Abstract
The title compound, C29H29ClFNO2, was synthesized by the reaction of 4-fluorobenzaldehyde, 5,5-dimethylcyclohexane-1,3-dione and 3-(4-chlorophenylamino)-5,5-dimethylcyclohex-2-enone in an ionic liquid (1-butyl-3-methylimidazolium bromide). X-ray analysis reveals that the 1,4-dihydropyridine ring adopts a boat conformation, while each of the attached partially saturated six-membered rings adopts a half-chair conformation. The structure is stabilized by weak C—H⋯O and C—H⋯F hydrogen bonds. The molecule has approximate mirror symmetry; the largest deviation from this symmetry concerns the fluoro- and chlorophenyl rings.
Related literature
For related literature, see: Dzierzbicka et al. (2001 ▶); Hutchins et al. (2003 ▶); Kamal et al. (2004 ▶); Li et al. (2003 ▶); Petříček et al. (2000 ▶); Srivastava & Nizamuddin (2004 ▶); Wang et al. (2002 ▶, 2003 ▶).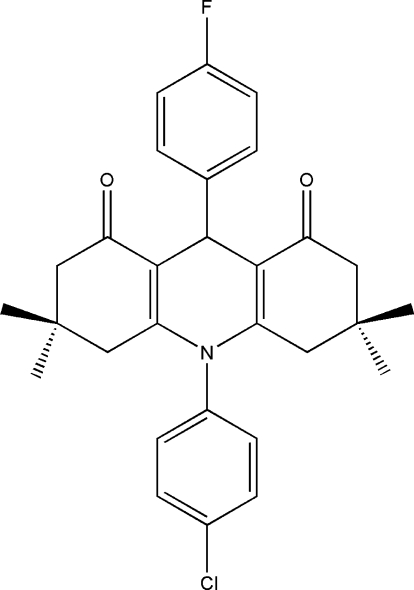
Experimental
Crystal data
C29H29ClFNO2
M r = 477.98
Monoclinic,
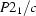
a = 12.0985 (12) Å
b = 10.9001 (10) Å
c = 19.4724 (18) Å
β = 101.231 (3)°
V = 2518.7 (4) Å3
Z = 4
Mo Kα radiation
μ = 0.18 mm−1
T = 113 (2) K
0.32 × 0.20 × 0.18 mm
Data collection
Rigaku Saturn diffractometer
Absorption correction: multi-scan (CrystalClear; Rigaku, 1999 ▶) T min = 0.943, T max = 0.967
30908 measured reflections
6016 independent reflections
5436 reflections with I > 2σ(I)
R int = 0.040
Refinement
R[F 2 > 2σ(F 2)] = 0.055
wR(F 2) = 0.137
S = 1.10
6016 reflections
312 parameters
H-atom parameters constrained
Δρmax = 0.42 e Å−3
Δρmin = −0.45 e Å−3
Data collection: CrystalClear (Rigaku, 1999 ▶); cell refinement: CrystalClear; data reduction: CrystalClear; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: CrystalStructure (Rigaku/MSC, 2003 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808025695/fb2102sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808025695/fb2102Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C16—H16C⋯O2i | 0.98 | 2.45 | 3.359 (2) | 153 |
| C11—H11⋯O2ii | 0.95 | 2.37 | 3.286 (2) | 160 |
| C10—H10⋯O1iii | 0.95 | 2.55 | 3.474 (2) | 165 |
| C16—H16A⋯O1iv | 0.98 | 2.59 | 3.528 (2) | 159 |
| C17—H17A⋯F1v | 0.98 | 2.45 | 3.373 (2) | 157 |
Symmetry codes: (i) 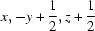 ; (ii)
; (ii) 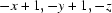 ; (iii)
; (iii) 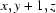 ; (iv)
; (iv) 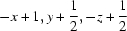 ; (v)
; (v) 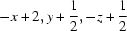 .
.
Acknowledgments
The authors are grateful to the Natural Science Foundation of Xuzhou Normal University (grant Nos. 06AXL10 and 06PYL04) and to the Natural Science Foundation of the Education Committee of Jiangsu Province (grant No. 04KJB150139) for financial support.
supplementary crystallographic information
Comment
Acridine derivatives are well-known compounds because of their pharmacological profile as anticancer agents (Hutchins et al., 2003) and potential DNA-binding agents (Kamal et al., 2004). Acridine derivatives have also been reported to possess antitumor activity (Dzierzbicka et al., 2001) as well as fungicidal activity (Srivastava & Nizamuddin, 2004). Here we report the crystal structure of 10-(4-chlorophenyl)-9-(4-fluorophenyl)-3,4,6,7-tetrahydro-3,3,6,6- tetramethylacridine-1,8(2H,5H,9H,10H)-dione.
