed] [Google Scholar]
- 21.Hathcock, K. S., H. Hirano, and R. J. Hodes. 1993. CD45 expression by murine B cells and T cells: alteration of CD45 isoforms in subpopulations of activated B cells. Immunol. Res. 12:21-36. [DOI] [PubMed] [Google Scholar]
- 22.Hill, A. B., M. Lobigs, R. V. Blanden, A. Kulkarni, and A. Mullbacher. 1993. The cellular immune response to flaviviruses, p. 363-428. In D. B. Thomas (ed.), Viruses and the cellular immune response. Marcel Dekker Inc., New York, N.Y.
- 23.Hill, A. B., A. Mullbacher, C. Parrish, G. Coia, E. G. Westaway, and R. V. Blanden. 1992. Broad cross-reactivity with marked fine specificity in the cytotoxic T cell response to flaviviruses. J. Gen. Virol. 73:1115-1123. [DOI] [PubMed] [Google Scholar]
- 24.Johnson, A. J., D. A. Martin, N. Karabatsos, and J. T. Roehrig. 2000. Detection of anti-arboviral immunoglobulin G by using a monoclonal antibody-based capture enzyme-linked immunosorbent assay. J. Clin. Microbiol. 38:1827-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kesson, A. M., R. V. Blanden, and A. Mullbacher. 1987. The primary in vivo murine cytotoxic T cell response to the flavivirus, West Nile. J. Gen. Virol. 68:2001-2006. [DOI] [PubMed] [Google Scholar]
- 26.Kesson, A. M., R. V. Blanden, and A. Mullbacher. 1988. The secondary in vitro murine cytotoxic T cell response to the flavivirus, West Nile. Immunol. Cell Biol. 66:23-32. [DOI] [PubMed] [Google Scholar]
- 27.Kohm, A. P., P. A. Carpentier, H. A. Anger, and S. D. Miller. 2002. CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol. 169:4712-4716. [DOI] [PubMed] [Google Scholar]
- 28.Koller, B. H., P. Marrack, J. W. Kappler, and O. Smithies. 1990. Normal development of mice deficient in β2M, MHC class I proteins, and CD8+ T cells. Science 248:1227-1230. [DOI] [PubMed] [Google Scholar]
- 29.Konishi, E., M. Yamaoka, W. Khin Sane, I. Kurane, and P. W. Mason. 1998. Induction of protective immunity against Japanese encephalitis in mice by immunization with a plasmid encoding Japanese encephalitis virus premembrane and envelope genes. J. Virol. 72:4925-4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulkarni, A. B., A. Mullbacher, and R. V. Blanden. 1991. Functional analysis of macrophages, B cells and splenic dendritic cells as antigen-presenting cells in West Nile virus-specific murine T lymphocyte proliferation. Immunol. Cell Biol. 69:71-80. [DOI] [PubMed] [Google Scholar]
- 31.Langevin, S. A., M. Bunning, B. Davis, and N. Komar. 2001. Experimental infection of chickens as candidate sentinels for West Nile virus. Emerg. Infect. Dis. 7:726-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ledbetter, J. A., R. V. Rouse, H. S. Micklem, and L. A. Herzenberg. 1980. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens. Two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J. Exp. Med. 152:280-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Licon Luna, R. M., E. Lee, A. Mullbacher, R. V. Blanden, R. Langman, and M. Lobigs. 2002. Lack of both Fas ligand and perforin protects from flavivirus-mediated encephalitis in mice. J. Virol. 76:3202-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu, Y., R. V. Blanden, and A. Mullbacher. 1989. Identification of cytolytic lymphocytes in West Nile virus-infected murine central nervous system. J. Gen. Virol. 70:565-573. [DOI] [PubMed] [Google Scholar]
