Abstract
Given the current difficulties generating vaccine-induced neutralizing antibodies to human immunodeficiency virus (HIV), the focus of the vaccine community has shifted toward creating cytotoxic-T-lymphocyte (CTL)-based vaccines. Recent reports of CTL-based vaccine trials in macaques challenged with simian/human immunodeficiency virus SHIV-89.6P have supported the notion that such vaccines can ameliorate the course of disease. However, almost all of these studies included Env as an immunogen and since SHIV-89.6P is sensitive to neutralizing antibodies it is difficult to determine the mechanism(s) of protection. Consequently, SHIV-89.6P challenge of macaques may be a poor model for determining vaccine efficacy in humans. To ascertain the effect of vaccine-induced multispecific mucosal CTL, in the absence of Env-specific antibody, on the control of an immunodeficiency virus challenge, we vaccinated Mamu-A*01+ macaques with constructs encoding a combination of CTL epitopes and full-length proteins (Tat, Rev, and Nef) by using a DNA prime/recombinant modified vaccinia virus Ankara (rMVA) boost regimen. The vaccination induced virus-specific CTL and CD4+ helper T lymphocytes with CTL frequencies as high as 20,000/million peripheral blood mononuclear cells. The final rMVA vaccination, delivered intravenously, engendered long-lived mucosal CTL. At 16 weeks after the final rMVA vaccination, the vaccinees and naive, Mamu-A*01+ controls were challenged intrarectally with SIVmac239. Massive early anamnestic cellular immune responses controlled acute-phase viral replication; however, the three vaccinees were unable to control virus replication in the chronic phase. The present study suggests that multispecific mucosal CTL, in the absence of neutralizing antibodies, can achieve a modicum of control over early viral replication but are unable to control chronic-phase viral replication after a high-dose mucosal challenge with a pathogenic simian immunodeficiency virus.
Recent studies have demonstrated protection from disease progression, offering hope that cytotoxic-T-lymphocyte (CTL)-based vaccines might ameliorate the course of human immunodeficiency virus (HIV) disease (6, 8, 50). After infection in these studies, some macaques had strong anamnestic CTL responses associated with reduced viral loads. Each of these studies used SHIV-89.6P, a chimeric simian immunodeficiency virus (SIV) expressing env, tat, rev, and vpu genes of HIV type 1 (HIV-1) isolate 89.6, as the challenge virus (41). However, unlike most primary HIV strains, simian/human immunodeficiency virus SHIV-89.6P causes acute CD4+-T-lymphocyte loss and is sensitive to neutralizing antibodies (35, 41). It is difficult, therefore, to extrapolate these studies to HIV infection in humans. Protection from the acute depletion of CD4+ T lymphocytes caused by SHIV-89.6P may allow for the development of an effective antibody response to arise that is capable of controlling SHIV-89.6P replication. Conversely, the molecular clone SIVmac239 is difficult to neutralize and infected macaques have a protracted decline in CD4+ T lymphocytes.
Mucosal tissues are an active site of replication for both HIV and SIV (48, 51). Ideally, vaccine-induced CTL could prevent the virus from spreading systemically by rapidly eliminating infected cells at the site of exposure. Studies in murine models suggest that mucosal, but not systemic, CTL are capable of protecting against mucosally transmitted viruses (9, 44). Since the majority of HIV infections occur across mucosal surfaces, targeting of immune responses to the mucosa may provide immediate effector cells at the site of infection. Furthermore, the importance of mucosal CTL may not only be spatial but also functional. In mice, vesicular stomatitis virus-specific memory T cells derived from nonlymphatic tissue have been shown to be more directly lytic than corresponding cells derived from lymphatic tissues (33). This suggests that HIV-specific memory T-lymphocytes, residing in nonlymphatic tissues (small intestine, vagina, colon, etc.), may be able to react immediately to HIV infection.
In a previous study we used a DNA prime/recombinant modified vaccinia virus Ankara (rMVA) vaccination regimen to induce a massive CTL response to a single Mamu-A*01-restricted epitope, Gag181-189CM9 (5). When vaccinated animals were challenged with SIVmac239, we observed no amelioration of disease. Subsequently, we described a Mamu-A*01-restricted CTL epitope in Tat that escaped early in infection, suggesting that this epitope was under selective pressure (3, 37). Since the Gag181-189CM9 epitope escapes only intermittently in chronic infection (1, 37), we hypothesized that this epitope might be under less selective pressure from CTLs and therefore a less attractive vaccine target than the Tat28-35SL8 epitope. We, therefore, designed a similar vaccine that induced Tat-specific CTLs and determined whether they could control acute virus replication (2). Despite a high-level anamnestic CTL response, the vaccinated macaques were also unable to control replication of SIVmac239. We then designed another vaccine, by using constructs encoding all proteins of SIV (including Env), which did result in lower viral replication in the acute phase (23). Unfortunately, we could not exclude the possibility that control was due to vaccine-induced Env-specific antibody, even though no SIVmac239 neutralizing antibodies were detected either before or after challenge.
We tested here whether CTLs directed against multiple epitopes could control replication of SIVmac239 in the absence of Env-specific antibody. We also sought to determine whether a vaccine regimen targeting CTLs to both systemic and mucosal tissues would be more effective against viral challenge than vaccines now in use. Therefore, we vaccinated three Mamu-A*01+ rhesus macaques with the Mamu-A*01-restricted CTL epitopes Gag181-189CM9 and Tat28-35SL8, along with full-length SIV Tat, Rev, and Nef, by using a DNA prime/rMVA boost regimen. A recent study showed that the intravenous (i.v.) administration of vaccinia virus could induce long-lived memory T cells in the mucosa (32). For this reason the final rMVA boost was delivered i.v.
MATERIALS AND METHODS
Animals.
Rhesus macaques (Macaca mulatta) were maintained in accordance with the NIH Guide to the Care and Use of Laboratory Animals, and under the approval of the University of Wisconsin and the University of California Research Animal Resource Center (RARC) review committees.
Peptides.
Overlapping peptides (20-mers, 15-mers, 10-mers, 9-mers, and 8-mers) were synthesized by Chiron (Raleigh, N.C.), the Natural and Medical Science Institute (University of Tuebingen, Germany), or the Biotechnology Center (University of Wisconsin-Madison) based on SIVmac239 protein sequences, with the exception of Pol peptides, which corresponded to the SIVmac251 sequence. Lyophilized peptides were resuspended in phosphate-buffered saline (PBS) with 10% dimethyl sulfoxide (Sigma Chemical Co., St. Louis, Mo.). Consecutive 20-mer, 15-mer, and 9-mer peptides overlap by 10, 11, or 8 amino acids, respectively. Pools of peptides contained 10 peptides at a final concentration of 1 mg/ml per peptide.
PBMC.
Peripheral blood mononuclear cells (PBMC) were separated from whole heparinized blood by Ficoll-diatrizoate (Histopaque; Sigma) density gradient centrifugation and cultured according to methods described previously (53).
B-LCL lines.
Rhesus monkey B-lymphoblastoid cell lines (B-LCL) were generated as described previously (52, 53) by incubating PBMC with herpesvirus papio produced by S594 cells.
Generation of chimeric HBcAg-CTL epitope expressing DNA vaccine.
The hepatitis B virus core antigen (HBcAg) carrier expression vector pHBc expresses HBcAg under the control of the cytomegalovirus (CMV) immediate-early promoter (PJV 7198; PowderJect Vaccines, Inc., Madison, Wis.). It contains a unique Bsp120I restriction site within the immunodominant loop of HBcAg between amino acids 80 and 81 and a unique NotI restriction site at the 3′ end of the HBcAg gene, facilitating the insertion of epitopes at either site (27). To construct chimeric HBcAg-epitope DNA vaccines, pHBc was digested with either Bsp120I or NotI. Oligonucleotides encoding Bsp120I- or NotI-flanked, codon-optimized SIV CTL epitopes were synthesized, annealed, and ligated into pHBc at the immunodominant region or carboxy terminus, respectively, of HBcAg. Clones containing inserts were identified by PCR as described previously (27) and sequenced to confirm insertion of the correct coding sequences and orientation.
Generation of the PJV7343, a SIVmacC8 Nef expressing DNA vaccine.