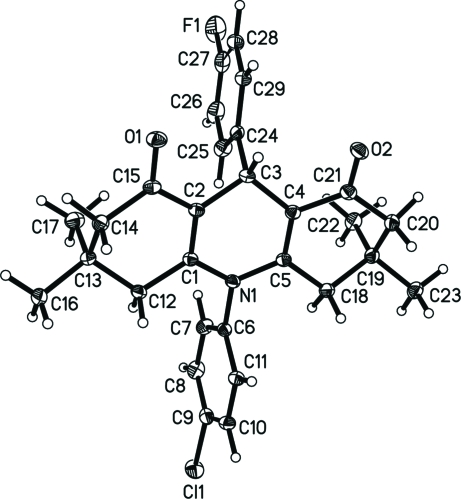
The X-ray crystal structure determination indicates that the central 1,4-dihydropyridine ring C1/C2···N1 is slightly distorted and adopts the boat conformation. The atoms C1, C2, C4 and C5 are coplanar, with C3 and N1 deviating from the plane by 0.286 (3) and 0.109 (2) Å, respectively. The similar distortions have been observed in the structures of 3,3,6,6-tetramethyl-9-(4-chlorophenyl)-10-(4-methylphenyl)- 1,2,3,4,5,6,7,8,9,10-decahydroacridine-1,8-dione [0.192 (3) and 0.091 (3) Å for C13 and N, respectively; Wang et al., 2003) and 3,3,6,6-tetramethyl-9-(3,4-methylenedioxylphenyl)-1,2,3,4,5,6,7,8,9,10- decahydroacridine-1,8-dione [0.313 (7) and 0.107 (7) Å for C7 and N1, respectively; Li et al., 2003).
The two outer six-membered rings of the acridine group adopt half-chair conformations; the atoms C13 and C19 deviate from the mean planes defined by C1, C2, C14, C15, C12 and C4, C5, C18, C20, C21 by 0.657 (2) and 0.668 (2) Å, respectively. A similar conformation has been found in the structure of 7,7-dimethyl-2-amino-3-cyano-4-(3,4-methylenedioxylphenyl)-5-oxo-5,6,7,8- tetrahydro-4H-benzo-[b]-pyran (Wang et al., 2002).
The 4-fluorophenyl ring is nearly perpendicular to the plane defined by the atoms C1—C2—C4—C5, forming a dihedral angle of 87.9 (1)°. The molecule has an approximate mirror symmetry. For example, the atoms C14 and C20 of the acridine groups are deviated by about 0.25Å. (The calculation has been carried out with the help of JANA2000 (Petříček et al., 2000). The largest deviation from this symmetry concerns the fluoro- and the chlorophenyl rings whose planes contain 9.3 (1)°.
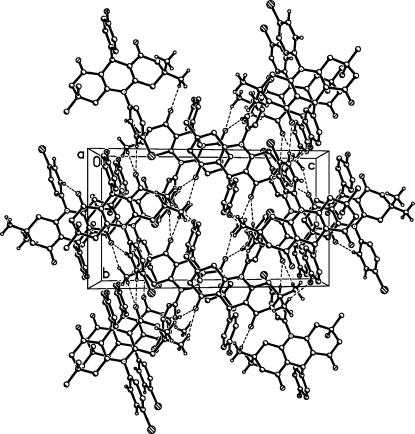
The weak hydrogen bonds of C—H···O and C—H···F are listed in Table 1. The weak intermolecular hydrogen bonds of C—H···O and C—H···F link the molecules (Fig. 2).
Experimental
The title compound was prepared by the reaction of 4-fluorobenzaldehyde (2 mmol, 0.248 g), 5,5-dimethyl-1,3-cyclohexanedione (2 mmol, 0.280 g) and 3-(4-chlorophenylamino)-5,5-dimethylcyclohex-2-enone (2 mmol, 0.498 g) in the ionic liquid of [Bmim]Br (1-butyl-3-methylimidazolium bromide) (10 ml) at 353 K. After the reaction had completed (monitored by TLC, about 6 h), the reactants were cooled to room temperature. The generated yellow solid was filtered off, and washed with small amount of water. The block crystals (about 0.2 mm in length, width and height respectively) suitable for X-ray diffraction were obtained by slow evaporation from ethanol solution. M.p. 583–585 K.
Refinement
In the structure all the H atoms were discernible in the difference electron density map. However, they were constrained by the riding model approximation. C—Hmethyl = 0.98 Å; C—Hmethylene = 0.99 Å; C—Hmethine = 1.00 Å; C—Haryl = 0.95 Å; UisoHmethyl = 1.5Ueq(Cmethyl); UisoHaryl = 1.2Ueq(Caryl, Cmethylene and Cmethine).
Figures
Fig. 1.
The title molecule with the displacement ellipsoids shown at the 50% probability level.
Fig. 2.
The molecular packing including the hydrogen-bonding network.