- 35.Liu, Y., N. King, A. Kesson, R. V. Blanden, and A. Mullbacher. 1989. Flavivirus infection up-regulates the expression of class I and class II major histocompatibility antigens on and enhances T cell recognition of astrocytes in vitro. J. Neuroimmunol. 21:157-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu, Y., N. King, A. Kesson, R. V. Blanden, and A. Mullbacher. 1988. West Nile virus infection modulates the expression of class I and class II MHC antigens on astrocytes in vitro. Ann. N. Y. Acad. Sci. 540:483-485. [DOI] [PubMed] [Google Scholar]
- 37.Lobigs, M., C. E. Arthur, A. Mullbacher, and R. V. Blanden. 1994. The flavivirus nonstructural protein NS3 is a dominant source of cytotoxic T cell peptide determinants. Virology 202:195-201. [DOI] [PubMed] [Google Scholar]
- 38.Lobigs, M., R. V. Blanden, and A. Mullbacher. 1996. Flavivirus-induced up-regulation of MHC class I antigens: implications for the induction of CD8+ T-cell-mediated autoimmunity. Immunol. Rev. 152:5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lobigs, M., A. Mullbacher, and M. Regner. 2003. MHC class I up-regulation by flaviviruses: immune interaction with unknown advantage to host or pathogen. Immunol. Cell Biol. 81:217-223. [DOI] [PubMed] [Google Scholar]
- 40.Lowenthal, J. W., R. H. Zubler, M. Nabholz, and H. R. MacDonald. 1985. Similarities between interleukin-2 receptor number and affinity on activated B and T lymphocytes. Nature 315:669-672. [DOI] [PubMed] [Google Scholar]
- 41.Lustig, S., U. Olshevsky, D. Ben-Nathan, B. E. Lachmi, M. Malkinson, D. Kobiler, and M. Halevy. 2000. A live attenuated West Nile virus strain as a potential veterinary vaccine. Viral Immunol. 13:401-410. [DOI] [PubMed] [Google Scholar]
- 42.Marianneau, P., M. Flamand, V. Deubel, and P. Despres. 1998. Induction of programmed cell death (apoptosis) by dengue virus in vitro and in vivo. Acta Cient. Venez. 49(Suppl. 1):13-17. [PubMed] [Google Scholar]
- 43.Monath, T. P. 1986. Pathobiology of the flaviviruses, p. 375-440. In S. Schlesinger and M. Schlesinger (ed.), The Togaviridae and Flaviviridae. Plenum Press, New York, N.Y.
- 44.Müllbacher, A., M. Lobigs, and E. Lee. 2003. Immunobiology of mosquito-borne encephalitic flaviviruses. Adv. Virus Res. 60:87-120. [DOI] [PubMed]
- 45.Nakayama, K., I. Negishi, K. Kuida, M. C. Louie, O. Kanagawa, H. Nakauchi, and D. Y. Loh. 1994. Requirement for CD8 β chain in positive selection of CD8-lineage T cells. Science 263:1131-1133. [DOI] [PubMed] [Google Scholar]
- 46.Nash, D., F. Mostashari, A. Fine, J. Miller, D. O'Leary, K. Murray, A. Huang, A. Rosenberg, A. Greenberg, M. Sherman, S. Wong, and M. Layton. 2001. The outbreak of West Nile virus infection in the New York City area in 1999. N. Engl. J. Med. 344:1807-1814. [DOI] [PubMed] [Google Scholar]
- 47.Petersen, L. R., and J. T. Roehrig. 2001. West Nile virus: a reemerging global pathogen. Emerg. Infect. Dis. 7:611-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price, W. H., and I. S. Thind. 1972. The mechanism of cross-protection afforded by dengue virus against West Nile virus in hamsters. J. Hyg. (London) 70:611-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reddehase, M. J., W. Mutter, K. Munch, H. J. Buhring, and U. H. Koszinowski. 1987. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J. Virol. 61:3102-3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Regner, M., M. Lobigs, R. V. Blanden, P. Milburn, and A. Mullbacher. 2001. Antiviral cytotoxic T cells cross-reactively recognize disparate peptide determinants from related viruses but ignore more similar self- and foreign determinants. J. Immunol. 166:3820-3828. [DOI] [PubMed] [Google Scholar]
