Abstract
Human immunodeficiency virus type 1 encapsidates two copies of viral genomic RNA in the form of a dimer. The dimerization process initiates via a 6-nucleotide palindrome that constitutes the loop of a viral RNA stem-loop structure (i.e., stem loop 1 [SL1], also termed the dimerization initiation site [DIS]) located within the 5′ untranslated region of the viral genome. We have now shown that deletion of the entire DIS sequence virtually eliminated viral replication but that this impairment was overcome by four second-site mutations located within the matrix (MA), capsid (CA), p2, and nucleocapsid (NC) regions of Gag. Interestingly, defective viral RNA dimerization caused by the ΔDIS deletion was not significantly corrected by these compensatory mutations, which did, however, allow the mutated viruses to package wild-type levels of this DIS-deleted viral RNA while excluding spliced viral RNA from encapsidation. Further studies demonstrated that the compensatory mutation T12I located within p2, termed MP2, sufficed to prevent spliced viral RNA from being packaged into the ΔDIS virus. Consistently, the ΔDIS-MP2 virus displayed significantly higher levels of infectiousness than did the ΔDIS virus. The importance of position T12 in p2 was further demonstrated by the identification of four point mutations,T12D, T12E, T12G, and T12P, that resulted in encapsidation of spliced viral RNA at significant levels. Taken together, our data demonstrate that selective packaging of viral genomic RNA is influenced by the MP2 mutation and that this represents a major mechanism for rescue of viruses containing the ΔDIS deletion.
The hallmark of all retroviruses, including human immunodeficiency virus type 1 (HIV-1), is the need to generate a full-length double-stranded proviral DNA molecule from plus-strand RNA, after which integration of this DNA into the host cell genome takes place. Given that the length of the retroviral genome is >9 kb and that most viral RNA molecules are nicked, one might expect that the successful generation of complete proviral DNA from such a genome would represent a difficult task. To overcome difficulties, retroviruses specifically package two copies of dimeric full-length viral genomic RNA that are noncovalently linked at their 5′ ends (48). The availability of two copies of the RNA genome provides viral reverse transcriptase with an alternate template, should this enzyme encounter a break during transcription, and also contributes to overall viral genetic variability (18).
The mechanisms of retroviral RNA dimerization have been extensively studied, particularly for HIV-1. The major determinant for HIV-1 RNA dimerization has been mapped to a stem-loop structure termed SL1, which is located within the 5′ untranslated region (UTR) of the viral genome (23, 32, 47). A 6-nucleotide (nt) palindrome sequence within the loop region of SL1 initiates dimerization though a “kissing-loop” mechanism that involves the formation of base pairs between the palindromes of two genomic RNA molecules (9, 24, 37, 39). The loose dimer is converted to a more stable extended duplex with the help of the viral nucleocapsid (NC) protein (13, 25, 37). Accordingly, SL1 has been termed the dimerization initiation site (DIS) (39).
The features of SL1 that allow this RNA structure to function as the DIS have been further explored in a number of genetic and structural studies. HIV-1 RNA dimerization is affected not only by mutation of the palindrome loop sequence but also by alteration of the stem region (9, 28, 46). These latter changes may either affect the appropriate presentation of the palindrome within the loop, which is needed to initiate RNA dimerization, or prevent the transition of RNA dimers from the loose to the stable form. Detailed structures of the RNA dimer formed by SL1 have shown that both loop and stem sequences contribute to stabilization of the RNA duplex (11, 12, 14, 35, 36, 49). As an example, the loop region contains three adenine residues that cannot form base pairs within the dimer and, as a consequence, might be expected to distort the RNA duplex. However, what actually happens is that these adenines initiate a distinctive pattern of interstrand stacking which helps to stabilize the dimer structure. These studies provide an explanation of why RNA dimerization is initiated at SL1 and not at other viral RNA structures, such as the poly(A) hairpin, whose loop region also contains a palindrome (27, 43).
Consistent with these observations, the mutation of DIS sequences can result in severely diminished viral infectiousness (2, 8, 16, 21, 26, 28, 38, 46). To further understand the role of the DIS, we previously generated two DNA constructs, BH-LD3 and BH-LD4, that lacked portions of the stem sequence of the DIS structure (29, 30). Replication of these two mutated viruses in permissive cells led to the outgrowth of revertant viruses that displayed wild-type virus infectiousness. Interestingly, these revertants retained the original DIS mutations but acquired compensatory mutations within the Gag region. However, the mechanisms that underlie this compensatory activity have not been characterized. In the present study, we show that the compensatory mutations involved were able to confer viability to viruses lacking the entire DIS sequences. Interestingly, restoration of viral replication was accompanied not by wild-type RNA dimerization but, rather, by the exclusion of spliced viral RNA from the infectious ΔDIS viruses that were produced, and MP2 played a major role in this process.
MATERIALS AND METHODS
Plasmid construction.
The infectious HIV-1 BH10 cDNA clone was used to generate the constructs described below, and all mutations were introduced by PCR-based strategies using the Pfu enzyme (Stratagene, La Jolla, Calif.). ΔDIS and ΔLoop are deletion mutations that were engineered by PCR using the sense primer pS (5′-AGA CCA GAT CTG AGC CTG GGAG-3′ [nt 14 to 35, numbering from the first nucleotide in the viral repeat region]) together with the antisense primers ΔDIS (5′-TAC TCA CCA GTC GCC GCC CTC CTG CGT CGA GAG AGC-3′ [nt 293 to 227]) and ΔLoop (5′-CGC CGC CCC TCG CCT CCT GCC GCA GCA AGC CGA GTC CTG C-3′ [nt 282 to 235]), respectively (Fig. 1). The PCR products thus generated were then used as primers, together with primer pApa-A (5′-CCT AGG GGC CCT GCA ATT TCT G-3′ [nt 1559 to 1538]), in a second round of PCR. Final PCR products were digested with the restriction enzymes NarI and ApaI and inserted into a BH10 vector that had been cut with the same enzymes. To generate constructs containing the ΔDIS or ΔLoop deletions or wild-type BH10, along with various combinations of compensatory mutations, the gag gene was replaced with that of previously generated constructs containing combinations of various compensatory mutations (Table 1) (30). Accordingly, ΔDIS-MP2, ΔDIS-MNC, ΔLoop-MP2, and ΔLoop-MNC were generated by substituting the gag gene from the previously described BH10-MP2 and BH10-MNC into the ΔDIS and ΔLoop constructs (41). The protease-negative (PR−) (34) and MD1 (42) plasmidsand constructs containing substitutions of all 20 amino acids in position 12 of p2 have also been described previously (41). All constructs generated were confirmed by sequencing.
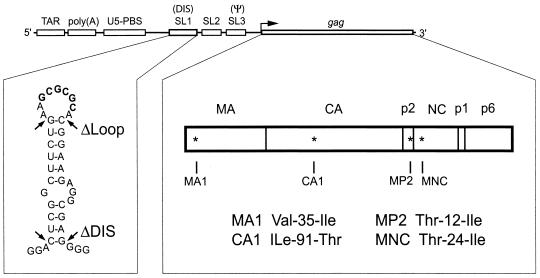
FIG. 1.