Crystal data
| C29H29ClFNO2 | F000 = 1008 |
| Mr = 477.98 | Dx = 1.260 Mg m−3 |
| Monoclinic, P21/c | Melting point = 583–585 K |
| Hall symbol: -P 2ybc | Mo Kα radiation λ = 0.71070 Å |
| a = 12.0985 (12) Å | Cell parameters from 8097 reflections |
| b = 10.9001 (10) Å | θ = 1.7–27.9º |
| c = 19.4724 (18) Å | µ = 0.19 mm−1 |
| β = 101.231 (3)º | T = 113 (2) K |
| V = 2518.7 (4) Å3 | Block, yellow |
| Z = 4 | 0.32 × 0.20 × 0.18 mm |
Data collection
| Rigaku Saturn diffractometer | 6016 independent reflections |
| Radiation source: rotating anode | 5436 reflections with I > 2σ(I) |
| Monochromator: confocal | Rint = 0.040 |
| Detector resolution: 14.63 pixels mm-1 | θmax = 27.9º |
| T = 113(2) K | θmin = 1.7º |
| ω scans | h = −15→15 |
| Absorption correction: multi-scan(CrystalClear; Rigaku, 1999) | k = −14→14 |
| Tmin = 0.943, Tmax = 0.967 | l = −25→25 |
| 30908 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.055 | H-atom parameters constrained |
| wR(F2) = 0.137 | w = 1/[σ2(Fo2) + (0.0579P)2 + 1.0431P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.10 | (Δ/σ)max = 0.001 |
| 6016 reflections | Δρmax = 0.42 e Å−3 |
| 312 parameters | Δρmin = −0.45 e Å−3 |
| 112 constraints | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.0058 (9) |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | 0.86649 (5) | 1.04288 (4) | 0.25315 (3) | 0.04213 (16) | |
| F1 | 1.03322 (14) | 0.02721 (16) | 0.07820 (8) | 0.0761 (5) | |
| O1 | 0.56729 (11) | 0.16766 (11) | 0.17692 (7) | 0.0326 (3) | |
| O2 | 0.57270 (11) | 0.31474 (12) | −0.06509 (7) | 0.0330 (3) | |
| N1 | 0.70646 (12) | 0.56013 (12) | 0.13279 (7) | 0.0229 (3) | |
| C1 | 0.67413 (13) | 0.47215 (14) | 0.17771 (8) | 0.0219 (3) | |
| C2 | 0.64094 (13) | 0.35886 (15) | 0.15353 (8) | 0.0223 (3) | |
| C3 | 0.64825 (14) | 0.31701 (14) | 0.08070 (9) | 0.0231 (3) | |
| H3 | 0.5793 | 0.2681 | 0.0614 | 0.028* | |
| C4 | 0.65130 (13) | 0.42790 (15) | 0.03444 (9) | 0.0231 (3) | |
| C5 | 0.68642 (13) | 0.53925 (14) | 0.06063 (9) | 0.0224 (3) | |
| C6 | 0.74535 (14) | 0.67913 (14) | 0.16009 (8) | 0.0228 (3) | |
| C7 | 0.85853 (15) | 0.69498 (17) | 0.18908 (10) | 0.0304 (4) | |
| H7 | 0.9099 | 0.6289 | 0.1895 | 0.036* | |
| C8 | 0.89647 (16) | 0.80758 (18) | 0.21746 (10) | 0.0336 (4) | |
| H8 | 0.9738 | 0.8194 | 0.2376 | 0.040* | |
| C9 | 0.82031 (15) | 0.90184 (16) | 0.21587 (9) | 0.0278 (4) | |
| C10 | 0.70815 (15) | 0.88861 (15) | 0.18595 (9) | 0.0269 (4) | |
| H10 | 0.6576 | 0.9557 | 0.1844 | 0.032* | |
| C11 | 0.67004 (14) | 0.77543 (15) | 0.15803 (9) | 0.0244 (3) | |
| H11 | 0.5927 | 0.7642 | 0.1376 | 0.029* | |
| C12 | 0.67473 (15) | 0.51183 (15) | 0.25200 (9) | 0.0263 (4) | |
| H12A | 0.6074 | 0.5631 | 0.2527 | 0.032* | |
| H12B | 0.7422 | 0.5631 | 0.2686 | 0.032* | |