The coding sequence for SIV Nef was amplified by PCR with the plasmid pNef C8 derived from the C8 isolate of SIVmac32H (a SIVmac251 derivative) (46) as a template with primers JF121 (5′-GGA AAG CTT GCA ATC ATG GGT GGA GCT ATT TCC AGG-3′) and JF124 (5′-GGT GGG CCC TCA GCG AGT TTC CTT CTT GTC AG-3′) by a standard PCR methodology (1× PCR core buffer with 15 mM MgCl2 [Promega, Madison, Wis.], 0.4 μM concentrations of primers, 200 μM concentrations of deoxynucleoside triphosphates, 2.5 U of Taq polymerase [Promega], 1.0 ng of template DNA, water to 100 μl, and a mineral oil overlay). After phenol-chloroform extraction and ethanol precipitation, the PCR product was resuspended in Tris-EDTA buffer and cut with Hind3 and Bsp120 I to generate an insert fragment. A vector fragment was prepared by removing the HBcAg coding region from plasmid pHBcAg (27) by cutting with HindIII and NotI. The two fragments were ligated together, resulting in PJV7343. The Nef insert in PJV7343 was sequenced, and no changes from the expected sequence were discovered.
Generation of the SIV Tat-expressing DNA vaccine.
Two PCRs were performed to create an intronless Tat coding fragment. PJV7135 (PowderJect Vaccines), a plasmid containing the SIV-17E-Fred genome served as the template and the Tat encoding regions were amplified with primers JF35 (5′-GCG CTA GCG AGA CAC CCT TGA GGG AG-3′) and JF37 (5′-CAA ACA ACA GAC CCA TAT CCA ACA GGA C-3′) and with primers JF36 (5′-ATG GGT CTG TTG TTT GAT GCA GAA GAT G-3′) and JF38 (5′-GCG GAT CCG TCT ATC TGC CAA GGC CAG GAG C-3) by using standard PCR conditions. The thermocycler conditions included an initial denaturation step at 95°C for 4 min, followed by 30 cycles of a 1-min denaturation at 95°C, 1 min 15 s of annealing at 55°C, and a 1-min extension at 72°C. After a final 10-min extension step at 72°C, the reactions were stored at 4°C. The two PCR products were electrophoresed on a 1% agarose gel, stained with ethidium bromide, excised from the gel, and soaked for 30 min at 65°C in 100 μl of water to elute the PCR fragments. One microliter of each gel eluate was used in a standard PCR with primers JF35and JF38 to amplify the complete Tat coding sequence. The resulting PCR product was purified and cut with NheI and BamHI for fragment insertion. A vector fragment was prepared by removing the HBcAg from a signal peptide-less version of plasmid pWRG7063 (27) by cutting with NheI and BamHI. The insert and vector were then ligated, resulting in PJV7271. The Tat insert in PJV7271 was sequenced, and one change (tyrosine to serine) from the expected sequence at position 44 was discovered.
DNA/rMVA vaccinations.
Animals were immunized six times with DNA by using the PowderJectXR1 device (PowderJect Vaccines). The first three DNA immunizations were given epidermally (eight sites) at intervals of 4 to 9 weeks; after a 7- to 14-week rest period, another three DNA immunizations were given epidermally (eight sites) and orally (four sites into the cheek pouch and four sites into the tongue) at intervals of 4 weeks. For the first three immunizations two plasmid vectors expressing SIV Nef (pJV7343; see above) and HBcAg with the CTL epitope Gag181-189CM9 (4) incorporated into the antigenic loop (see above) were used. For the next set of three DNA immunizations, an additional three vectors were used, expressing SIV Tat (pSIVTat; see above), SIV Rev (pSIVrev; see reference 19), and HBcAg with the CTL epitope Tat28-35SL8 (3) incorporated into its antigenic loop (see above). Equal amounts of each plasmid DNA were precipitated onto 1- to 3-μm gold particles (Degussa, Plainfield, N.J.) in the presence of 0.1 M spermidine (Sigma) and 2.5 M CaCl2 (Fujisawa, Inc., Melrose Park, Ill.) at a rate of 4 μg of DNA per mg of gold. One milligram of gold was delivered per site.
Generation and inoculation of rMVA vector vaccines.
rMVA constructs used in the present study separately express the tat, rev, or nef coding sequences of the SIVmac32H J5 clone (46) under the transcriptional control of the vaccinia virus early/late promoter P7.5. We also used an rMVA virus expressing the CTL epitope Gag181-189CM9 (4) as a minigene under the control of the P7.5 promoter. To generate vaccine preparations, recombinant and nonrecombinant MVA were amplified on chicken embryo fibroblast (CEF) cells derived from embryonated eggs of a specific-pathogen-free stock. CEF were grown in minimal essential medium (Biochrom, Berlin, Germany) supplemented with 10% fetal bovine serum (Biochrom) and maintained in a humidified air-5% CO2 atmosphere at 37°C. Viruses were purified by ultracentrifugation through a cushion of 36% (wt/vol) sucrose in 10 mM Tris-Cl (pH 8.0) and reconstituted in PBS, and titers were determined by immunostaining of virus-infected cell foci on CEF monolayers by using vaccinia virus-specific rabbit polyclonal antibody (Biogenesis, Ltd., Poole, United Kingdom). Virus preparations were divided into aliquots that contained 5 × 108 infectious units/ml and stored at −70°C. The vector vaccine preparations were tested in vitro for their capacity to synthesize SIV target antigens by Western blot analyses for Rev and Nef proteins, and Tat production was confirmed by assaying the transcriptional activation of HIV-long-terminal-repeat-controlled luciferase reporter gene expression (data not shown). About 14 to 28 weeks after the last DNA vaccination, all animals of the vaccine group were inoculated with rMVA vaccines encoding SIVmacJ5 (46) Nef, Rev, and Tat and with rMVA encoding the CTL epitope Gag181-189CM9 delivered intradermally (i.d.) and intranasally (i.n.). The animals received 108 infectious units of each rMVA vector vaccine. Control animals received equal amounts of nonrecombinant MVA. After a 33- to 35-week rest period, all animals were inoculated a second time with the same rMVA, but this time they were inoculated i.v. No side effects or lesions were found associated with the inoculations.
Peptide-specific T-cell lines.
Peptide-specific CD8+- and CD4+-T-cell lines were generated by using previously described methods (53). Briefly, at day 0 fresh PBMC were in vitro stimulated with peptide-pulsed, autologous B-LCL as stimulator cells. At day 7, CD8β+ cells and CD4+ cells were separated by using the Miltenyi Biotec MiniMACS system. The separated CD8β+ and CD4+ cells were again in vitro stimulated with peptide-pulsed, autologous B-LCL as stimulator cells. After a total of 14 days of in vitro stimulation the cells were used as effectors in intracellular cytokine staining (ICS) assays to test for the peptide-specific release of gamma interferon (IFN-γ).
ICS of fresh PBMC.
ICS assays were performed as previously described (23). Between 5 × 105 and 1 × 106 PBMC were incubated with either staphylococcal enterotoxin B (10 μg/ml; Sigma) as a positive control, pools of 10 15-mer and 20-mer peptides together, or individual peptides at a concentration of 5 μg/ml, along with 0.5 μg of anti-CD28 (clone L293; BD Biosciences, San Diego, Calif.) and 0.5 μg of anti-CD49d (clone 9F10; BD Pharmingen) in a total volume of 200 μl of R-10 (RPMI 1640 supplemented with 10% fetal calf serum, 2 mM l-glutamine, 25 mM HEPES buffer, 25 μM 2-mercaptoethanol, 50 μg of streptomycin/ml, and 50 U of penicillin/ml). Anti-CD28 and anti-CD49d antibodies were added to provide optimal costimulation (38). After 1.5 h at 37°C, 10 μg of Brefeldin A (Sigma)/ml was added to inhibit secretion of cytokines, and the cells were further incubated for 5 h at 37°C. Cells were washed twice with 1 ml of fluorescence-activated cell sorting (FACS) buffer (PBS plus 2% fetal calf serum) and then stained with 6 μl of CD8α-PerCP (clone SK1; Becton Dickinson) and 4 μl of CD4-allophycocyanin (APC) (clone SK3, Becton Dickinson) in 100 μl of FACS buffer for 40 min. After two washes with 1 ml of FACS buffer, the cells were fixed with 2% paraformaldehyde (PFA)-PBS solution overnight at 4°C. The cells were then washed once with FACS buffer, treated with permeabilization buffer (0.1% saponin in FACS buffer) for 10 min at room temperature, washed once more with 0.1% saponin buffer, and resuspended in 100 μl of 0.1% saponin buffer. Then, 1 μl of anti-human IFN-γ-fluorescein isothiocyanate (FITC) monoclonal antibody (clone 4S.B3; Pharmingen) and 1 μl of anti-human tumor necrosis factor alpha-phycoerythrin (PE)monoclonal antibody (clone MAb11; Pharmingen) were added. After 50 min of incubation at room temperature, the cells were washed two times with 0.1% saponin buffer, followed by a 10-min incubation before the last spin, and then fixed with 2% PFA-PBS. A total of 100,000 to 200,000 lymphocyte-gated events were acquired on a FACSCalibur flow cytometer (Becton Dickinson) and analyzed by using FlowJo software (Treestar). The background level of IFN-γ staining in PBMC (induced by the control influenza peptide SNEGSYFFG) varied from animal to animal but was typically <0.05% in CD8+ lymphocytes and <0.02% in CD4+ lymphocytes. Only samples displaying IFN-γ staining at least twice that of the background or in which there was a distinct population of IFN-γ (bright)-positive cells (also positive for TNF-α) were considered positive. All values are reported after subtraction of the background level staining.