Illustration of the various RNA structural elements located within the HIV-1 5′ region, including TAR, poly(A), U5-PBS, SL1 (DIS), SL2, SL3 (ψ), and the gag gene. The secondary structure of SL1 is shown below, with the 257-GCGCGC-262 loop palindrome highlighted in boldface. Nucleotides that were deleted in the ΔDIS and ΔLoop mutants are indicated by arrows, with ΔLoop lacking the 9 nt that comprise the loop of SL1 and ΔDIS missing all 35 nt contained within SL1. The MA, CA, p2, NC, p1, and p6 domains of Gag are shown, with the positions of the MA1, CA1, MP2, and MNC compensatory point mutations indicated by asterisks.
TABLE 1.
Viruses generated in this study
| Virus | Deletion | Compensatory mutations |
|---|---|---|
| ΔDIS | ΔDIS | None |
| DA | ΔDIS | MNC-MP2 |
| DB | ΔDIS | MNC-CA1 |
| DC | ΔDIS | MNC-MP2-CA1 |
| DD | ΔDIS | MNC-MP2-CA1-MA1 |
| ΔLoop | ΔLoop | None |
| LA | ΔLoop | MNC-MP2 |
| LB | ΔLoop | MNC-CA1 |
| LC | ΔLoop | MNC-MP2-CA1 |
| LD | ΔLoop | MNC-MP2-CA1-MA1 |
| BH10-D | None | MNC-MP2-CA1-MA1 |
To generate antisense riboprobes for use in RNase protection assays (RPA), DNA fragments of 486, 449, and 477 bp were amplified from BH10, ΔDIS, and ΔLoop proviral DNA, respectively, using primers RPA-S (5′-Cag ggc ccG AGA GCT GCA TCC GGAG-3′ [nt −164 to −140]), which was modified to contain an ApaI restriction enzyme site (shown in lowercase type), and RPA-A (5′-CCT CCG gaa ttc AAA ATT TTT GGC G-3′ [nt 321 to 297]), which was modified to contain an EcoRI restriction enzyme site (shown in lowercase type), as previously described (42). The resulting PCR products were digested with ApaI and EcoRI and inserted into the pBluescript II KS(+) cloning vector (Stratagene), which had been cut with the same enzymes to generate constructs RPA1, RPA-DIS, and RPA-Loop.
Cell culture, transfection, and infection.
COS-7 cells were grown in Dulbecco’s modified Eagle’s medium. HeLa-CD4-LTR-β-gal cells were obtained from Michael Emerman (22) through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases and were cultured in Dulbecco’s modified Eagle’s medium containing 0.1 mg of G418 per ml and 0.05 mg of hygromycin B per ml. MT-2 and Jurkat cells were grown in RPMI 1640 medium containing 2 mM l-Glu. All cells were supplemented with 10% fetal calf serum. Cord blood mononuclear cells (CBMCs) were grown in RPMI 1640 supplemented with 10% fetal calf serum and 20 U of interleukin-2 per ml. Transfection of COS-7 cells was performed using Lipofectamine (Invitrogen, Burlington, Ontario, Canada). Quantities of progeny viruses were quantified based on p24 antigen levels by enzyme-linked immunosorbert assays (Vironostika HIV-1 Antigen Microelisa System; Organon Teknika Corp., Durham, N.C.).
MT-2 or Jurkat cells (5 × 105) were incubated in 2 ml of medium at 37°C for 2 h with aliquots of virus equivalent to 5 ng of p24. The cells were then washed twice and maintained in 10 ml of medium. CBMC infections were performed as described previously (6). Culture fluids were collected at various times to determine levels of reverse transcriptase (RT) activity. Viral infectivity was also measured using a multinuclear activation of a galactosidase indicator (MAGI) assay (22). HeLa-CD4-LTR-β-gal cells were plated in 24-well plates (4 × 10 4 cells/well) and cultured for 1 day before being infected in triplicate with an amount of virus equivalent to 10 ng of p24 (CA) in the presence of 20 μg of DEAE-dextran per ml. At 48 h after infection, the cells were fixed with 1% formaldehyde-0.2% glutaraldehyde in phosphate-buffered saline and stained with 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal). Infectivity was scored based on the number of blue cells counted per well as described previously (22).
Native Northern blotting.
Progeny viruses generated by transfected COS-7 cells were first clarified by centrifugation in a Beckman GR-6S centrifuge at 3,000 rpm for 30 min at 4°C and then pelleted through a 20% sucrose cushion by ultracentrifugation in a Beckman XL-80 ultracentrifuge with an SW41 rotor at 40,000 rpm for 1 h at 4°C. Virus pellets were suspended in 300 μl of Tris-NaCl (pH 7.8) (TN) buffer; a 2-μl portion was removed for p24 determination, and the remaining material was treated with virus lysis buffer (50 mM Tris-HCl [pH 7.4], 10 mM EDTA, 1% sodium dodecyl sulfate, 100 mM NaCl, 50 μg of yeast tRNA/ml, 100 μg of proteinase K/ml) for 20 min at 37°C. Samples were then extracted twice with phenol-chloroform-isoamyl alcohol (25:24:1) and once with chloroform. Viral RNA was precipitated in 2.5 volumes of 95% ethanol together with 0.1 volume of 3M sodium acetate (pH 5.2). RNA pellets were washed with 70% ethanol and dissolved in Tris-EDTA (pH 7.5) (TE) buffer. An amount of viral RNA equivalent to 150 ng of HIV-1 p24 was fractionated on 0.9% native agarose gels in 1× Tris-borate-EDTA (TBE) buffer at 100 V for 4 h at 4°C and analyzed by Northern blotting (44), using an [α-32P]dCTP (ICN, Irvine, Calif.)-labeled 2-kb HIV-1 DNA fragment (nt 1 to 2000) as a probe. Bands were visualized by autoradiography and quantified by digital image analysis using the AlphaImager v5.5 program. Briefly, object boxes were selected at the smallest size necessary to encase the largest band to be measured in a series. The same box was then used to measure all bands within that series. Based on the manufacturer's recommendations, the Autobackground setting, which calculated an independent background for each object box and was determined on the basis of the average intensity of the 10 lowest pixels within each box, was used.
RPA.
Preparation of riboprobes and RPA experiments were performed as described previously (42). Briefly, radiolabeled probes were transcribed in vitro from BspE1-linearized RPA1, RPA-DIS, and RPA-Loop plasmids by using the T7-MEGAshortscript kit (Ambion Inc., Austin, Tex.) in the presence of [α-32P]UTP (ICN). RNA was isolated from viruses as described above. Amounts of virion RNA equivalent to 25 ng of p24 capsid (CA) antigen were treated with 10 U of DNase I (Invitrogen) for 30 min at 37°C to remove any plasmid contamination and then subjected to phenol-chloroform extraction and ethanol precipitation before being analyzed with the RPA II kit (Ambion Inc.). RNA was then incubated at 42°C overnight with an excess of labeled riboprobe (105 cpm), followed by digestion with single-strand-specific RNases. Protected fragments were separated on 5% polyacrylamide-8 M urea gels, visualized by autoradiography, and quantified by digital image analysis using the AlphaImager v5.5 program.
RESULTS
Compensatory mutations increase the replication capacity of the ΔDIS mutant.