| C13 | 0.67515 (15) | 0.40361 (15) | 0.30251 (9) | 0.0264 (4) | |
| C14 | 0.58278 (15) | 0.31382 (16) | 0.26947 (9) | 0.0274 (4) | |
| H14A | 0.5842 | 0.2417 | 0.3006 | 0.033* | |
| H14B | 0.5085 | 0.3541 | 0.2656 | 0.033* | |
| C15 | 0.59618 (13) | 0.27108 (15) | 0.19831 (9) | 0.0242 (3) | |
| C16 | 0.65091 (19) | 0.45153 (17) | 0.37181 (10) | 0.0365 (4) | |
| H16A | 0.5787 | 0.4952 | 0.3632 | 0.055* | |
| H16B | 0.7111 | 0.5077 | 0.3931 | 0.055* | |
| H16C | 0.6474 | 0.3825 | 0.4035 | 0.055* | |
| C17 | 0.78922 (16) | 0.33872 (18) | 0.31572 (11) | 0.0367 (4) | |
| H17A | 0.8487 | 0.3977 | 0.3342 | 0.055* | |
| H17B | 0.8031 | 0.3043 | 0.2717 | 0.055* | |
| H17C | 0.7890 | 0.2725 | 0.3497 | 0.055* | |
| C18 | 0.70364 (15) | 0.64547 (15) | 0.01412 (9) | 0.0263 (4) | |
| H18A | 0.7669 | 0.6965 | 0.0386 | 0.032* | |
| H18B | 0.6350 | 0.6970 | 0.0056 | 0.032* | |
| C19 | 0.72913 (15) | 0.60365 (16) | −0.05647 (9) | 0.0276 (4) | |
| C20 | 0.63778 (15) | 0.51338 (17) | −0.08888 (9) | 0.0293 (4) | |
| H20A | 0.5663 | 0.5589 | −0.1036 | 0.035* | |
| H20B | 0.6584 | 0.4777 | −0.1314 | 0.035* | |
| C21 | 0.61825 (14) | 0.41026 (16) | −0.04108 (9) | 0.0264 (4) | |
| C22 | 0.84573 (16) | 0.54320 (18) | −0.04596 (10) | 0.0338 (4) | |
| H22A | 0.8465 | 0.4697 | −0.0168 | 0.051* | |
| H22B | 0.9029 | 0.6011 | −0.0228 | 0.051* | |
| H22C | 0.8622 | 0.5201 | −0.0916 | 0.051* | |
| C23 | 0.72650 (17) | 0.71613 (17) | −0.10400 (10) | 0.0347 (4) | |
| H23A | 0.7434 | 0.6909 | −0.1491 | 0.052* | |
| H23B | 0.7829 | 0.7758 | −0.0818 | 0.052* | |
| H23C | 0.6515 | 0.7537 | −0.1114 | 0.052* | |
| C24 | 0.75157 (15) | 0.23635 (15) | 0.08100 (9) | 0.0262 (4) | |
| C25 | 0.85885 (16) | 0.28331 (19) | 0.10536 (10) | 0.0350 (4) | |
| H25 | 0.8671 | 0.3649 | 0.1227 | 0.042* | |
| C26 | 0.95412 (18) | 0.2128 (2) | 0.10467 (11) | 0.0443 (5) | |
| H26 | 1.0274 | 0.2449 | 0.1214 | 0.053* | |
| C27 | 0.9395 (2) | 0.0961 (2) | 0.07926 (11) | 0.0480 (6) | |
| C28 | 0.8360 (2) | 0.0456 (2) | 0.05529 (11) | 0.0479 (6) | |
| H28 | 0.8289 | −0.0363 | 0.0382 | 0.057* | |
| C29 | 0.74091 (18) | 0.11699 (17) | 0.05649 (10) | 0.0357 (4) | |
| H29 | 0.6680 | 0.0834 | 0.0403 | 0.043* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.0539 (3) | 0.0306 (3) | 0.0401 (3) | −0.0193 (2) | 0.0050 (2) | −0.00797 (19) |
| F1 | 0.0758 (10) | 0.1001 (13) | 0.0550 (9) | 0.0666 (10) | 0.0195 (8) | 0.0108 (8) |
| O1 | 0.0356 (7) | 0.0234 (6) | 0.0404 (7) | −0.0077 (5) | 0.0110 (6) | −0.0044 (5) |
| O2 | 0.0345 (7) | 0.0322 (7) | 0.0303 (7) | −0.0003 (5) | 0.0012 (5) | −0.0087 (5) |
| N1 | 0.0274 (7) | 0.0189 (6) | 0.0234 (7) | −0.0022 (5) | 0.0071 (5) | −0.0024 (5) |
| C1 | 0.0220 (8) | 0.0207 (8) | 0.0236 (8) | 0.0005 (6) | 0.0060 (6) | −0.0010 (6) |
| C2 | 0.0210 (8) | 0.0212 (8) | 0.0255 (8) | 0.0014 (6) | 0.0062 (6) | −0.0019 (6) |