ICS with T-cell lines for fine mapping.
When T-cell lines were analyzed by ICS, the method described above (for fresh PBMC) was modified so that 105 B-LCL were used instead of anti-CD28 and anti-CD49d. The background level of ICS in T-cell lines (induced by the control influenza peptide SNEGSYFFG) was usually <0.5% and was subtracted from all values.
Challenge with molecularly cloned SIVmac239/nef-open.
At 17 weeks after the last rMVA boost, three vaccinated animals and three controls were challenged intrarectally (i.r.) with a molecularly cloned virus, SIVmac239/nef-open (40), with a dose of approximately 10 i.r. monkey infectious doses (MID50) (36), as described previously (23).
Viral load analysis.
Viral RNA from SIV was quantitated by real-time PCR by using the TaqMan assay kit (Perkin-Elmer Applied Biosystems, Foster City, Calif.) and evaluated on an ABI Prism 7000 (Perkin-Elmer Applied Biosystems, Foster City, CA) apparatus. Primer and probe sequences were as follows: forward primer SIV-61F, 5′-CCACCTACCATTAAGCCCGA-3′; reverse primer SIV-143R, 5′-CTGGCACTACTTCTGCTCCAAA-3′; and probe SIV-84T (FAM reporter, TAMRA quencher), 5′-CATTAAATGCCTGGGTAAAATTGATAGAGGA(GA)AAGAA-3′. The reaction mixture contained 1× TaqMan EZ buffer, 3 mM magnesium acetate, 1.2 mM concentrations of deoxynucleoside triphosphates, 100 nM SIV-84T probe, 400 nM final forward primer, 800 nM final reverse primer, 2.5 U of rTth, and 2 μl of RNA sample or RNA standard. Cycling conditions were as follows: 50°C for 2 min, 60°C for 30 min, and 95°C for 5 min, followed by 40 cycles of 95°C 15 s, 60°C for 1 min, and 25°C for 2 min. The data were collected during the extension phase only.
Statistical analysis.
Viral load differences between groups were tested for statistical significance by using Student t tests after log transformation of the data to improve normality and homoscedasticity. In addition, Levene's test for homoscedasticity was conducted and, if differences were found to be significant, the Welch correction for unequal variances was used. Finally, to further examine the robustness of the results, a nonparametric test, the Mann-Whitney U test, was performed. The P values for the nonparametric tests were calculated by exact methods. All of the P values are two tailed.
Neutralizing antibody assays.
Neutralization of a T-cell line-adapted stock of SIVmac251 or molecularly cloned SIVmac239/nef-open was measured in CEMx174 cells as described previously (34). Briefly, titers of neutralizing antibodies in this assay are reported as the reciprocal plasma dilution at which 50% of cells were protected from virus-induced killing as measured by neutral red uptake. The assay stock of SIVmac251 in this case was generated in H9 cells and is highly sensitive to neutralization. Neutralization of molecularly cloned SIVmac239/nef-open was also measured in human PBMC as a reduction in p27 Gag antigen synthesis (34). The assay stock of SIVmac239/nef-open was generated in human PBMC and was derived from the animal challenge stock.
Lymphocyte isolation from pinch biopsies.
Six to eight pinch biopsies approximately 2 by 2 by 2 mm in size were collected from the sigmoid colon by using a Fujinon FG-100PE pediatric gastroscope. The biopsies were incubated three successive times in an orbital shaker at 37°C for 30 min in R10 containing 15 μg of collagenase type II (Sigma)/ml. The supernatant after each incubation period was collected and pooled. Lymphocytes were isolated by overlaying the collected cells on an isotonic Percoll (Amersham-Pharmacia, Piscataway, N.J.) gradient (40% layered over 100%) and centrifuging them for 30 min at 800 rpm. Lymphocytes were collected from the 40%-100% Percoll interface and washed with R10.
Tetramer staining.
We used a previously described method (23) to stain lymphocytes. Briefly, 5 × 105 to 1 × 106 fresh, unstimulated lymphocytes were suspended in a 100-μl volume of FACS buffer. The cells were stained in the dark for 40 min at room temperature with either the Mamu-A*01/CM9 or Mamu-A*01/SL8 tetramer labeled with PE or APC (5 μg/ml), anti-human CD3ɛ-FITC (SP34; Pharmingen), and anti-CD8α-PerCP (clone SK1; Becton Dickinson). In certain cases tetramer-positive cells were also phenotyped for the presence of mucosal homing and retention markers by staining them with a mixture of APC-labeled tetramers, CD8α-PerCP, and antibodies to α4β7 (PE labeled; Millenium Pharmaceuticals) and αEβ7 (CD103 [Coulter Immunotech], FITC labeled). The cells were then washed two times with 1 ml of FACS buffer and fixed by adding 2% PFA-PBS solution. Sample data were acquired on a FACSCalibur instrument and analyzed by using CellQuest software (Becton Dickinson). Background tetramer staining of fresh, unstimulated PBMC from naive Mamu-A*01-positive animals was routinely less than 0.08%.
RESULTS
Immunization.
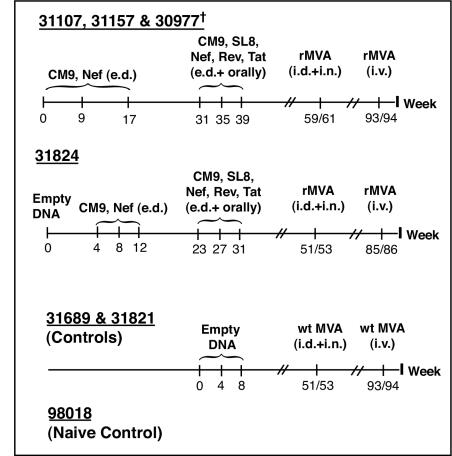
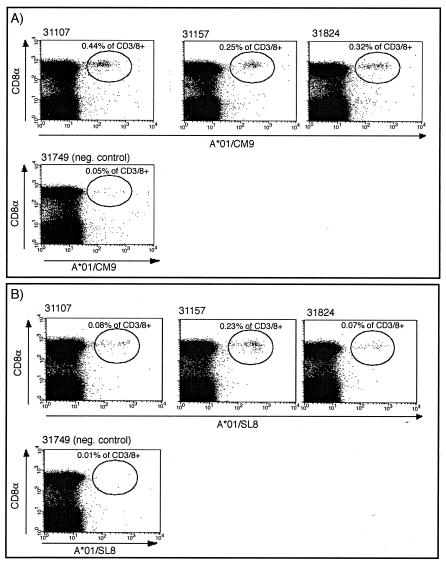
Our DNA/rMVA vaccine regimen was designed to induce systemic and mucosal CTL against multiple epitopes in the absence of Env-specific antibodies (Fig. 1). Three Mamu-A*01+ rhesus macaques were immunized with DNA encoding SIV Tat, Rev, Nef, and two immunodominant Mamu-A*01 restricted epitopes, Gag181-189CM9 and Tat28-35SL8. A fourth animal started the vaccination regimen but was euthanized prior to completion of the study due to unrelated health problems. At 2 weeks after the first DNA immunization, we detected Mamu-A*01/CM9 tetramer-positive cells in fresh PBMC in all four macaques at frequencies between 0.12 and 0.75% of the CD3+ CD8+ lymphocytes. After the DNA immunizations, both CTL and helper T-lymphocyte (HTL) responses were detected in each animal by ICS after in vitro stimulation. The first rMVA immunization was administered both i.n. and i.d. Virus-specific CD8+ lymphocytes were clearly detectable in PBMC after the boost (Fig. 2 and Table 1).
FIG. 1.
Immunization schedule. e.d., epidermal. †, Animal euthanized 4 weeks after the first rMVA boost.