We have previously identified mutations within the Gag protein that were able to rescue the deletion of stem sequences within the DIS (29, 30). We now wished to determine whether the complete absence of DIS sequences could still be compensated by second-site mutations. Accordingly, the DIS region spanning nt 243 to 277 was removed to generate a construct termed ΔDIS (Fig. 1). Mutated DNA was transfected into COS-7 cells, and the virus particles thus generated were used to infect MT-2 cells. The results in Fig. 2A show that RT activity was detectable in the wild-type BH10 virus culture by day 4, which coincided with the first appearance of cytopathic effects (CPE), and that this peaked at day 6. In contrast, the ΔDIS mutant culture was negative for RT activity and did not show CPE over 3 months. Similar results in regard to the ΔDIS mutant were obtained with Jurkat cells and human CBMC (Fig. 2B). Thus, ΔDIS viruses were unable to establish persistent infection in culture and did not have the opportunity to accumulate second-site mutations to improve infectiousness.
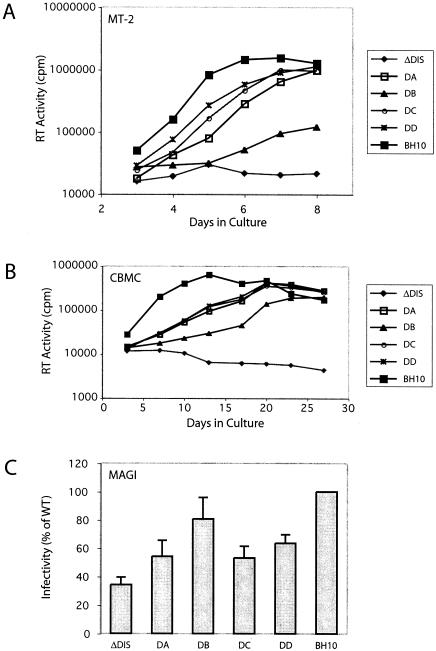
FIG. 2.
Viral replication kinetics of the ΔDIS virus in the presence of various combinations of compensatory mutations. (A and B) MT-2 cells (A) or CBMCs (B) were infected with an amount of progeny virus containing 5 or 200 ng of p24 antigen, respectively. Viral replication was monitored based on observations of syncytium formation and RT activity in culture supernatants at various times. (C) For MAGI assays, viruses equivalent to 10 ng of p24 antigen were incubated with 4 × 104 HeLa-CD4-LTR-β-gal cells/well in triplicate for 48 h. The numbers of blue cells in each well were determined (multinuclear syncytia were scored as one) and expressed as a percentage of the wild type to generate a bar graph representing relative infectivity. The number of β-galactosidase-positive cells ranged from 50 to 200/well, and the mock-infected wells contained a mean of 9 ± 3 cells. See Table 1 for the combinations of compensatory mutations represented by DA, DB, DC, and DD.
We further studied the production of p24 by Jurkat cells that had been infected by the ΔDIS virus and found low levels of capsid protein (approximately 35 pg/ml, as opposed to an average of 100,000 pg/ml for wild-type virus) during 2 weeks of culture. In the case of the ΔDIS, this subsequently became negative and remained so over 2 months (data not shown), suggesting that the virus was able to replicate at low levels, but too low to establish a persistent infection.
We next asked whether the compensatory mutations that had previously been identified with the BH-LD3 and BH-LD4 deletions were able to rescue the ΔDIS deletion. The results in Fig. 2A show that the combination of ΔDIS deletion with the compensatory mutations restored viral replication and that as few as two of these mutations (i.e., MNC-MP2 in DA or MNC-CA1 in DB) were sufficient in this regard. It should be noted that the DB mutant, which included the CA1 mutation instead of MP2, showed a lower rate of virus replication than did viruses containing other combinations of the compensatory mutations. This suggests that the MP2 mutation played a more important role in viral reversion than did CA1. When combinations of three (DC; MNC-MP2-CA1) or all four (DD; MNC-MP2-CA1-MA1) compensatory mutations were incorporated into the ΔDIS mutant, the resultant viruses grew to significantly higher levels than did the ΔDIS mutant alone, with rates of replication comparable to that of the wild type (Fig. 2A). The positive roles of these compensatory mutations were further confirmed by infection studies performed with CBMC (Fig. 2B) and Jurkat cells (data not shown).
We further quantified the levels of infectivity of our mutants in a single-round infection experiment termed the MAGI assay (22). The results in Fig. 2C show that the ΔDIS mutant exhibited an approximately threefold decrease in viral infectivity compared to the wild-type BH10. In agreement with the results of the spread infection studies shown in Fig. 2A and B, the infectivity of the DIS-deleted viruses was significantly increased in the presence of the compensatory mutations (Fig. 2C). It was also noted that the DB mutant, which lacks the MP2 mutation, displayed the greatest increase in infectivity in the MAGI assay among the four mutants DA, DB, DC, and DD, as opposed to the lowest replication rate of DB in each of the MT-2 and Jurkat cells and CBMC. Since the MAGI assay measures the transactivation levels of HIV-1 long terminal repeat by the Tat protein, the observed discrepancy indicates that compensation of ΔDIS by the four suppressor mutations, particularly by MP2, occurs, to a large extent, at the late stages of viral replication.
Compensatory mutations do not restore wild-type RNA dimerization to ΔDIS.
The DIS is the major signal for viral RNA dimerization (23, 32, 47). Not surprisingly, the ΔDIS mutant showed a decreased level of dimerized RNA compared to wild-type BH10 (Fig. 3A, compare lane 1 with lane 6). Moreover, ΔDIS RNA formed complexes that migrated slower on gels than did RNA dimers, a defect not seen with wild-type BH10 RNA. We next assessed whether the compensatory mutations could repair these RNA dimerization defects by native Northern blotting and found that introduction of the substitutions had only modest effects. Notably, approximately 55% of viral RNA was still in monomeric form, compared with only 10% monomeric presence in the case of BH10 (Fig. 3A). However, large aggregates of viral RNA associated with ΔDIS were resolved by the presence of the compensatory mutations, particularly when at least three were present (lanes 4 and 5). Therefore, the compensatory mutations did not lead to wild-type RNA dimerization but did have positive effects with regard to formation of RNA complexes.
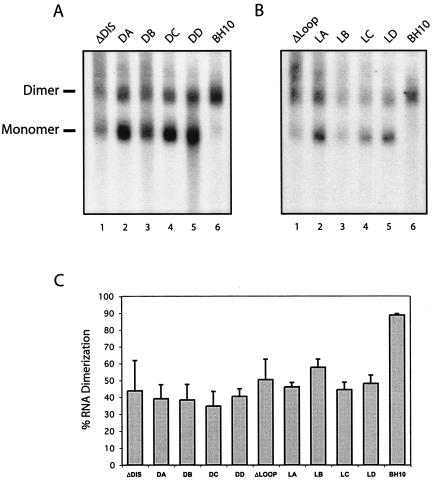
FIG. 3.