- 51.Regner, M., M. Lobigs, R. V. Blanden, and A. Mullbacher. 2001. Effector cytolotic function but not IFN-γ production in cytotoxic T cells triggered by virus-infected target cells in vitro. Scand. J. Immunol. 54:366-374. (Erratum, 54: 640-641). [DOI] [PubMed] [Google Scholar]
- 52.Smithburn, K. C., T. P. Hughes, A. W. Burke, and J. H. Paul. 1940. A neurotropic virus isolated from the blood of a native of Uganda. Am. J. Trop. Med. Hyg. 20:471-492. [Google Scholar]
- 53.Steele, K. E., M. J. Linn, R. J. Schoepp, N. Komar, T. W. Geisbert, R. M. Manduca, P. P. Calle, B. L. Raphael, T. L. Clippinger, T. Larsen, J. Smith, R. S. Lanciotti, N. A. Panella, and T. S. McNamara. 2000. Pathology of fatal West Nile virus infections in native and exotic birds during the 1999 outbreak in New York City, New York. Vet. Pathol. 37:208-224. [DOI] [PubMed] [Google Scholar]
- 54.Stefanini, M., C. de Martino, and L. Zamboni. 1967. Fixation of ejaculated spermatozoa for electron microscopy. Nature 216:173-174. [DOI] [PubMed] [Google Scholar]
- 55.Wang, T., J. F. Anderson, L. A. Magnarelli, S. J. Wong, R. A. Koski, and E. Fikrig. 2001. Immunization of mice against West Nile virus with recombinant envelope protein. J. Immunol. 167:5273-5277. [DOI] [PubMed] [Google Scholar]
- 56.Wang, Y., Y. P. Wang, Y. C. Tay, and D. C. Harris. 2000. Progressive adriamycin nephropathy in mice: sequence of histologic and immunohistochemical events. Kidney Int. 58:1797-1804. [DOI] [PubMed] [Google Scholar]
- 57.Wang, Y., Y. P. Wang, Y. C. Tay, and D. C. Harris. 2001. Role of CD8+ cells in the progression of murine adriamycin nephropathy. Kidney Int. 59:941-949. [DOI] [PubMed] [Google Scholar]
- 58.Weiner, L. P., G. A. Cole, and N. Nathanson. 1970. Experimental encephalitis following peripheral inoculation of West Nile virus in mice of different ages. J. Hyg. 68:435-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wineman, J. P., G. L. Gilmore, C. Gritzmacher, B. E. Torbett, and C. E. Muller-Sieburg. 1992. CD4 is expressed on murine pluripotent hematopoietic stem cells. Blood 80:1717-1724. [PubMed] [Google Scholar]
- 60.Wu, L., R. Scollay, M. Egerton, M. Pearse, G. J. Spangrude, and K. Shortman. 1991. CD4 expressed on earliest T-lineage precursor cells in the adult murine thymus. Nature 349:71-74. [DOI] [PubMed] [Google Scholar]
- 61.Yap, K. L., G. L. Ada, and I. F. McKenzie. 1978. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature 273:238-239. [DOI] [PubMed] [Google Scholar]
- 62.Yokoyama, W. M., F. Koning, P. J. Kehn, G. M. Pereira, G. Stingl, J. E. Coligan, and E. M. Shevach. 1988. Characterization of a cell surface-expressed disulfide-linked dimer involved in murine T cell activation. J. Immunol. 141:369-376. [PubMed] [Google Scholar]
- 63.Zijlstra, M., M. Bix, N. E. Simister, J. M. Loring, D. H. Raulet, and R. Jaenisch. 1990. β 2-microglobulin-deficient mice lack CD4-8+ cytolytic T cells. Nature 344:742-746. [DOI] [PubMed] [Google Scholar]
- 64.Zinkernagel, R. M., and P. C. Doherty. 1973. Cytotoxic thymus-derived lymphocytes in cerebrospinal fluid of mice with lymphocytic choriomeningitis. J. Exp. Med. 138:1266-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]