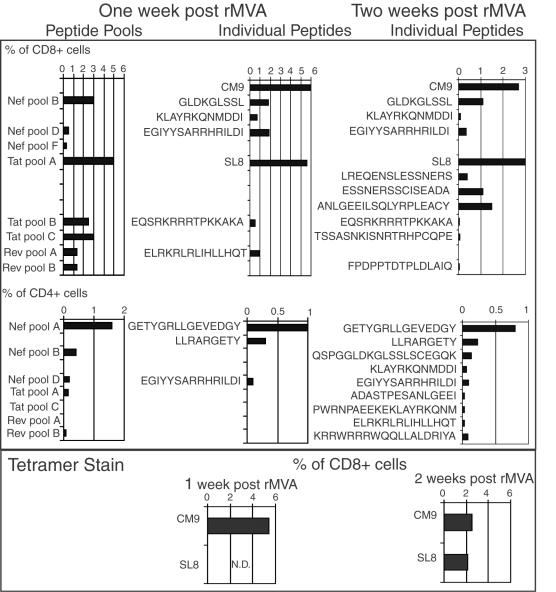
FIG. 2.
Frequencies of CD8 and CD4 responses after the first rMVA boost in fresh PBMC as determined by ICS and tetramer staining. Fresh PBMC were tested for SIV-specific cellular responses by using previously mapped peptides or peptide pools as stimuli in ICS 1 week after administration of rMVA (two left columns) and all individual peptides contained in positive pools or previously mapped epitopes (right column) at week 2 after administration of rMVA. In some cases, two overlapping peptides were included and are reported as the combination of the two peptides rather than as two independent peptides. In addition, we determined the frequency of Mamu-A*01/CM9 and Mamu-A*01/SL8 tetramer-positive cells (bottom panels). Animal 31157 is shown as an example. All animals were analyzed by this method.
TABLE 1.
Frequencies of SIV-specific CD8 and CD4 responses 1 week after rMVA boost 1 (i.d. and i.n.) as determined by ICS
| Animal no. | T-cell marker | Frequency (%) of:
|
|||||
|---|---|---|---|---|---|---|---|
| CM9 | SL8 | Tat (other than SL8) | Rev | Nef | Total Ag specifica | ||
| 30977 | CD8 | 1.0 | 0.43 | 0.26 | 0.2 | 0.22 | 2.11 |
| CD4 | 0 | 0.12 | 0.06 | 0.18 | |||
| 31107 | CD8 | 4.0 | 0.3 | 0 | 0 | 0.4 | 4.7 |
| CD4 | 0.05 | 0.04 | 0.02 | 0.1 | |||
| 31157 | CD8 | 6.0 | 5.6 | 0.5 | 2.2 | 4.75 | 19 |
| CD4 | 0.13 | 0.08 | 1.8 | 2 | |||
| 31824 | CD8 | 4.2 | 0.14 | 0.46 | 0 | 0.53 | 5.33 |
| CD4 | 0 | 0.08 | 0.06 | 0.13 | |||
Ag, antigen.
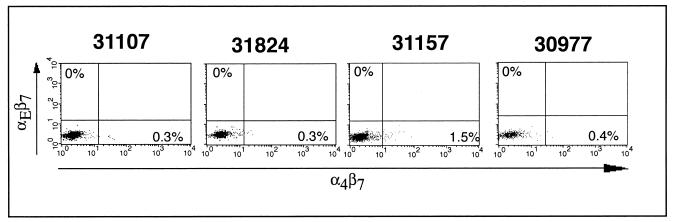
We also assayed vaccine-induced CD8+ T cells for expression of mucosal homing and retention markers. Effector and memory lymphocytes acquire “tissue-specific” adhesion and chemoattractant receptors based on their site of primary activation (13). This selective tissue tropism allows lymphocytes to maximize their chances of reencountering their specific antigen (12). α4β7 and αE(CD103)β7 are involved in lymphocyte homing to and retention within the gut. α4β7 is upregulated on lymphocytes primed in mucosal tissues and signal cells that will ultimately return to the gastrointestinal tract (13, 54), whereas αEβ7 retains intraepithelial lymphocytes (IEL) within the mucosal tissue by binding to its ligand, E-cadherin, on the surface of epithelial cells (14, 22). After the DNA immunizations, only a small subset of tetramer-positive CD8+ lymphocytes expressed α4β7 or αEβ7 (data not shown). Indeed, the rationale for delivering part of the first rMVA boost i.n. was to stimulate mucosal CTLs. Surprisingly, 1 week after the boost αEβ7 expression was not detectable and α4β7 was only detectable on a small fraction of tetramer-positive lymphocytes in the blood (Fig. 3). In animal 30977, euthanized 3 weeks after the first rMVA boost, Mamu-A*01/CM9 and Mamu-A*01/SL8 tetramer-positive cells were detected in lymph nodes throughout the body (Table 2). The vaccine-induced CTL responses were long-lived in the remaining three vaccinees. Mamu-A*01/CM9 and Mamu-A*01/SL8 tetramer-positive cells could be detected in PBMC 31 weeks after boosting with rMVA (Fig. 4), although the expression of mucosal homing and retention markers remained low (data not shown).
FIG. 3.
Tetramer-positive lymphocytes in the peripheral blood lack mucosal surface and retention markers after rMVA boost 1 (given i.d. and i.n.). Fresh PBMC obtained 1 week after the first rMVA boost were stained with anti-CD8, anti-α4β7, and anti-αEβ7 antibodies and Mamu-A*01/CM9 tetramer. Only the results for CD8+ tetramer-positive cells are shown.
TABLE 2.
Tetramer staining in PBMC and lymph nodes in animal 30977 3 weeks after rMVA boost 1 (i.d. and i.n.)
| Sample sourcea | A*01/CM9 (%)b | A*01/SL8 (%)b |
|---|---|---|
| PBMC | 0.28 | 0.36 |
| Mesenteric LN | 0.05 | 0.17 |
| Inguinal LN | 0.22 | 0.3 |
| Axillary LN | 0.1 | 0.13 |
| Sublingual LN | 0.11 | 0.25 |
LN, lymph nodes.
Percentage of CD8+ lymphocytes.
FIG. 4.
Tetramer-positive lymphocytes in the peripheral blood are detectable 31 weeks after rMVA boost 1 (given i.d. and i.n.). Fresh PBMC obtained 30 weeks after the first rMVA boost were stained with anti-CD3, anti-CD8, Mamu-A*01/CM9 tetramer (A) and Mamu-A*01/SL8 tetramer (B). The frequencies are listed as the percentage of tetramer staining in the CD3+ CD8+ lymphocyte population.
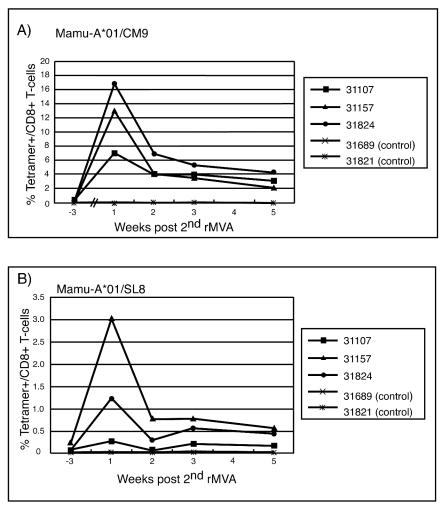
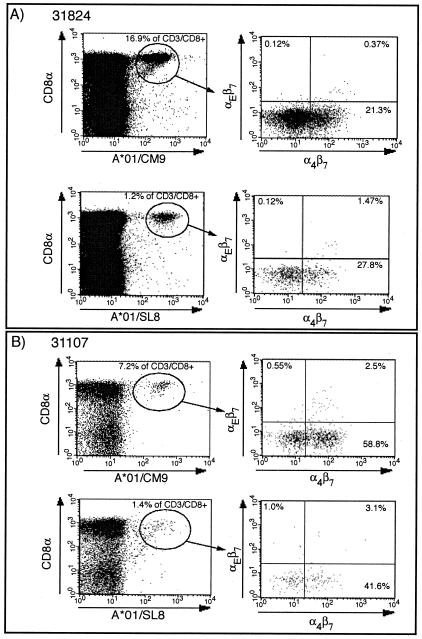
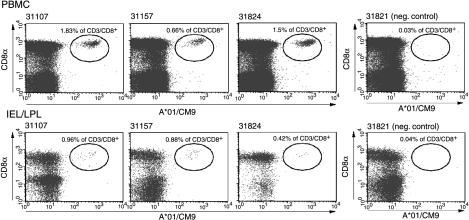
Based on recently published data demonstrating that i.v. administration of vaccinia induced a sustained mucosal response (32), we boosted the animals once more i.v. with rMVA. In contrast to previous studies (5, 21), in which the second rMVA boost, given via the same route as the first, did not increase the frequency of antigen-specific lymphocytes over the initial rMVA boost, the i.v. rMVA boost resulted in very high levels of tetramer-positive lymphocytes (Fig. 5). Mamu-A*01/CM9-specific CTLs reached levels of between 7 and 17% of all CD8+ T lymphocytes (Fig. 5A). There was also a boosting of the Mamu-A*01/SL8-specific response (Fig. 5B). Other previously defined CD8+- and CD4+-T-lymphocyte responses to SIV were also detectable by ICS (Table 3). Importantly, we were now able to detect tetramer-positive cells expressing the mucosal homing and retention markers α4β7 and αEβ7 (Fig. 6A). Virus-specific T cells expressing these markers were also detectable on lymphocytes isolated from pinch biopsies taken from the sigmoid colon (Fig. 6B). The i.v. administration of rMVA induced sustained, virus-specific CTL responses in both the blood and the sigmoid colon at 10 weeks postboost (Fig. 7).