| C3 | 0.0240 (8) | 0.0199 (8) | 0.0255 (8) | −0.0012 (6) | 0.0051 (6) | −0.0040 (6) |
| C4 | 0.0214 (8) | 0.0228 (8) | 0.0249 (8) | 0.0013 (6) | 0.0042 (6) | −0.0019 (6) |
| C5 | 0.0215 (8) | 0.0215 (8) | 0.0248 (8) | 0.0021 (6) | 0.0058 (6) | −0.0007 (6) |
| C6 | 0.0272 (8) | 0.0195 (8) | 0.0225 (8) | −0.0033 (6) | 0.0069 (6) | −0.0011 (6) |
| C7 | 0.0247 (9) | 0.0297 (9) | 0.0361 (10) | 0.0017 (7) | 0.0042 (7) | 0.0027 (7) |
| C8 | 0.0251 (9) | 0.0359 (10) | 0.0374 (10) | −0.0087 (7) | 0.0000 (7) | 0.0017 (8) |
| C9 | 0.0338 (9) | 0.0255 (8) | 0.0242 (8) | −0.0101 (7) | 0.0058 (7) | −0.0005 (7) |
| C10 | 0.0297 (9) | 0.0215 (8) | 0.0310 (9) | −0.0012 (7) | 0.0093 (7) | −0.0027 (7) |
| C11 | 0.0231 (8) | 0.0227 (8) | 0.0281 (8) | −0.0023 (6) | 0.0065 (6) | −0.0025 (6) |
| C12 | 0.0355 (9) | 0.0201 (8) | 0.0248 (8) | −0.0015 (7) | 0.0097 (7) | −0.0032 (6) |
| C13 | 0.0335 (9) | 0.0199 (8) | 0.0253 (8) | −0.0007 (7) | 0.0046 (7) | −0.0001 (6) |
| C14 | 0.0311 (9) | 0.0244 (8) | 0.0282 (9) | −0.0023 (7) | 0.0091 (7) | 0.0014 (7) |
| C15 | 0.0203 (8) | 0.0207 (8) | 0.0314 (9) | 0.0002 (6) | 0.0044 (6) | −0.0022 (6) |
| C16 | 0.0572 (13) | 0.0263 (9) | 0.0267 (9) | −0.0019 (8) | 0.0100 (8) | 0.0006 (7) |
| C17 | 0.0349 (10) | 0.0314 (10) | 0.0394 (11) | 0.0016 (8) | −0.0038 (8) | −0.0039 (8) |
| C18 | 0.0303 (9) | 0.0230 (8) | 0.0268 (9) | 0.0019 (7) | 0.0084 (7) | 0.0003 (7) |
| C19 | 0.0307 (9) | 0.0282 (9) | 0.0249 (8) | 0.0044 (7) | 0.0081 (7) | 0.0028 (7) |
| C20 | 0.0307 (9) | 0.0318 (9) | 0.0243 (8) | 0.0053 (7) | 0.0028 (7) | 0.0005 (7) |
| C21 | 0.0217 (8) | 0.0286 (9) | 0.0283 (9) | 0.0059 (7) | 0.0039 (6) | −0.0034 (7) |
| C22 | 0.0292 (9) | 0.0388 (10) | 0.0356 (10) | 0.0058 (8) | 0.0121 (8) | 0.0053 (8) |
| C23 | 0.0415 (11) | 0.0330 (10) | 0.0309 (10) | 0.0053 (8) | 0.0106 (8) | 0.0067 (8) |
| C24 | 0.0332 (9) | 0.0248 (8) | 0.0221 (8) | 0.0069 (7) | 0.0096 (7) | 0.0026 (6) |
| C25 | 0.0299 (10) | 0.0371 (10) | 0.0395 (11) | 0.0063 (8) | 0.0107 (8) | 0.0043 (8) |
| C26 | 0.0334 (11) | 0.0602 (14) | 0.0423 (12) | 0.0169 (10) | 0.0146 (9) | 0.0142 (10) |
| C27 | 0.0546 (14) | 0.0618 (15) | 0.0310 (10) | 0.0382 (12) | 0.0168 (9) | 0.0116 (10) |
| C28 | 0.0770 (17) | 0.0372 (11) | 0.0303 (11) | 0.0299 (11) | 0.0124 (10) | −0.0012 (8) |
| C29 | 0.0515 (12) | 0.0278 (9) | 0.0273 (9) | 0.0104 (8) | 0.0058 (8) | −0.0018 (7) |
Geometric parameters (Å, °)
| Cl1—C9 | 1.7449 (18) | C14—H14A | 0.9900 |
| F1—C27 | 1.364 (2) | C14—H14B | 0.9900 |
| O1—C15 | 1.229 (2) | C16—H16A | 0.9800 |
| O2—C21 | 1.227 (2) | C16—H16B | 0.9800 |
| N1—C5 | 1.397 (2) | C16—H16C | 0.9800 |
| N1—C1 | 1.404 (2) | C17—H17A | 0.9800 |
| N1—C6 | 1.445 (2) | C17—H17B | 0.9800 |
| C1—C2 | 1.354 (2) | C17—H17C | 0.9800 |