Effects of the ΔDIS and ΔLoop deletions on viral RNA dimerization. (A and B) Viral RNA was prepared from mutant viruses ΔDIS, DA, DB, DC, DD (A, lanes 1 to 5), ΔLoop, LA, LB, LC, LD (B, lanes 1 to 5), and wild-type virus BH10 (A and B, lanes 6), equivalent to 150 ng of p24 antigen, fractionated on native agarose gels, and subjected to Northern blot analysis. Dimers and monomers are indicated on the left side of the gels. The gels shown are from one representative experiment. (C) Band intensities of dimer and monomer signals were measured using the AlphaImager v5.5 program, and relative dimer levels were plotted for each construct. The results represent pooled data from three Northern blots using virion-derived RNA from three independent transfection experiments. The interassay variation is reflected by the error bars; for instance, the DA, DC, and DD mutants may display as few as 30% RNA dimers. See Table 1 for the combinations of compensatory mutations represented by DA, DB, DC, and DD.
An important determinant for RNA dimerization within the DIS is the palindrome loop sequence 257-GCGCGC-262, which triggers dimerization by formation of “Waston-Crick” base pairs (9, 24, 37, 39). Removal of the loop sequence in the construct ΔLoop (shown in Fig. 1) affected RNA dimerization, but to a lesser extent than did the ΔDIS deletion (Fig. 3A and B). We next tested whether the compensatory mutations were able to correct the defect in RNA dimerization caused by ΔLoop. The results showed that significant levels of monomeric RNA were still associated with each of the viruses that contained the ΔLoop deletion in combination with various compensatory mutations (Fig. 3B). However, the latter viruses contained less of the high-molecular-weight RNA, which migrated slower than dimers on gels. Hence, wild-type RNA dimerization was not restored by compensatory mutations within Gag.
The compensatory mutations restore wild-type RNA packaging to the ΔDIS and ΔLoop viruses while excluding spliced viral RNA from virions.
As stated, the compensatory mutations did not correct dimerization defects associated with ΔDIS but did lead to more intense dimer and monomer bands on gels (Fig. 3A). This suggests that these mutations may have helped to increase the overall levels of viral RNA within the ΔDIS viruses. To validate this notion, we next assessed our viral RNA samples by RPA. The results showed that all mutants in transfected cells produced approximately equal levels of viral RNA, with ratios of genomic to spliced viral RNA that were similar to those of the wild type (data not shown). When RNA was extracted from the wild-type and mutant viruses, it was found by RPA that the ΔDIS mutant packaged approximately 35% less viral genomic RNA than did wild-type BH10 (Fig. 4B, compare lane 1 with lane 8, and Fig. 4D). The presence of the compensatory mutations resulted in increased levels of genomic RNA packaged into the ΔDIS mutant (Fig. 4B, lanes 2 to 5, and Fig. 4D), and the DD virus (which contained all four compensatory mutations) showed the highest level in this regard (97%). Therefore, the compensatory mutations increased the overall packaging efficiency of viral RNA, which may have accounted for the increased infectiousness of the ΔDIS virus. As a control, the BH10-D virus, which contained all four compensatory mutations in the context of wild-type 5′ RNA sequences, was also analyzed by RPA and showed similar levels of viral RNA to wild-type virus (Fig. 4B, lane 6). Thus, the effects of the compensatory mutations on the packaging efficiency of the ΔDIS mutant were specific to this deletion.
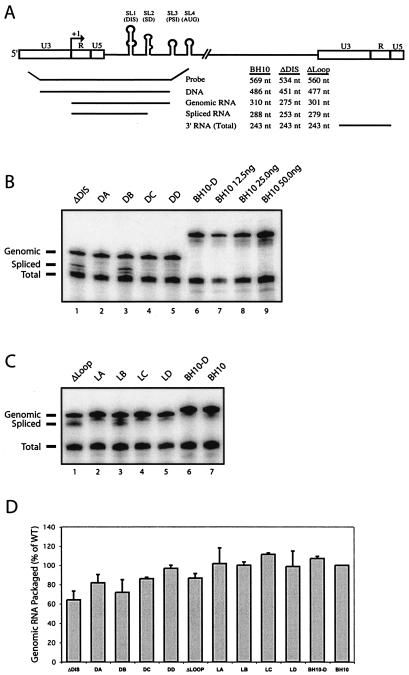
FIG. 4.
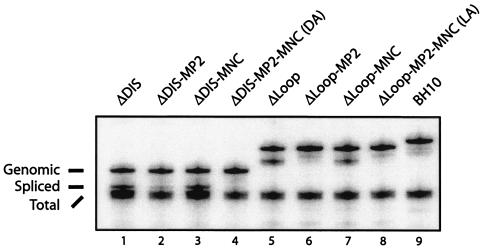
Effects of the ΔDIS and ΔLoop deletions on viral RNA packaging efficiency and specificity. (A) Illustration of the RPA system. At the top are the 5′ and 3′ long terminal repeat sequences and stem-loops 1, 2, 3, and 4. Below are the respective probes used to detect the BH10, ΔDIS, and ΔLoop viral RNA molecules as well as the sizes of the protected fragments. The probes thus designed allow the detection of proviral DNA contamination of the virion-derived RNA. RNA species detected include genomic RNA (B, top band), spliced RNA (B, middle band), and a total viral RNA band derived from binding of the probe to the 3′ U3 and R sequences found on all viral RNA species (B, bottom band). In some experiments, spliced and total RNA-protected fragments appear as a doublet. In the case of the spliced RNA, this may result from heterogeneity in the initial sequences of the various spliced exons immediately following the major splice donor, allowing one or two extra nucleotides of homology to the probe. It is also suggested in the RPA II kit literature that AU-rich sequences, often found in the 3′ UTR of many transcripts, can be susceptible to RNase digestion due to local denaturation of the double-stranded RNA hybrid. Such doublets have been reported elsewhere (4, 16, 50, 51). (B and C) Quantification of viral RNA by RPA. Viral RNA was prepared from mutant viruses ΔDIS, DA, DB, DC, DD, BH10-D (B, lanes 1 to 6), ΔLoop, LA, LB, LC, LD (C, lanes 1 to 5), and wild-type virus BH10 (B, lanes 7 to 9, and C, lane 7). An amount of viral RNA equivalent to 25 ng of p24 was annealed to 105 cpm of α-UTP-labeled riboprobe and digested with RNases specific for single-stranded RNA, and protected fragments were separated by denaturing polyacrylamide gel electrophoresis (5% polyacrylamide). A dilution series of wild-type RNA (12.5, 25.0, and 50.0 ng of p24 [B, lanes 7 to 9, respectively]) was analyzed to show the linear range of the assay. Two samples containing 10 μg of yeast tRNA with or without RNase were included in all RPA experiments to demonstrate probe specificity but are not shown due to the large difference in size between the probe and the relevant bands. One representative gel is shown from three independent experiments. (D) Relative packaging efficiency. Band intensities were measured using the AlphaImager v5.5 program. Packaging levels of mutant viral genomic RNA are expressed as a percentage of wild-type BH10 (arbitrarily set at 100%). The bar graph represents pooled data from three RPA gels, using RNA from three independent transfections of each mutant. See Table 1 for the combinations of compensatory mutations represented by DA, DB, DC, and DD.