FIG. 5.
Frequencies of tetramer-positive lymphocytes in the peripheral blood after rMVA boost 2 (i.v.). Fresh PBMC obtained at different time points after the second rMVA boost (i.v.) were stained with anti-CD3, anti-CD8, and Mamu-A*01/CM9 tetramers (A) or Mamu-A*01/SL8 tetramers (B). Percentage of tetramer-positive cells in the CD3+ CD8+ lymphocyte population. All control animals were negative for tetramer staining (less than the background [i.e., 0.08%]).
TABLE 3.
Frequencies of SIV-specific CD8 and CD4 responses after rMVA boost 2 (i.v.) as determined by ICS.
| Animal no. | CD type | Frequency (%) of CD8+ lymphocytes
|
|||||
|---|---|---|---|---|---|---|---|
| CM9 | SL8 | Tat | Rev | Nef | Total Ag specifica | ||
| 31107 | CD8 | 8.5 | 0.52 | 0.3 | 0.3 | 0.5 | 10.12 |
| CD4 | 0.61 | 0.18 | 0.92 | 1.71 | |||
| 31157 | CD8 | 8.4 | 1.8 | 0.4 | 1 | 1.3 | 12.1 |
| CD4 | 0.03 | 0.08 | 2.94 | 3.05 | |||
| 31824 | CD8 | 18 | 1.5 | 0.4 | 0 | 3.6 | 23.5 |
| CD4 | 0.38 | 1.6 | 0.1 | 2.08 | |||
Ag, antigen.
FIG. 6.
Tetramer-positive lymphocytes after rMVA boost 2 (i.v.) express mucosal homing and retention markers. Fresh PBMC (A) or IEL/LPL obtained from intestinal punch biopsies (B) at 1 week after the second rMVA boost (i.v.) were stained with anti-CD8, Mamu-A*01/CM9 or Mamu-A*01/SL8 tetramer and antibodies to α4β7 and αEβ7.
FIG. 7.
Tetramer-positive lymphocytes are still detectable in PBMC and IEL/LPL at 10 weeks after the rMVA boost (i.v.). Fresh PBMC (upper panel) or IEL/LPL obtained from sigmoid colon punch biopsies (lower panel) at 10 weeks after the second rMVA boost (i.v.) were stained with anti-CD3, anti-CD8, and Mamu-A*01/CM9 tetramer.
Immune responses after mucosal challenge with SIVmac239.
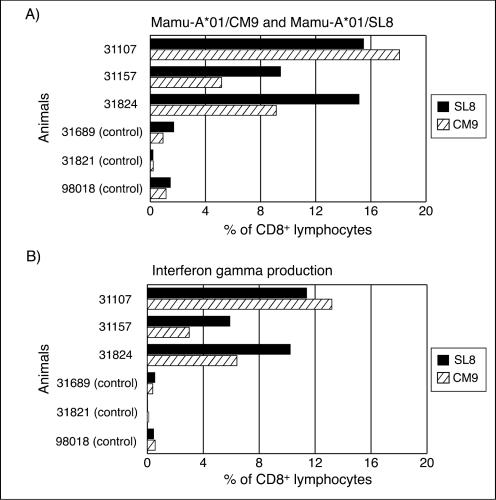
To determine whether these long-lived cellular immune responses could control SIVmac239 replication, we challenged the three vaccinated animals, along with three naive Mamu-A*01+ controls, i.r. with 10 MID50 SIVmac239 at 16 weeks after the final rMVA boost. The vaccinated animals made robust anamnestic immune responses. In animal 31107 the Mamu-A*01/CM9-specific CTLs attained levels of 18.1% of CD8+ lymphocytes in the PBMC by 2 weeks postchallenge (Fig. 8). In comparison, the highest Mamu-A*01/CM9 tetramer levels observed in any of the controls was 1.25%. Moreover, although Mamu-A*01/SL8-specific CTL levels were modest after the final rMVA boost in comparison to Mamu-A*01/CM9-specific CTL, in two of three vaccinated animals at 2 weeks postchallenge, Mamu-A*01/SL8 tetramer-positive lymphocytes were present at a higher frequency than were Mamu-A*01/CM9 tetramer-positive lymphocytes (Fig. 8A). ICS assays with the corresponding peptides, Gag181-189CM9 and Tat28-35SL8, showed comparable, yet somewhat lower, frequencies of CD8+ lymphocytes than were observed by tetramer staining (compare Fig. 8A and B). ICS also showed that, in addition to Gag181-189CM9 and Tat28-35SL8, other regions of Tat, Rev, and Nef that were recognized after immunization were still recognized 2 weeks postchallenge, including several CD4+-T-cell responses (data not shown).
FIG. 8.
Cellular immune response to Gag181-189CM9 and Tat28-35SL8 at 2 weeks postchallenge. Fresh PBMC was isolated at 2 weeks postinfection and tested for IFN-γ production upon peptide stimulation with Gag181-189CM9 and Tat28-35SL8. The frequency of CD8+ tetramer-positive lymphocytes (A) correlates with the proportion of CD8+ lymphocytes producing IFN-γ in response to the respective peptides (B), but at a somewhat lower frequency.
Vaccinated macaques do not control pathogenic SIVmac239.
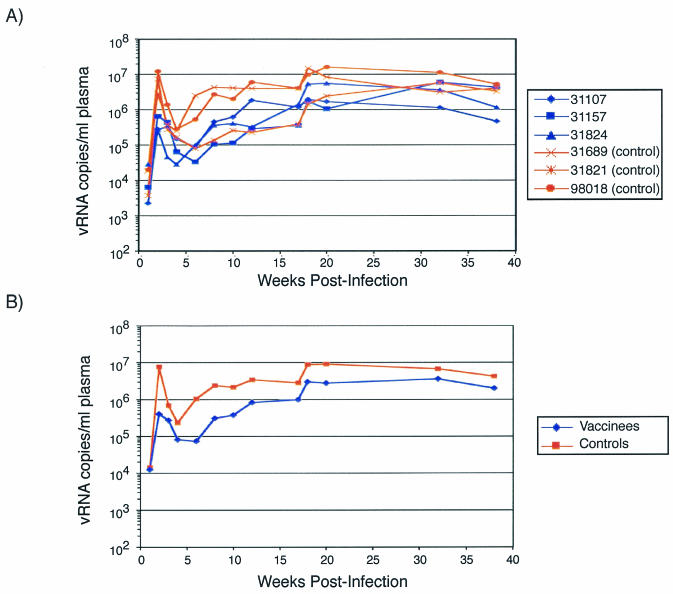
After mucosal challenge with SIVmac239, control animals experienced peak viremia at 2 weeks postchallenge of between 3 × 106 and 1.2 × 107 viral RNA/ml of plasma, with a mean of 7.6 × 106 copies/ml (Fig. 9). In contrast, vaccinated animals demonstrated significantly lower peak viral loads than the controls, with a mean viral load of 4 × 105 viral copies/ml, >1 log less than the mean viral load of the controls (P = 0.005). At 3 weeks postinfection and at various time points thereafter, the difference in viral loads between the vaccinees and controls lost statistical significance. This suggests that the vaccine-induced CTL were able to reduce initial viral replication but were unable to control replication once the infection became established.
FIG. 9.