| C1—C12 | 1.509 (2) | C18—C19 | 1.535 (2) |
| C2—C15 | 1.467 (2) | C18—H18A | 0.9900 |
| C2—C3 | 1.509 (2) | C18—H18B | 0.9900 |
| C3—C4 | 1.512 (2) | C19—C20 | 1.522 (3) |
| C3—C24 | 1.527 (2) | C19—C23 | 1.533 (2) |
| C3—H3 | 1.0000 | C19—C22 | 1.534 (2) |
| C4—C5 | 1.353 (2) | C20—C21 | 1.507 (3) |
| C4—C21 | 1.460 (2) | C20—H20A | 0.9900 |
| C5—C18 | 1.509 (2) | C20—H20B | 0.9900 |
| C6—C11 | 1.385 (2) | C22—H22A | 0.9800 |
| C6—C7 | 1.387 (2) | C22—H22B | 0.9800 |
| C7—C8 | 1.387 (3) | C22—H22C | 0.9800 |
| C7—H7 | 0.9500 | C23—H23A | 0.9800 |
| C8—C9 | 1.376 (3) | C23—H23B | 0.9800 |
| C8—H8 | 0.9500 | C23—H23C | 0.9800 |
| C9—C10 | 1.376 (2) | C24—C29 | 1.383 (2) |
| C10—C11 | 1.390 (2) | C24—C25 | 1.390 (3) |
| C10—H10 | 0.9500 | C25—C26 | 1.388 (3) |
| C11—H11 | 0.9500 | C25—H25 | 0.9500 |
| C12—C13 | 1.535 (2) | C26—C27 | 1.364 (3) |
| C12—H12A | 0.9900 | C26—H26 | 0.9500 |
| C12—H12B | 0.9900 | C27—C28 | 1.364 (4) |
| C13—C17 | 1.527 (2) | C28—C29 | 1.393 (3) |
| C13—C16 | 1.528 (2) | C28—H28 | 0.9500 |
| C13—C14 | 1.530 (2) | C29—H29 | 0.9500 |
| C14—C15 | 1.500 (2) | ||
| C5—N1—C1 | 120.03 (13) | H16A—C16—H16B | 109.5 |
| C5—N1—C6 | 119.75 (13) | C13—C16—H16C | 109.5 |
| C1—N1—C6 | 119.62 (13) | H16A—C16—H16C | 109.5 |
| C2—C1—N1 | 120.39 (15) | H16B—C16—H16C | 109.5 |
| C2—C1—C12 | 122.73 (15) | C13—C17—H17A | 109.5 |
| N1—C1—C12 | 116.85 (13) | C13—C17—H17B | 109.5 |
| C1—C2—C15 | 120.44 (15) | H17A—C17—H17B | 109.5 |
| C1—C2—C3 | 122.33 (15) | C13—C17—H17C | 109.5 |
| C15—C2—C3 | 117.23 (14) | H17A—C17—H17C | 109.5 |
| C2—C3—C4 | 109.33 (13) | H17B—C17—H17C | 109.5 |
| C2—C3—C24 | 111.60 (13) | C5—C18—C19 | 112.63 (14) |
| C4—C3—C24 | 110.34 (13) | C5—C18—H18A | 109.1 |
| C2—C3—H3 | 108.5 | C19—C18—H18A | 109.1 |
| C4—C3—H3 | 108.5 | C5—C18—H18B | 109.1 |
| C24—C3—H3 | 108.5 | C19—C18—H18B | 109.1 |
| C5—C4—C21 | 120.15 (15) | H18A—C18—H18B | 107.8 |
| C5—C4—C3 | 122.35 (15) | C20—C19—C23 | 109.80 (15) |
| C21—C4—C3 | 117.47 (14) | C20—C19—C22 | 110.63 (15) |
| C4—C5—N1 | 120.36 (15) | C23—C19—C22 | 109.55 (15) |
| C4—C5—C18 | 122.15 (15) | C20—C19—C18 | 107.87 (14) |
| N1—C5—C18 | 117.48 (14) | C23—C19—C18 | 108.60 (14) |
| C11—C6—C7 | 120.61 (15) | C22—C19—C18 | 110.36 (14) |
| C11—C6—N1 | 120.28 (14) | C21—C20—C19 | 114.50 (14) |
| C7—C6—N1 | 119.11 (15) | C21—C20—H20A | 108.6 |
| C6—C7—C8 | 119.77 (17) | C19—C20—H20A | 108.6 |
| C6—C7—H7 | 120.1 | C21—C20—H20B | 108.6 |
| C8—C7—H7 | 120.1 | C19—C20—H20B | 108.6 |
| C9—C8—C7 | 118.91 (16) | H20A—C20—H20B | 107.6 |
| C9—C8—H8 | 120.5 | O2—C21—C4 | 120.67 (16) |
| C7—C8—H8 | 120.5 | O2—C21—C20 | 120.69 (16) |
| C10—C9—C8 | 122.13 (16) | C4—C21—C20 | 118.61 (15) |
| C10—C9—Cl1 | 118.55 (14) | C19—C22—H22A | 109.5 |
| C8—C9—Cl1 | 119.32 (14) | C19—C22—H22B | 109.5 |