Consistent with previous studies showing that reduced HIV-1 genomic RNA packaging was constantly accompanied by increased incorporation of spliced viral RNA (8, 45), a strong band corresponding to the spliced viral RNA was found to be associated with the ΔDIS virus, as distinct from the exclusive packaging of genomic RNA by wild-type BH10 (Fig. 4B). This suggests that ΔDIS caused defects in packaging efficiency and packaging specificity as well, both of which might have been related to its inability to replicate in culture. The presence of the compensatory mutations helped the ΔDIS virus to exclude spliced RNA molecules from being packaged (Fig. 4B), although they did not all function equally well in this regard. The DA (ΔDIS-MNC-MP2), DC (ΔDIS-MNC-MP2-CA1), and DD (ΔDIS-MNC-MP2-CA1-MA1) viruses recruited only trace amounts of spliced viral RNA (Fig. 4B, lanes 2, 4, and 5); in contrast, the DB (ΔDIS-MNC-CA1) virus exhibited high levels of spliced viral RNA concomitant with deficient genomic RNA packaging. More importantly, the DB virus replicated at a significantly lower rate than did any of DA, DC, or DD (Fig. 2). These results strongly suggest that correction of both packaging efficiency and specificity was important for compensation to occur.
The positive roles of these compensatory mutations in overcoming the deficits of packaging specificity were further confirmed by experiments performed in the context of the ΔLoop deletion (Fig. 4C). Similar to the DB virus, the LB virus packaged significant levels of spliced viral RNA. However, it was noteworthy that DB and LB are the only viruses that did not contain the MP2 mutation (Table 1); this suggests that MP2 is responsible for restoration of wild-type packaging to the ΔDIS and ΔLoop viruses.
The MP2 mutation alone corrects defects in packaging specificity seen in the ΔDIS and ΔLoop mutants.
We next generated four DNA constructs that contained the ΔDIS or ΔLoop deletions, in combination with either the MP2 or MNC compensatory mutations. Analysis of virion-derived RNA from these mutants showed that ΔDIS-MP2 barely packaged any spliced viral RNA compared with ΔDIS and ΔDIS-MNC (Fig. 5). Similarly, MP2 alone sufficed to restore normal packaging specificity to the ΔLoop mutant (Fig. 5, compare lane 5 with lane 6). Consistently, the ΔDIS-MP2 virus resulted in CPE by day 14 after infection of MT-2 cells and showed peak levels of RT activity on day 17, in contrast to wild-type virus, which showed peak RT activity on day 7 (data not shown). These data demonstrate a role for MP2 in restoration of wild-type packaging specificity to ΔDIS, as well as the importance of this mutation in augmenting the infectiousness of this virus.
FIG. 5.
Effects of the MP2 and MNC mutations on selective packaging of the full-length ΔDIS and ΔLoop RNA molecules. RPA was performed as described in the legend to Fig. 4, using virion-derived RNA from the ΔDIS (lane 1) and ΔLoop (lane 5) mutants in the presence of MP2 (lanes 2 and 6), MNC (lanes 3 and 7), or both compensatory mutations in combination (lanes 4 and 8), along with viral RNA from wild-type BH10 (lane 9). One representative gel is shown of two independent experiments.
Mutation of T12 within p2 causes defective packaging of wild-type genomic RNA.
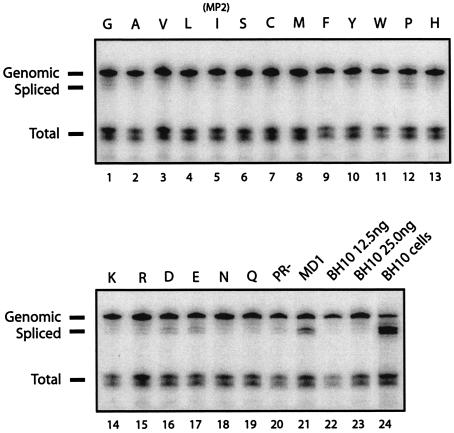
The positive effect of MP2 on selective packaging of full-length versus spliced ΔDIS RNA suggests a role for the T12 amino acid position in HIV-1 RNA packaging. To assess this possibility, we performed RPA on a series of viruses containing all 20 amino acid substitutions at position 12 of p2 in the context of wild-type RNA packaging signals (41). The results show that wild-type BH10 virus packaged more than 95% full-length genomic RNA, although spliced viral RNA species represented the majority of viral RNA that was present in the cytoplasm of BH10-transfected cells (Fig. 6, compare lane 23 with lane 24). Most of the substitutions at position 12 of p2 had no effect on the selective packaging of full-length viral RNA (Fig. 6; Table 2), but exceptions were the replacement of T by G, P, D, and E, which caused significant increases in the levels of the 288-nt band representing spliced viral RNA (Fig. 6, lanes 1, 12, 16, and 17; Table 2).
FIG. 6.
Effects of amino acid substitutions at position 12 in p2 on the packaging specificity of wild-type RNA. RPA was performed as described in the legend to Fig. 4, using virion-derived RNA from a panel of HIV-1 mutants containing each of the 20 amino acids at position 12 in p2 (41). Thr is shown in the wild-type BH10 (lane 23), and single-letter codes are given for the other 19 amino acids (lanes 1 to 19). Lane 5 shows T121, previously identified as MP2. The PR− and ψ− mutant MD1 (containing wild-type sequence except for deletion of the SL3 loop sequence 5′-GGAG-3′ [nt 317 to 320]) were run as controls (lanes 20 and 21, respectively). A 12.5-ng p24 equivalent of wild-type BH10 RNA was run to show the range of the assay (lane 22). A 250-ng sample of cytoplasmic RNA from transfection of wild-type BH10 was also analyzed to show the abundance of spliced RNA in the transfected cells and to confirm the identity of the spliced band reported in other lanes (lane 24). Band intensities were measured using the AlphaImager v5.5 program, and the data are summarized in Table 2. One representative gel of two independent experiments is shown.
TABLE 2.
Effects of substitutions at T12 of p2 on overall versus specific packaging
| Amino acid | % Packaginga,c specificity | % Genomeb,c packaging |
|---|---|---|
| G | 43 | 87 |
| A | 75 | 77 |
| V | 65 | 87 |
| L | 83 | 81 |
| I | 96 | 82 |
| S | 65 | 87 |
| C | 76 | 90 |
| M | 75 | 86 |
| F | 103 | 70 |
| Y | 84 | 87 |
| W | 94 | 72 |
| P | 49 | 78 |
| H | 52 | 79 |
| K | 97 | 90 |
| R | 80 | 107 |
| D | 48 | 91 |
| E | 40 | 82 |
| N | 100 | 98 |
| Q | 92 | 93 |
| T(BH10) | 100 | 100 |
The ratio of genomic to spliced viral RNA incorporated into virions was expressed as a percentage of wild-type BH10 based on digital image analysis of RPA gels.
Viral genomic RNA packaged, as a percentage of wild-type BH10.
All values represent the average from two independent experiments. Interassay variation was less than 10%.