Viral loads for all animals. (A) The virus load over time was plotted for each animal. Blue traces, vaccinees; red traces, controls. (B) Geometric mean virus loads for vaccine (blue) and control (red) groups. The viral loads were determined by kinetic PCR.
Neutralizing antibodies.
Since Env was not included as an immunogen in the vaccination regimen, we did not expect neutralizing antibodies to be present at the time of challenge. However, since vaccinees did initially control virus replication relative to controls, we tested for the presence of neutralizing antibodies 6 months after challenge. Little or no neutralizing antibody activity against SIVmac239 was seen in the sera of either vaccinated or control animals tested against a human PBMC-grown stock of SIVmac239 and measured with CEMx174 as the target cells. However, all of the animals did show neutralizing antibody responses to a T-cell-line-adapted strain of SIVmac251, as measured on CEMx174 cells (Table 4).
TABLE 4.
Neutralizing antibodies from vaccinated and control animals at 6 months postchallenge with SIVmac239
| Animal | Nab titer toa:
|
|
|---|---|---|
| SIVmac251/TCLAb | SIVmac239 | |
| Vaccinated | ||
| 31107 | 8,321 | <20 |
| 31157 | 5,921 | 25 |
| 31824 | 5,411 | <20 |
| Control | ||
| 31689 | 8,655 | <20 |
| 31821 | 7,209 | <20 |
| 98018 | 7,168 | 47 |
Nab titer, the reciprocal serum dilution at which 50% of the cells were protected from virus-induced killing, as measured by neutral red uptake.
TCLA, T-cell line adapted.
DISCUSSION
We used a DNA prime/rMVA boost regimen to induce HTL and a strong, multispecific CTL response. These CTL responses were detected in both systemic and mucosal tissues. By delivering two rMVA boosts via different routes, we were able to elicit the highest SIV epitope-specific CTL responses reported thus far for the DNA/rMVA strategy. In vaccinees, an average of 12% of their CD3+ CD8+ lymphocytes were Mamu-A*01/CM9 tetramer-positive 1 week after an i.v. boost with rMVA. These vaccine-induced Gag181-189CM9-specific tetramer levels were up to 2-fold higher than those achieved in a similar study using a DNA prime/rMVA boost strategy (6), >10-fold higher than tetramer levels in a cytokine-augmented DNA vaccination regimen (8), and approximately half of those induced by a DNA/CRL1005 prime/adenovirus boost regimen (50). Moreover, the i.v. boost induced long-lived mucosal CTL, as shown by the expression of mucosal homing and retention markers on PBMC and detection of Gag181-189CM9-specific CTLs in the sigmoid colon for up to 10 weeks postboost.
Our vaccine strategy differed from those reported previously in that we used the second i.v. rMVA boost to target CTL to the mucosa. Targeting vaccine-induced immune responses to mucosal surfaces may be important for several reasons. Mucosal surfaces provide the first line of defense against sexually transmitted HIV, and locating virus-specific CTL in the mucosa may facilitate a rapid response after exposure. In a murine study, virus-specific memory T cells derived from tertiary lymphoid tissues, including the intestinal mucosa, are more directly lytic than their splenic counterparts. Therefore, vaccine-induced mucosal CTL may be more effective at limiting early HIV/SIV replication. Since the vast majority of HIV infections occur across mucosal barriers, it may be crucial for successful HIV vaccines to elicit both systemic and mucosal responses. Our vaccine regimen successfully elicited α4β7+ and αEβ7+ CTL and tetramer-positive CD8+ cells in mucosal tissues.
After i.r. challenge with a high dose of SIVmac239, a strong anamnestic CTL response significantly reduced peak viremia in the vaccinated animals (P = 0.005). After peak viremia, the difference in the viral loads between the vaccinees and the controls lost statistical significance and none of the vaccinated animals were able to control viral replication in the chronic phase. Neutralizing antibodies to SIVmac239 were not detected in any of the animals at 6 months postchallenge. It is possible that with an increased number of animals in the study a statistically significant difference in the viral loads may have been maintained by the vaccinees into the chronic phase of infection. However, even with a small sample size, the present study suggests that multispecific CTLs are capable of providing a degree of protection against acute viral replication but, without neutralizing antibodies, they are unable to control chronic viral replication after a high-dose mucosal challenge of pathogenic SIV.
Taken together, our data suggest that even very vigorous CTL responses, targeted both systemically and mucosally, cannot alone control a pathogenic SIV challenge. In contrast, some recent reports have suggested that CTL-based vaccines can ameliorate the course of immunodeficiency diseases (6-8, 50). These studies used the chimeric virus SHIV-89.6P as the challenge, and the discrepancy between our results and those of other groups can likely be attributed to fundamental differences between SHIV-89.6P and SIVmac239. First, SHIV-89.6P and SIVmac239 exhibit different cell tropisms. HIV, SIV, and SHIV can be phenotyped based on the coreceptor used for cell attachment (10). The major coreceptors for HIV and SIV are the chemokine receptors CCR5 and CXCR4 (11). SIVmac239 is a CCR5-utilizing (R5) virus, and infected macaques typically show a gradual loss of peripheral CD4+ T cells; this loss is analogous to that seen in the course of most HIV infections (18, 25, 28, 29, 31, 55).SHIV-89.6P, meanwhile, expresses an env gene derived from a dualtropic virus that could use CCR5 or CXCR4 for entry (R5X4) (42, 55). Macaques infected with SHIV-89.6P show a rapid and irreversible loss of CD4+ T lymphocytes in the peripheral lymphoid tissues that is similar to that seen in infections with CXCR4-using (X4) strains of HIV (17, 26, 30, 41-43, 47, 49). Moreover, SHIV-89.6P, unlike most primary strains of HIV, is sensitive to neutralizing antibodies (35, 41), whereas our data and those of others show that SIVmac239 is difficult to neutralize (16, 23, 24). A previous study linked the ability of macaques to mount an antibody response to SHIV-89.6P to longer survival (30), suggesting that antibodies play a significant role in the observed protection from disease progression. Thus, in recent vaccine studies with SHIV-89.6P as the challenge virus (6-8, 45, 50), protection of CD4+ T cells from rapid depletion may have been the key to the vaccinees' long-term survival. If preserved, CD4+ T cells could provide adequate help to B cells, enabling them to mount an effective antibody response against neutralization-susceptible SHIV-89.6P. Furthermore, in at least three of these previous vaccine studies the viral envelope was used as an immunogen and, as a result, cross-reactive neutralizing antibodies may have developed rapidly after challenge. It is difficult to understand the rationale for using SHIV-89.6P in vaccine studies designed to test the efficacy of CTL-based vaccines when several SIV strains (such as SIVmac251, SIVmac239, and SIVmacE660) are readily available. In contrast to SHIV-89.6P, SIVmac239 resembles HIV in that it is very difficult to neutralize with antibodies (23).
Despite strong CTL responses, including mucosally located responses, vaccinated macaques lost control of SIVmac239 by the chronic phase. This failure to control virus replication may be the result of several factors. Both the vaccinees and the controls were infected with a single, high dose of SIVmac239. Although several factors play a role in HIV transmission, recent studies have suggested that the viral load of the infected individual is the chief predictor of heterosexual transmission (20, 39). These studies show that the probability of transmission increases with rising virus levels. In addition, an empirical model of heterosexual HIV-1 transmission predicts that, when seminal levels in plasma are high (>100,000 copies/ml), transmission occurs in 1 of 100 sexual encounters, whereas the probability of transmission declines rapidly with decreasing seminal viral loads (15). Thus, it may be that the single dose of SIVmac239 used to infect macaques is unnaturally high and does not accurately reflect the transmission of HIV in humans. In a challenge more closely imitating physiological conditions, vaccine-induced CTL, such as those we observed, may be able to control viral replication. However, the development of virus-specific CTL alone may not be sufficient to limit viral replication. Additional help from neutralizing antibodies and the innate immune system may be necessary to control HIV replication. Moreover, the induction of particularly strong immunodominant CTL responses, like those against Gag181-189CM9 and Tat28-35SL8, may hinder the stimulation of subdominant CTL responses during infection. The lack of activation of subdominant CTL responses may impede the immune system's ability to mount an effective response.
In conclusion, the DNA prime/rMVA boost vaccination regimen generated HTL and long-lived, multispecific systemic and mucosal CTL. After mucosal challenge with the highly pathogenic SIVmac239, a massive anamnestic CTL response was observed in the limited number of vaccinees, and these animals controlled peak viral replication (P = 0.005). In contrast to similar studies with SHIV-89.6P as the challenge virus, our vaccinated animals were unable to control viral replication in the chronic phase of infection. The present study suggests that multispecific CTL, in the absence of neutralizing antibodies, can achieve a modicum of control over early viral replication but are unable to control chronic viral replication after a high dose mucosal challenge with a pathogenic SIV.