| C9—C10—C11 | 118.90 (16) | H22A—C22—H22B | 109.5 |
| C9—C10—H10 | 120.6 | C19—C22—H22C | 109.5 |
| C11—C10—H10 | 120.6 | H22A—C22—H22C | 109.5 |
| C6—C11—C10 | 119.66 (15) | H22B—C22—H22C | 109.5 |
| C6—C11—H11 | 120.2 | C19—C23—H23A | 109.5 |
| C10—C11—H11 | 120.2 | C19—C23—H23B | 109.5 |
| C1—C12—C13 | 113.13 (13) | H23A—C23—H23B | 109.5 |
| C1—C12—H12A | 109.0 | C19—C23—H23C | 109.5 |
| C13—C12—H12A | 109.0 | H23A—C23—H23C | 109.5 |
| C1—C12—H12B | 109.0 | H23B—C23—H23C | 109.5 |
| C13—C12—H12B | 109.0 | C29—C24—C25 | 118.83 (17) |
| H12A—C12—H12B | 107.8 | C29—C24—C3 | 121.26 (16) |
| C17—C13—C16 | 109.41 (15) | C25—C24—C3 | 119.90 (15) |
| C17—C13—C14 | 109.67 (14) | C26—C25—C24 | 121.0 (2) |
| C16—C13—C14 | 109.88 (15) | C26—C25—H25 | 119.5 |
| C17—C13—C12 | 110.72 (15) | C24—C25—H25 | 119.5 |
| C16—C13—C12 | 109.02 (14) | C27—C26—C25 | 118.1 (2) |
| C14—C13—C12 | 108.12 (14) | C27—C26—H26 | 121.0 |
| C15—C14—C13 | 112.68 (14) | C25—C26—H26 | 121.0 |
| C15—C14—H14A | 109.1 | F1—C27—C26 | 118.0 (2) |
| C13—C14—H14A | 109.1 | F1—C27—C28 | 118.9 (2) |
| C15—C14—H14B | 109.1 | C26—C27—C28 | 123.11 (19) |
| C13—C14—H14B | 109.1 | C27—C28—C29 | 118.3 (2) |
| H14A—C14—H14B | 107.8 | C27—C28—H28 | 120.8 |
| O1—C15—C2 | 120.69 (16) | C29—C28—H28 | 120.8 |
| O1—C15—C14 | 121.55 (15) | C24—C29—C28 | 120.6 (2) |
| C2—C15—C14 | 117.72 (14) | C24—C29—H29 | 119.7 |
| C13—C16—H16A | 109.5 | C28—C29—H29 | 119.7 |
| C13—C16—H16B | 109.5 | ||
| C5—N1—C1—C2 | 11.0 (2) | C1—C12—C13—C17 | 71.36 (18) |
| C6—N1—C1—C2 | −177.87 (14) | C1—C12—C13—C16 | −168.23 (15) |
| C5—N1—C1—C12 | −166.96 (14) | C1—C12—C13—C14 | −48.81 (19) |
| C6—N1—C1—C12 | 4.1 (2) | C17—C13—C14—C15 | −64.45 (19) |
| N1—C1—C2—C15 | −173.71 (14) | C16—C13—C14—C15 | 175.25 (14) |
| C12—C1—C2—C15 | 4.2 (2) | C12—C13—C14—C15 | 56.38 (19) |
| N1—C1—C2—C3 | 6.5 (2) | C1—C2—C15—O1 | −179.08 (15) |
| C12—C1—C2—C3 | −175.64 (15) | C3—C2—C15—O1 | 0.7 (2) |
| C1—C2—C3—C4 | −22.1 (2) | C1—C2—C15—C14 | 3.3 (2) |
| C15—C2—C3—C4 | 158.08 (14) | C3—C2—C15—C14 | −176.84 (14) |
| C1—C2—C3—C24 | 100.24 (18) | C13—C14—C15—O1 | 147.61 (16) |
| C15—C2—C3—C24 | −79.58 (18) | C13—C14—C15—C2 | −34.8 (2) |
| C2—C3—C4—C5 | 23.5 (2) | C4—C5—C18—C19 | −25.5 (2) |
| C24—C3—C4—C5 | −99.62 (18) | N1—C5—C18—C19 | 155.53 (14) |
| C2—C3—C4—C21 | −158.60 (14) | C5—C18—C19—C20 | 52.13 (18) |
| C24—C3—C4—C21 | 78.30 (18) | C5—C18—C19—C23 | 171.08 (15) |
| C21—C4—C5—N1 | 173.05 (14) | C5—C18—C19—C22 | −68.83 (19) |
| C3—C4—C5—N1 | −9.1 (2) | C23—C19—C20—C21 | −169.46 (14) |
| C21—C4—C5—C18 | −5.9 (2) | C22—C19—C20—C21 | 69.51 (19) |
| C3—C4—C5—C18 | 171.93 (15) | C18—C19—C20—C21 | −51.28 (19) |
| C1—N1—C5—C4 | −9.7 (2) | C5—C4—C21—O2 | −170.52 (16) |
| C6—N1—C5—C4 | 179.21 (15) | C3—C4—C21—O2 | 11.5 (2) |