To confirm that these bands represent packaged spliced RNA, as opposed to degradation products of the probe, the previously described MD1 mutant, which contains wild-type sequence except for deletion of the SL3 loop sequence 5′-GGAG-3′ [nt 317 to 320] and which packages significant levels of spliced viral RNA (42), was included as a control (Fig. 6, lane 21). As expected, the spliced RNA bands seen in the G, P, D, and E lanes correspond to 288-nt spliced viral RNA observed with the MD1 mutant. Since the mutated residue is located close to the protease (PR) cleavage site between p2 and NC, we also included a PR− control to rule out the possibility that any of the observed phenotypes might have been caused by improper Gag cleavage. The results in Fig. 6 (lane 20) show that PR− virus particles barely packaged any spliced viral RNA. This result also confirms that packaging specificity is indeed conferred by the Gag precursor prior to cleavage by viral protease. Taken together, these data demonstrate that T12 within p2 participates in the selection of full-length HIV-1 RNA for packaging.
DISCUSSION
The DIS represents the major dimerization signal for HIV-1 RNA (23, 32, 47). Partial deletion of DIS sequences, as found for the BH-LD3 and BH-LD4 deletions (29, 30), severely attenuated viral replication. However, the mutated viruses were able to establish persistent infection in permissive cells and eventually regained wild-type replication capacity due to the emergence of four compensatory mutations in Gag (29, 30). In contrast, removal of the entire DIS effectively destroyed viral replication capacity. Interestingly, infectivity of the ΔDIS virus could be restored to near wild-type levels by the same four compensatory mutations that were originally associated with the rescue of the BH-LD3 and BH-LD4 deletions. Thus, HIV-1 can survive the loss of the DIS, reflecting the highly plastic nature of the virus and its genome.
It is of interest to understand how compensatory mutations within Gag, which are distal to the original deletions, could have rescued ΔDIS. In agreement with previous studies, the ΔDIS deletion severely compromised HIV-1 RNA dimerization, a defect that must have led to dramatic reductions in viral infectivity (2, 8, 16, 21, 25, 26, 28, 38, 46). It is reasonable to assume that the four compensatory mutations, which restored wild-type infectiousness to the ΔDIS virus, should also have corrected this dimerization defect, but this was not the case. Conceivably, the four compensatory mutations could still have promoted the association of the ΔDIS RNA, and the binding between the mutated RNA molecules may have been too weak to resist extraction and electrophoresis procedures. However, even if this is the case, we can still conclude that tightly associated RNA dimers, as seen within wild-type viruses, are not a strict prerequisite for efficient viral replication, since the ΔDIS viruses could replicate in the presence of the compensatory mutations.
Although the compensatory mutations did not help ΔDIS RNA to dimerize in wild-type fashion, they did exert positive effects on the folding and association of ΔDIS RNA molecules. In this context, Fig. 3 shows that significant levels of ΔDIS viral RNA migrated on gels at a rate lower than that of the dimer complexes. This migration defect was overcome by the compensatory mutations (Fig. 3). It is likely that lack of the ΔDIS element not only prevented RNA dimerization but also may have led to abnormal RNA folding. In support of this view, large viral RNA complexes have also been detected for a variety of mutations within the DIS element (8, 46). Although the compensatory mutations were unable to rescue the dimerization function of the deleted DIS motif, they did help to reorganize ΔDIS RNA molecules within virus particles to promote discrete dimer or monomer forms.
Aside from its role in viral RNA dimerization, the DIS acts in concert with other viral RNA sequences, such as SL3, to regulate the specific encapsidation of viral RNA (2, 7, 8, 15, 16, 26, 33, 38; for a review, see reference 3). Our data show that deleting the DIS interfered with viral RNA packaging, as shown by increased levels of spliced viral RNA associated with the ΔDIS and ΔLoop viruses (Fig. 4). Interestingly, the MP2 mutation was able to help the ΔDIS virus to exclude spliced viral RNA from being packaged. Since the selective encapsidation of two copies of full-length viral RNA is normally achieved by specific interactions between NC residues and RNA packaging signals located within the 5′ UTR of viral RNA (3), we were surprised to find that the excessive encapsidation of spliced viral RNA into the ΔDIS virus was repaired by changing a single amino acid at position T12 within p2 (i.e., MP2) rather than by mutations at NC residues, such as the MNC mutation. This demonstrates the pivotal role of the p2 region in HIV-1 RNA packaging, and this conclusion is further supported by the identification of four mutations, T12D, T12E, T12G, and T12P, that led to packaging of spliced viral RNA in the context of wild-type BH10 virus (Fig. 6).
Consistent with its role in viral RNA packaging, the MP2 mutation was also able to increase the viability of the ΔDIS virus to significant levels. This suggests that the correction of nonspecific viral RNA packaging may represent a major mechanism for compensation. The importance of MP2 in rescue of the ΔDIS deletion is also supported by its role in rescue of other mutated viruses that are deleted within the 5′ UTR of HIV-1, e.g. U5 (43), the region immediately downstream of the primer binding site (31), as well as a GA-rich sequence adjacent to SL3 (unpublished data). More importantly, excessive packaging of spliced viral RNA caused by deletion of this GA-rich region could be corrected by the MP2 mutation (data not shown). Thus, MP2 is capable of repairing the defects in viral RNA packaging that are caused by mutation of RNA packaging signals within the 5′ UTR. This may involve binding of modified Gag protein containing MP2 to viral RNA elements distinct from 5′ packaging signals. This might then reestablish selectivity for mutated viral RNA.
The role of p2 in RNA packaging is also suggested by one study demonstrating that the presence of HIV-1 p2 within HIV-1/HIV-2 chimeric Gag viruses significantly enhanced the packaging of HIV-1 versus HIV-2 RNA (20). Since viral RNA is recruited into virus particles prior to the processing of Gag by PR, this indicates that p2 may regulate viral RNA packaging in concert with the downstream NC domain either through direct interaction with viral RNA or indirectly by helping NC to adopt a correct conformation.
Since maximal rescue of the ΔDIS deletion was seen when all four compensatory mutations were present, the correction of viral RNA packaging by the MP2 mutation may not represent the sole mechanism for compensation. Consistent with this belief, DIS sequences may have been involved in the regulation of HIV-1 reverse transcription (2, 38, 46), viral protein translation (5), and other activities in either a direct or indirect manner. The DIS can also participate in the overall folding of the HIV-1 5′ UTR, which can then assume distinctive conformations and perform more than one function, depending on the stage of viral replication (1, 19, 40). Conceivably, the DIS may be necessary for multiple steps of the viral life cycle. This may explain the fact that the ΔDIS mutant was able to infect a significant proportion of cells in the MAGI assay but failed to establish a productive infection during continuous culture. It is also possible that compensatory mutations may have improved the function of viral components other than the DIS to stimulate viral replication. For example, a replication defect caused by insertion of an AUG translation initiation codon into HIV-1 5′ UTR was overcome by second-site mutations within the Env protein, which presumably improved Env function (10).
Interestingly, a recent article by Hill et al. (17) reported that the HIV-1 DIS stem-loop was dispensable for viral replication in peripheral blood mononuclear cells, which is contrary to the noninfectiousness of our ΔDIS mutated viruses in CBMCs (Fig. 2B). This apparent discrepancy could be attributable to the fact that our ΔDIS deletion lacked the complete DIS stem-loop, including the palindrome, whereas the DIS mutants described in the paper by Hill et al. contained either the wild-type or an arbitrary palindrome sequence in place of SL1.