Acknowledgments
T.U.V. and M.R.R. contributed equally to this study.
We thank Ronald Desrosiers for providing the SIVmac239, Marianne Löwel for expertise in preparing the MVA, Sarah Fuenger for help with ICS, Kim Schmidt for technical assistance, and Thomas Friedrich for help in preparation of the manuscript. The anti-α4β7 antibody was generously provided by Millennium Pharmaceuticals.
This work is supported by NIH grants AI41913, AI46366, AI45461, RR15371, and RR00169 and by grants from the Deutsche Forschungsgemeinschaft and the European Community (QLK2-2000-1040 to G.S.). D.I.W. is a recipient of an Elizabeth Glaser scientist award.
REFERENCES
- 1.Allen, T. M., P. Jing, B. Calore, H. Horton, D. H. O'Connor, T. Hanke, M. Piekarczyk, R. Ruddersdorf, B. R. Mothe, C. Emerson, N. Wilson, J. D. Lifson, I. M. Belyakov, J. A. Berzofsky, C. Wang, D. B. Allison, D. C. Montefiori, R. C. Desrosiers, S. Wolinsky, K. J. Kunstman, J. D. Altman, A. Sette, A. J. McMichael, and D. I. Watkins. 2002. Effects of cytotoxic T lymphocytes (CTL) directed against a single simian immunodeficiency virus (SIV) Gag CTL epitope on the course of SIVmac239 infection. J. Virol. 76:10507-10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, T. M., L. Mortara, B. R. Mothe, M. Liebl, P. Jing, B. Calore, M. Piekarczyk, R. Ruddersdorf, D. H. O'Connor, X. Wang, C. Wang, D. B. Allison, J. D. Altman, A. Sette, R. C. Desrosiers, G. Sutter, and D. I. Watkins. 2002. Tat-vaccinated macaques do not control simian immunodeficiency virus SIVmac239 replication. J. Virol. 76:4108-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, T. M., D. H. O'Connor, P. Jing, J. L. Dzuris, B. R. Mothe, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viremia. Nature 407:386-390. [DOI] [PubMed] [Google Scholar]
- 4.Allen, T. M., J. Sidney, M. F. del Guercio, R. L. Glickman, G. L. Lensmeyer, D. A. Wiebe, R. DeMars, C. D. Pauza, R. P. Johnson, A. Sette, and D. I. Watkins. 1998. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from simian immunodeficiency virus. J. Immunol. 160:6062-6071. [PubMed] [Google Scholar]
- 5.Allen, T. M., T. U. Vogel, D. H. Fuller, B. R. Mothe, S. Steffen, J. E. Boyson, T. Shipley, J. Fuller, T. Hanke, A. Sette, J. D. Altman, B. Moss, A. J. McMichael, and D. I. Watkins. 2000. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J. Immunol. 164:4968-4978. [DOI] [PubMed] [Google Scholar]
- 6.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 7.Amara, R. R., F. Villinger, S. I. Staprans, J. D. Altman, D. C. Montefiori, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Different patterns of immune responses but similar control of a simian-human immunodeficiency virus 89.6P mucosal challenge by modified vaccinia virus Ankara (MVA) and DNA/MVA vaccines. J. Virol. 76:7625-7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 9.Belyakov, I. M., J. D. Ahlers, J. D. Clements, W. Strober, and J. A. Berzofsky. 2000. Interplay of cytokines and adjuvants in the regulation of mucosal and systemic HIV-specific CTL. J. Immunol. 165:6454-6462. [DOI] [PubMed] [Google Scholar]
- 10.Berger, E. A., R. W. Doms, E. M. Fenyo, B. T. Korber, D. R. Littman, J. P. Moore, Q. J. Sattentau, H. Schuitemaker, J. Sodroski, and R. A. Weiss. 1998. A new classification for HIV-1. Nature 391:240. [DOI] [PubMed] [Google Scholar]
- 11.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 12.Butcher, E. C., and L. J. Picker. 1996. Lymphocyte homing and homeostasis. Science 272:60-66. [DOI] [PubMed] [Google Scholar]
- 13.Campbell, D. J., and E. C. Butcher. 2002. Rapid acquisition of tissue-specific homing phenotypes by CD4+ T cells activated in cutaneous or mucosal lymphoid tissues. J. Exp. Med. 195:135-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cepek, K. L., S. K. Shaw, C. M. Parker, G. J. Russell, J. S. Morrow, D. L. Rimm, and M. B. Brenner. 1994. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the αEβ7 integrin. Nature 372:190-193. [DOI] [PubMed] [Google Scholar]
- 15.Chakraborty, H., P. K. Sen, R. W. Helms, P. L. Vernazza, S. A. Fiscus, J. J. Eron, B. K. Patterson, R. W. Coombs, J. N. Krieger, and M. S. Cohen. 2001. Viral burden in genital secretions determines male-to-female sexual transmission of HIV-1: a probabilistic empiric model. AIDS 15:621-627. [DOI] [PubMed] [Google Scholar]
- 16.Choi, W. S., C. Collignon, C. Thiriart, D. P. Burns, E. J. Stott, K. A. Kent, and R. C. Desrosiers. 1994. Effects of natural sequence variation on recognition by monoclonal antibodies neutralize simian immunodeficiency virus infectivity. J. Virol. 68:5395-5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connor, R. I., K. E. Sheridan, D. Ceradini, S. Choe, and N. R. Landau. 1997. Change in coreceptor use coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 185:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feinberg, M. B., and J. P. Moore. 2002. AIDS vaccine models: challenging challenge viruses. Nat. Med. 8:207-210. [DOI] [PubMed] [Google Scholar]
- 19.Fuller, D. H., L. Simpson, K. S. Cole, J. E. Clements, D. L. Panicali, R. C. Montelaro, M. Murphey-Corb, and J. R. Haynes. 1997. Gene gun-based nucleic acid immunization alone or in combination with recombinant vaccinia vectors suppresses virus burden in rhesus macaques challenged with a heterologous SIV. Immunol. Cell Biol. 75:389-396. [DOI] [PubMed] [Google Scholar]
- 20.Gray, R. H., M. J. Wawer, R. Brookmeyer, N. K. Sewankambo, D. Serwadda, F. Wabwire-Mangen, T. Lutalo, X. Li, T. vanCott, and T. C. Quinn. 2001. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet 357:1149-1153. [DOI] [PubMed] [Google Scholar]
- 21.Hanke, T., R. V. Samuel, T. J. Blanchard, V. C. Neumann, T. M. Allen, J. E. Boyson, S. A. Sharpe, N. Cook, G. L. Smith, D. I. Watkins, M. P. Cranage, and A. J. McMichael. 1999. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vaccinia virus Ankara boost vaccination regimen. J. Virol. 73:7524-7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins, J. M., D. A. Mandlebrot, S. K. Shaw, G. J. Russell, E. A. Murphy, Y. T. Chen, W. J. Nelson, C. M. Parker, and M. B. Brenner. 1998. Direct and regulated interaction of integrin αEβ7 with E-cadherin. J. Cell Biol. 140:197-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horton, H., T. U. Vogel, D. K. Carter, K. Vielhuber, D. H. Fuller, T. Shipley, J. T. Fuller, K. J. Kunstman, G. Sutter, D. C. Montefiori, V. Erfle, R. C. Desrosiers, N. Wilson, L. J. Picker, S. M. Wolinsky, C. Wang, D. B. Allison, and D. I. Watkins. 2002. Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. J. Virol. 76:7187-7202. [DOI] [PMC free article] [PubMed]
- 24.Joag, S. V., M. G. Anderson, J. E. Clements, M. F. McEntee, D. P. Sharma, R. J. Adams, and O. Narayan. 1993. Antigenic variation of molecularly cloned SIVmac239 during persistent infection in a rhesus macaque. Virology 195:406-412. [DOI] [PubMed] [Google Scholar]
- 25.Kestler, H., T. Kodama, D. Ringler, M. Marthas, N. Pedersen, A. Lackner, D. Regier, P. Sehgal, M. Daniel, N. King, et al. 1990. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science 248:1109-1112. [DOI] [PubMed] [Google Scholar]
- 26.Koot, M., I. P. Keet, A. H. Vos, R. E. de Goede, M. T. Roos, R. A. Coutinho, F. Miedema, P. T. Schellekens, and M. Tersmette. 1993. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann. Intern. Med. 118:681-688. [DOI] [PubMed] [Google Scholar]