| C1—N1—C5—C18 | 169.32 (14) | C5—C4—C21—C20 | 7.5 (2) |
| C6—N1—C5—C18 | −1.8 (2) | C3—C4—C21—C20 | −170.44 (14) |
| C5—N1—C6—C11 | 79.2 (2) | C19—C20—C21—O2 | −159.05 (16) |
| C1—N1—C6—C11 | −91.90 (19) | C19—C20—C21—C4 | 22.9 (2) |
| C5—N1—C6—C7 | −101.61 (19) | C2—C3—C24—C29 | 120.14 (17) |
| C1—N1—C6—C7 | 87.3 (2) | C4—C3—C24—C29 | −118.09 (17) |
| C11—C6—C7—C8 | 1.2 (3) | C2—C3—C24—C25 | −60.7 (2) |
| N1—C6—C7—C8 | −177.99 (16) | C4—C3—C24—C25 | 61.0 (2) |
| C6—C7—C8—C9 | −0.3 (3) | C29—C24—C25—C26 | 0.6 (3) |
| C7—C8—C9—C10 | −1.2 (3) | C3—C24—C25—C26 | −178.51 (17) |
| C7—C8—C9—Cl1 | 178.58 (14) | C24—C25—C26—C27 | 0.1 (3) |
| C8—C9—C10—C11 | 1.7 (3) | C25—C26—C27—F1 | 179.58 (18) |
| Cl1—C9—C10—C11 | −178.07 (13) | C25—C26—C27—C28 | −0.7 (3) |
| C7—C6—C11—C10 | −0.7 (3) | F1—C27—C28—C29 | −179.85 (18) |
| N1—C6—C11—C10 | 178.50 (15) | C26—C27—C28—C29 | 0.4 (3) |
| C9—C10—C11—C6 | −0.7 (3) | C25—C24—C29—C28 | −0.9 (3) |
| C2—C1—C12—C13 | 20.1 (2) | C3—C24—C29—C28 | 178.23 (16) |
| N1—C1—C12—C13 | −161.93 (14) | C27—C28—C29—C24 | 0.4 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C16—H16C···O2i | 0.98 | 2.45 | 3.359 (2) | 153 |
| C11—H11···O2ii | 0.95 | 2.37 | 3.286 (2) | 160 |
| C10—H10···O1iii | 0.95 | 2.55 | 3.474 (2) | 165 |
| C16—H16A···O1iv | 0.98 | 2.59 | 3.528 (2) | 159 |
| C17—H17A···F1v | 0.98 | 2.45 | 3.373 (2) | 157 |
Symmetry codes: (i) x, −y+1/2, z+1/2; (ii) −x+1, −y+1, −z; (iii) x, y+1, z; (iv) −x+1, y+1/2, −z+1/2; (v) −x+2, y+1/2, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: FB2102).
References
- Dzierzbicka, K., Kolodziejczyk, A. M., Wysocka-Skrzela, B., Mysliwski, A. & Sosnowska, D. (2001). J. Med. Chem.44, 3606–3615. [DOI] [PubMed]
- Hutchins, R. A., Crenshaw, J. M., Graves, D. E. & Denny, W. A. (2003). Biochemistry, 42, 13754–13761. [DOI] [PubMed]
- Kamal, A., Srinivas, O., Ramulu, P., Ramesh, G. & Kumar, P. P. (2004). Bioorg. Med. Chem. Lett.14, 4107–4111. [DOI] [PubMed]
- Li, Y., Wang, X., Shi, D., Du, B. & Tu, S. (2003). Acta Cryst. E59, o1446–o1448.
- Petříček, V., Dušek, M. & Palatinus, L. (2000). JANA2000 Institute of Physics, Czech Academy of Science, Czech Republic.
- Rigaku (1999). CrystalClear Rigaku Corporation, Tokyo, Japan.
- Rigaku/MSC (2003). CrystalStructure Rigaku/MSC, The Woodlands, Texas, USA.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Srivastava, A. & Nizamuddin, C. (2004). Indian J. Heterocycl. Chem.13, 261–264.
- Wang, X., Shi, D. & Tu, S. (2003). Acta Cryst. E59, o1139–o1140.
- Wang, X. S., Shi, D. Q., Tu, S. J., Yao, C. S. & Wang, Y. C. (2002). Chin. J. Struct. Chem.21, 146–149.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808025695/fb2102sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808025695/fb2102Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report