In summary, we have demonstrated that HIV-1 is able to replicate efficiently in the absence of the DIS through modification of Gag protein sequences. The modified Gag did not restore wild-type RNA dimerization but was able to augment the selective packaging of full-length versus spliced viral RNA molecules into virus particles.
Acknowledgments
We thank Lawrence Kleiman and Nicholas Acheson for helpful suggestions and Maureen Oliveira for technical assistance.
Rodney S. Russell is the recipient of a Canadian Institutes of Health Research (CIHR) doctoral fellowship award, and Chen Liang is a CIHR Young Investigator. This work was supported by grants from the CIHR, the FRSQ, and the Canadian Foundation for Innovation.
REFERENCES
- 1.Abbink, T. E., and B. Berkhout. 2003. A novel long distance base-pairing interaction in human immunodeficiency virus type 1 RNA occludes the Gag start codon. J. Biol. Chem. 278:11601-11611. [DOI] [PubMed] [Google Scholar]
- 2.Berkhout, B., and J. L. van Wamel. 1996. Role of the DIS hairpin in replication of human immunodeficiency virus type 1. J. Virol. 70:6723-6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berkowitz, R. D., and S. P. Goff. 1996. RNA packaging. Curr. Top. Microbiol. Immunol. 214:177-218. [DOI] [PubMed] [Google Scholar]
- 4.Berkowitz, R. D., A. Ohagen, S. Hoglund, and S. P. Goff. 1995. Retroviral nucleocapsid domains mediate the specific recognition of genomic viral RNAs by chimeric Gag polyproteins during RNA packaging in vivo. J. Virol. 69:6445-6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brasey, A., M. Lopez-Lastra, T. Ohlmann, N. Beerens, B. Berkhout, J. L. Darlix, and N. Sonenberg. 2003. The leader of human immunodeficiency virus type 1 genomic RNA harbors an internal ribosome entry segment that is active during the G 2/M phase of the cell cycle. J. Virol. 77:3939-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner, B. G., J. P. Routy, M. Petrella, D. Moisi, M. Oliveira, M. Detorio, B. Spira, V. Essabag, B. Conway, R. Lalonde, R. P. Sekaly, and M. A. Wainberg. 2002. Persistence and fitness of multidrug-resistant human immunodeficiency virus type 1 acquired in primary infection. J. Virol. 76:1753-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clever, J. L., D. Mirandar, Jr., and T. G. Parslow. 2002. RNA structure and packaging signals in the 5′ leader region of the human immunodeficiency virus type 1 genome. J. Virol. 76:12381-12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clever, J. L., and T. G. Parslow. 1997. Mutant human immunodeficiency virus type 1 genomes with defects in RNA dimerization or encapsidation. J. Virol. 71:3407-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clever, J. L., M. L. Wong, and T. G. Parslow. 1996. Requirements for kissing-loop-mediated dimerization of human immunodeficiency virus RNA. J. Virol. 70:5902-5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das, A. T., A. P. van Dam, B. Klaver, and B. Berkhout. 1998. Improved envelope function selected by long-term cultivation of a translation-impaired HIV-1 mutant. Virology 244:552-562. [DOI] [PubMed] [Google Scholar]
- 11.Ennifar, E., P. Walter, B. Ehresmann, C. Ehresmann, and P. Dumas. 2001. Crystal structures of coaxially stacked kissing complexes of the HIV-1 RNA dimerization initiation site. Nat. Struct. Biol. 8:1064-1068. [DOI] [PubMed] [Google Scholar]
- 12.Ennifar, E., M. Yusupov, P. Walter, R. Marquet, B. Ehresmann, C. Ehresmann, and P. Dumas. 1999. The crystal structure of the dimerization initiation site of genomic HIV-1 RNA reveals an extended duplex with two adenine bulges. Struct. Fold Design. 7:1439-1449. [DOI] [PubMed] [Google Scholar]
- 13.Feng, Y. X., T. D. Copeland, L. E. Henderson, R. J. Gorelick, W. J. Bosche, J. G. Levin, and A. Rein. 1996. HIV-1 nucleocapsid protein induces “maturation” of dimeric retroviral RNA in vitro. Proc. Natl. Acad. Sci. USA 93:7577-7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girard, F., F. Barbault, C. Gouyette, T. Huynh-Dinh, J. Paoletti, and G. Lancelot. 1999. Dimer initiation sequence of HIV-1Lai genomic RNA: NMR solution structure of the extended duplex. J. Biomol. Struct. Dyn. 16:1145-1157. [DOI] [PubMed] [Google Scholar]
- 15.Greatorex, J., J. Gallego, G. Varani, and A. Lever. 2002. Structure and stability of wild-type and mutant RNA internal loops from the SL-1 domain of the HIV-1 packaging signal. J. Mol. Biol. 322:543-557. [DOI] [PubMed] [Google Scholar]
- 16.Harrison, G. P., G. Miele, E. Hunter, and A. M. Lever. 1998. Functional analysis of the core human immunodeficiency virus type 1 packaging signal in a permissive cell line. J. Virol. 72:5886-5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill, M. K., M. Shehu-Xhilaga, S. M. Campbell, P. Poumbourios, S. M. Crowe, and J. Mak. 2003. The dimer initiation sequence stem-loop of human immunodeficiency virus type 1 is dispensable for viral replication in peripheral blood mononuclear cells. J. Virol. 77:8329-8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu, W. S., and H. M. Temin. 1990. Genetic consequences of packaging two RNA genomes in one retroviral particle: pseudodiploidy and high rate of genetic recombination. Proc. Natl. Acad. Sci. USA 87:1556-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huthoff, H., and B. Berkhout. 2002. Multiple secondary structure rearrangements during HIV-1 RNA dimerization. Biochemistry 41:10439-10445 [DOI] [PubMed] [Google Scholar]
- 20.Kaye, J. F., and A. M. Lever. 1998. Nonreciprocal packaging of human immunodeficiency virus type 1 and type 2 RNA: a possible role for the p2 domain of Gag in RNA encapsidation. J. Virol. 72:5877-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim, H. J., K. Lee, and J. J. O'Rear. 1994. A short sequence upstream of the 5′ major splice site is important for encapsidation of HIV-1 genomic RNA. Virology 198:336-340. [DOI] [PubMed] [Google Scholar]
- 22.Kimpton, J., and M. Emerman. 1992. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of an integrated β-galactosidase gene. J. Virol. 66:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laughrea, M., and L. Jette. 1994. A 19-nucleotide sequence upstream of the 5′ major splice donor is part of the dimerization domain of human immunodeficiency virus 1 genomic RNA. Biochemistry 33:13464-13474. [DOI] [PubMed] [Google Scholar]
- 24.Laughrea, M., and L. Jette. 1996. Kissing-loop model of HIV-1 genome dimerization: HIV-1 RNAs can assume alternative dimeric forms, and all sequences upstream or downstream of hairpin 248-271 are dispensable for dimer formation. Biochemistry 35:1589-1598. [DOI] [PubMed] [Google Scholar]
- 25.Laughrea, M., and L. Jette. 1997. HIV-1 genome dimerization: kissing-loop hairpin dictates whether nucleotides downstream of the 5′ splice junction contribute to loose and tight dimerization of human immunodeficiency virus RNA. Biochemistry 36:9501-9508. [DOI] [PubMed] [Google Scholar]