- 27.Lesinski, G. B., S. L. Smithson, N. Srivastava, D. Chen, G. Widera, and M. A. Westerink. 2001. A DNA vaccine encoding a peptide mimic of Streptococcus pneumoniae serotype 4 capsular polysaccharide induces specific anti-carbohydrate antibodies in BALB/c mice. Vaccine 19:1717-1726. [DOI] [PubMed] [Google Scholar]
- 28.Levy, J. A. 1993. Pathogenesis of human immunodeficiency virus infection. Microbiol. Rev. 57:183-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis, M. G., S. Bellah, K. McKinnon, J. Yalley-Ogunro, P. M. Zack, W. R. Elkins, R. C. Desrosiers, and G. A. Eddy. 1994. Titration and characterization of two rhesus-derived SIVmac challenge stocks. AIDS Res. Hum. Retrovir. 10:213-220. [DOI] [PubMed] [Google Scholar]
- 30.Lu, Y., C. D. Pauza, X. Lu, D. C. Montefiori, and C. J. Miller. 1998. Rhesus macaques that become systemically infected with pathogenic SHIV 89.6-PD after intravenous, rectal, or vaginal inoculation and fail to make an antiviral antibody response rapidly develop AIDS. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 19:6-18. [DOI] [PubMed] [Google Scholar]
- 31.Luciw, P. A., K. E. Shaw, R. E. Unger, V. Planelles, M. W. Stout, J. E. Lackner, E. Pratt-Lowe, N. J. Leung, B. Banapour, and M. L. Marthas. 1992. Genetic and biological comparisons of pathogenic and nonpathogenic molecular clones of simian immunodeficiency virus (SIVmac). AIDS Res. Hum. Retrovir. 8:395-402. [DOI] [PubMed] [Google Scholar]
- 32.Masopust, D., J. Jiang, H. Shen, and L. Lefrancois. 2001. Direct analysis of the dynamics of the intestinal mucosa CD8 T-cell response to systemic virus infection. J. Immunol. 166:2348-2356. [DOI] [PubMed] [Google Scholar]
- 33.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413-2417. [DOI] [PubMed] [Google Scholar]
- 34.Montefiori, D. C., T. W. Baba, A. Li, M. Bilska, and R. M. Ruprecht. 1996. Neutralizing and infection-enhancing antibody responses do not correlate with the differential pathogenicity of SIVmac239delta3 in adult and infant rhesus monkeys. J. Immunol. 157:5528-5535. [PubMed] [Google Scholar]
- 35.Montefiori, D. C., K. A. Reimann, M. S. Wyand, K. Manson, M. G. Lewis, R. G. Collman, J. G. Sodroski, D. P. Bolognesi, and N. L. Letvin. 1998. Neutralizing antibodies in sera from macaques infected with chimeric simian-human immunodeficiency virus containing the envelope glycoproteins of either a laboratory-adapted variant or a primary isolate of human immunodeficiency virus type 1. J. Virol. 72:3427-3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy, C. G., W. T. Lucas, R. E. Means, S. Czajak, C. L. Hale, J. D. Lifson, A. Kaur, R. P. Johnson, D. M. Knipe, and R. C. Desrosiers. 2000. Vaccine protection against simian immunodeficiency virus by recombinant strains of herpes simplex virus. J. Virol. 74:7745-7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Connor, D. H., T. M. Allen, T. U. Vogel, P. Jing, I. P. DeSouza, E. Dodds, E. J. Dunphy, C. Melsaether, B. Mothe, H. Yamamoto, H. Horton, N. Wilson, A. L. Hughes, and D. I. Watkins. 2002. Acute phase cytotoxic T lymphocyte escape is a hallmark of simian immunodeficiency virus infection. Nat. Med. 8:493-499. [DOI] [PubMed] [Google Scholar]
- 38.Picker, L. J., M. K. Singh, Z. Zdraveski, J. R. Treer, S. L. Waldrop, P. R. Bergstresser, and V. C. Maino. 1995. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood 86:1408-1419. [PubMed] [Google Scholar]
- 39.Quinn, T. C., M. J. Wawer, N. Sewankambo, D. Serwadda, C. Li, F. Wabwire-Mangen, M. O. Meehan, T. Lutalo, R. H. Gray, et al. 2000. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N. Engl. J. Med. 342:921-929. [DOI] [PubMed] [Google Scholar]
- 40.Regier, D. A., and R. C. Desrosiers. 1990. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res. Hum. Retrovir. 6:1221-1231. [DOI] [PubMed] [Google Scholar]
- 41.Reimann, K. A., J. T. Li, R. Veazey, M. Halloran, I. W. Park, G. B. Karlsson, J. Sodroski, and N. L. Letvin. 1996. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J. Virol. 70:6922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reimann, K. A., J. T. Li, G. Voss, C. Lekutis, K. Tenner-Racz, P. Racz, W. Lin, D. C. Montefiori, D. E. Lee-Parritz, Y. Lu, R. G. Collman, J. Sodroski, and N. L. Letvin. 1996. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J. Virol. 70:3198-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reimann, K. A., A. Watson, P. J. Dailey, W. Lin, C. I. Lord, T. D. Steenbeke, R. A. Parker, M. K. Axthelm, and G. B. Karlsson. 1999. Viral burden and disease progression in rhesus monkeys infected with chimeric simian-human immunodeficiency viruses. Virology 256:15-21. [DOI] [PubMed] [Google Scholar]
- 44.Rose, J. R., M. B. Williams, L. S. Rott, E. C. Butcher, and H. B. Greenberg. 1998. Expression of the mucosal homing receptor α4β7 correlates with the ability of CD8+ memory T cells to clear rotavirus infection. J. Virol. 72:726-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 46.Rud, E. W., M. Cranage, J. Yon, J. Quirk, L. Ogilvie, N. Cook, S. Webster, M. Dennis, and B. E. Clarke. 1994. Molecular and biological characterization of simian immunodeficiency virus macaque strain 32H proviral clones containing nef size variants. J. Gen. Virol. 75(Pt. 3):529-543. [DOI] [PubMed] [Google Scholar]
- 47.Scarlatti, G., E. Tresoldi, A. Bjorndal, R. Fredriksson, C. Colognesi, H. K. Deng, M. S. Malnati, A. Plebani, A. G. Siccardi, D. R. Littman, E. M. Fenyo, and P. Lusso. 1997. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nat. Med. 3:1259-1265. [DOI] [PubMed] [Google Scholar]
- 48.Schneider, T., R. Ullrich, and M. Zeitz. 1996. The immunologic aspects of human immunodeficiency virus infection in the gastrointestinal tract. Semin. Gastrointest. Dis. 7:19-29. [PubMed] [Google Scholar]
- 49.Schuitemaker, H., M. Koot, N. A. Kootstra, M. W. Dercksen, R. E. de Goede, R. P. van Steenwijk, J. M. Lange, J. K. Schattenkerk, F. Miedema, and M. Tersmette. 1992. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J. Virol. 66:1354-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 51.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 52.Vogel, T., S. Norley, B. Beer, and R. Kurth. 1995. Rapid screening for Mamu-A1-positive rhesus macaques using a SIVmac Gag peptide-specific cytotoxic T-lymphocyte assay. Immunology 84:482-487. [PMC free article] [PubMed] [Google Scholar]
- 53.Vogel, T. U., T. C. Friedrich, D. H. O'Connor, W. Rehrauer, E. J. Dodds, H. Hickman, W. Hildebrand, J. Sidney, A. Sette, A. Hughes, H. Horton, K. Vielhuber, R. Rudersdorf, I. P. De Souza, M. R. Reynolds, T. M. Allen, N. Wilson, and D. I. Watkins. 2002. Escape in one of two cytotoxic T-lymphocyte epitopes bound by a high-frequency major histocompatibility complex class I molecule, Mamu-A*02: a paradigm for virus evolution and persistence? J. Virol. 76:11623-11636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.von Andrian, U. H., and C. R. Mackay. 2000. T-cell function and migration: two sides of the same coin. N. Engl. J. Med. 343:1020-1034. [DOI] [PubMed] [Google Scholar]
- 55.Zhang, Y., B. Lou, R. B. Lal, A. Gettie, P. A. Marx, and J. P. Moore. 2000. Use of inhibitors to evaluate coreceptor usage by simian and simian/human immunodeficiency viruses and human immunodeficiency virus type 2 in primary cells. J. Virol. 74:6893-6910. [DOI] [PMC free article] [PubMed] [Google Scholar]