- 26.Laughrea, M., L. Jette, J. Mak, L. Kleiman, C. Liang, and M. A. Wainberg. 1997. Mutations in the kissing-loop hairpin of human immunodeficiency virus type 1 reduce viral infectivity as well as genomic RNA packaging and dimerization. J. Virol. 71:3397-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laughrea, M., N. Shen, L. Jette, J. L. Darlix, L. Kleiman, and M. A. Wainberg. 2001. Role of distal zinc finger of nucleocapsid protein in genomic RNA dimerization of human immunodeficiency virus type 1: no role for the palindrome crowning the R-U5 hairpin. Virology 281:109-116. [DOI] [PubMed] [Google Scholar]
- 28.Laughrea, M., N. Shen, L. Jette, and M. A. Wainberg. 1999. Variant effects of non-native kissing-loop hairpin palindromes on HIV replication and HIV RNA dimerization: role of stem-loop B in HIV replication and HIV RNA dimerization. Biochemistry 38:226-234. [DOI] [PubMed] [Google Scholar]
- 29.Liang, C., L. Rong, M. Laughrea, L. Kleiman, and M. A. Wainberg. 1998. Compensatory point mutations in the human immunodeficiency virus type 1 Gag region that are distal from deletion mutations in the dimerization initiation site can restore viral replication. J. Virol. 72:6629-6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang, C., L. Rong, Y. Quan, M. Laughrea, L. Kleiman, and M. A. Wainberg. 1999. Mutations within four distinct gag proteins are required to restore replication of human immunodeficiency virus type 1 after deletion mutagenesis within the dimerization initiation site. J. Virol. 73:7014-7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang, C., L. Rong, R. S. Russell, and M. A. Wainberg. 2000. Deletion mutagenesis downstream of the 5′ long terminal repeat of human immunodeficiency virus type 1 is compensated for by point mutations in both the U5 region and gag gene. J. Virol. 74:6251-6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marquet, R., J. C. Paillart, E. Skripkin, C. Ehresmann, and B. Ehresmann. 1994. Dimerization of human immunodeficiency virus type 1 RNA involves sequences located upstream of the splice donor site. Nucleic Acids Res. 22:145-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McBride, M. S., and A. T. Panganiban. 1996. The human immunodeficiency virus type 1 encapsidation site is a multipartite RNA element composed of functional hairpin structures. J. Virol. 70:2963-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morin, N., E. Cherry, X. Li, and M. A. Wainberg. 1998. Cotransfection of mutated forms of human immunodeficiency virus type 1 Gag-Pol with wild-type constructs can interfere with processing and viral replication. J. Hum. Virol. 1:240-247. [PubMed] [Google Scholar]
- 35.Mujeeb, A., J. L. Clever, T. M. Billeci, T. L. James, and T. G. Parslow. 1998. Structure of the dimer initiation complex of HIV-1 genomic RNA. Nat. Struct. Biol. 5:432-436. [DOI] [PubMed] [Google Scholar]
- 36.Mujeeb, A., T. G. Parslow, A. Zarrinpar, C. Das, and T. L. James. 1999. NMR structure of the mature dimer initiation complex of HIV-1 genomic RNA. FEBS Lett. 458:387-392. [DOI] [PubMed] [Google Scholar]
- 37.Muriaux, D., P. Fosse, and J. Paoletti. 1996. A kissing complex together with a stable dimer is involved in the HIV-1 Lai RNA dimerization process in vitro. Biochemistry 35:5075-5082. [DOI] [PubMed] [Google Scholar]
- 38.Paillart, J. C., L. Berthoux, M. Ottmann, J. L. Darlix, R. Marquet, B. Ehresmann, and C. Ehresmann. 1996. A dual role of the putative RNA dimerization initiation site of human immunodeficiency virus type 1 in genomic RNA packaging and proviral DNA synthesis. J. Virol. 70:8348-8354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paillart, J. C., E. Skripkin, B. Ehresmann, C. Ehresmann, and R. Marquet. 1996. A loop-loop “kissing” complex is the essential part of the dimer linkage of genomic HIV-1 RNA. Proc. Natl. Acad. Sci. USA 93:5572-5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paillart, J. C., E. Skripkin, B. Ehresmann, C. Ehresmann, and R. Marquet. 2002. In vitro evidence for a long range pseudoknot in the 5′-untranslated and matrix coding regions of HIV-1 genomic RNA. J. Biol. Chem. 277:5995-6004. [DOI] [PubMed] [Google Scholar]
- 41.Rong, L., R. S. Russell, J. Hu, Y. Guan, L. Kleiman, C. Liang, and M. A. Wainberg. 2001. Hydrophobic amino acids in the human immunodeficiency virus type 1 p2 and nucleocapsid proteins can contribute to the rescue of deleted viral RNA packaging signals. J. Virol. 75:7230-7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russell, R. S., J. Hu, V. Beriault, A. J. Mouland, M. Laughrea, L. Kleiman, M. A. Wainberg, and C. Liang. 2003. Sequences downstream of the 5′ splice donor site are required for both packaging and dimerization of human immunodeficiency virus type 1 RNA. J. Virol. 77:84-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell, R. S., J. Hu, M. Laughrea, M. A. Wainberg, and C. Liang. 2002. Deficient dimerization of human immunodeficiency virus type 1 RNA caused by mutations of the U5 RNA sequences. Virology 303:152-163. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 45.Schwartz, M. D., D. Fiore, and A. T. Panganiban. 1997. Distinct functions and requirements for the Cys-His boxes of the human immunodeficiency virus type 1 nucleocapsid protein during RNA encapsidation and replication. J. Virol. 71:9295-9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen, N., L. Jette, C. Liang, M. A. Wainberg, and M. Laughrea. 2000. Impact of human immunodeficiency virus type 1 RNA dimerization on viral infectivity and of stem-loop B on RNA dimerization and reverse transcription and dissociation of dimerization from packaging. J. Virol. 74:5729-5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skripkin, E., J. C. Paillart, R. Marquet, B. Ehresmann, and C. Ehresmann. 1994. Identification of the primary site of the human immunodeficiency virus type 1 RNA dimerization in vitro. Proc. Natl. Acad. Sci. USA 91:4945-4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swanstrom, R., and J. W. Wills. 1997. Synthesis, assembly, and processing of viral proteins, p. 263. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [PubMed]
- 49.Yuan, Y., D. J. Kerwood, A. C. Paoletti, M. F. Shubsda, and P. N. Borer. 2003. Stem of SL1 RNA in HIV-1: structure and nucleocapsid protein binding for a 1×3 internal loop. Biochemistry 42:5259-5269. [DOI] [PubMed] [Google Scholar]
- 50.Zhang, Y., and E. Barklis. 1995. Nucleocapsid protein effects on the specificity of retrovirus RNA encapsidation. J. Virol. 69:5716-5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang, Y., and E. Barklis. 1997. Effects of nucleocapsid mutations on human immunodeficiency virus assembly and RNA encapsidation. J. Virol. 71:6765-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]