Abstract
In the present study we report on evolution of calicivirus RNA from a patient with chronic diarrhea (i.e., lasting >2 years) and viral shedding. Partial sequencing of open reading frame 1 (ORF1) from 12 consecutive isolates revealed shedding of a genogroup II virus with relatively few nucleotide changes during a 1-year period. The entire capsid gene (ORF2) was also sequenced from the same isolates and found to contain 1,647 nucleotides encoding a protein of 548 amino acids with similarities to the Arg320 and Mx strains. Comparative sequence analysis of ORF2 revealed 32 amino acid changes during the year. It was notable that the vast majority of the cumulative amino acid changes (8 of 11) appeared within residues 279 to 405 located within the hypervariable domain (P2) of the capsid protein and hence were subject to immune pressure. An interesting and novel observation was that the accumulated amino acid changes in the P2 domain resulted in predicted structural changes, including disappearance of a helix structure, and thus a possible emergence of a new phenotype. FUT2 gene polymorphism characterization revealed that the patient is heterozygous at nucleotide 428 and thus Secretor+, a finding in accordance with the hypothesis of FUT2 gene polymorphism and calicivirus susceptibility. To our knowledge, this is the first report of RNA evolution of calicivirus in a single individual, and our data suggest an immunity-driven mechanism for viral evolution. We also report on chronic virus excretion, immunoglobulin treatment, and modification of clinical symptoms; our observations from these studies, together with the FUT2 gene characterization, may lead to a better understanding of calicivirus pathogenesis.
Acute diarrheal diseases are common in humans and are associated with significant morbidity worldwide and substantial mortality in developing countries. In recent years, caliciviruses have emerged as an important cause of gastroenteritis in all age groups and are now recognized as the main cause of gastroenteritis in nursing homes and hospitals (3, 8, 12, 14, 15, 27).
Caliciviruses are classified into four genera: norovirus, sapovirus, vesivirus, and logovirus. Human caliciviruses are found within the norovirus and sapovirus genera, which contain a wide range of genetically distinct strains. The sequence heterogeneity of these strains may be a result of the high mutation rate caused by the lack of proofreading of the polymerase. However, the sequence variation has also raised the possibility that calicivirus RNA undergoes constant genetic drift through immune-response-driven mechanisms, leading to the emergence of new strains. However, at present there is no information on human calicivirus mutation rates over time, nor has the importance of immune-response-driven mutations been reported.
The clinical features of a calicivirus infection includes an incubation period of 12 to 48 h characterized by acute onset of nausea, vomiting, abdominal cramps and diarrhea that generally lasts for about 48 h. Recent studies have shown, however, that diarrhea can persist for up to 4 weeks (15, 21, 22). Furthermore, it was previously believed that a person remained contagious for 48 to 72 h after recovery from a Norwalk-like infection (24). This has been challenged, and a recent study reports that virus can be excreted for up to 3 weeks (21). Thus, the more recent studies show clearly that not only can the virus be excreted for much longer periods than previously thought but also that the diarrhea can also last much longer than was previously thought. It should be noted, however, that all current information is obtained from immunocompetent individuals, and no information is yet available about the clinical manifestation of calicivirus infections in immunosuppressed or immunodeficient individuals; it is also not known how calicivirus evolution and excretion is affected in these individuals.
We report here on polymerase and capsid gene evolution of the calicivirus in vivo. The most novel observations from sequencing the capsid gene from 12 consecutive isolates from a single individual were that the vast majority of the cumulative nucleotide changes (8 of 11) appeared within the region from amino acids 304 to 404 located on the surface of the capsid protein and within the hypervariable region, resulting in predicted structural changes in the capsid. To our knowledge, this is the first report of chronic calicivirus shedding and RNA evolution, and the results support the hypothesis of an immune-response-driven mechanism for calicivirus RNA evolution. We also provide host genetic information that support the hypothesis of specific histo-blood group antigens and calicivirus resistance (6, 13, 23).
MATERIALS AND METHODS
Patient and clinical samples.
The patient received a heart transplant in 1994 due to severe heart failure and malignant arrhythmias after a myocardial infarction. After transplantation he subsequently received immunosuppressive treatment with cyclosporine, azathioprine, and prednisolone. The immunosuppressive treatment resulted in a low total number of lymphocytes (8%; normal range, 50 to 80%), a low number of CD4+ cells (0.15; normal, 0.4), and a low CD4/CD8 ratio of 0.37 (normal, >1.0) but normal immunoglobulin concentrations. During the following years the patient developed renal insufficiency, nephrosclerosis, possibly due to cyclosporine toxicity, as shown on renal biopsies. In 1998 he became dialysis dependent. Since 1999 he has been on hemodialysis three times weekly, while waiting a cadaveric renal transplantation.
Fecal samples were collected from July 2000 to July 2001, and 12 urine samples and one serum sample were collected from 1 June 2001 to 31 July 2001.
EM.
All fecal samples and urine samples were initially screened for viruses by electron microscopy (EM) as previously described (3).
Calicivirus RT-PCR.
Twenty-two of the specimens were investigated for the presence of Norwalk-like human calicivirus by a reverse transcription-PCR (RT-PCR) as previously described by Vinje et al. (26, 27). Briefly, RNA was extracted from 50 μl of a 10% stool suspension by using the guanidinium thiocyanate-silica extraction method (1). RT was performed as follows. First, 5 μl of extracted RNA was annealed with 50 pmol of JV13 (5′-TCA TCA TCA CCA TAG AAA GAG) in a total volume of 9 μl and then added to a reaction mixture containing 10 mM Tris (pH 8.3), 50 mM KCl, 3 mM MgCl2, 1 mM deoxynucleoside triphosphate (dNTP), and 100 U of Moloney murine leukemia virus reverse transcriptase (Superscript; Life Technologies). Reaction mixtures were incubated for 1 h at 42°C. A total of 5 μl of the RT reaction was added to a PCR mix composed of 10 mM Tris (pH 9.2), 75 mM KCl, 1.5 mM MgCl2, 0.2 mM dNTP, 15 pmol of JV12 (5′-ATA CCA CTA TGA TGC AGA TTA), and 2.5 U of Taq polymerase. Forty reaction cycles were carried out for 1 min at 94°C, 1.5 min at 37°C, and 1 min at 74°C, followed by a final incubation at 74°C for 7 min. One-fifth of the reaction volume was analyzed on agarose gels.
RT-PCR and molecular cloning of open reading frame 2 (ORF2).
RNA was initially extracted from the stool suspension (sample 2004/00) by using the guanidinium thiocyanate-silica extraction method (1). RT was performed as follows. A total of 5 μl of extracted RNA was annealed with 50 pmol LV 6717 primer (5′-AGT ACC TTG TTC CGC TCC A) in a total volume of 9 μl and then added to a reaction mixture containing 10 mM Tris (pH 8.3), 50 mM KCl, 3 mM MgCl2, 1 mM dNTP, and 100 U of Moloney murine leukemia virus reverse transcriptase (Life Technologies). Reaction mixtures were incubated for 1 h at 42°C. Next, 5 μl of the RT reaction mixture was added to a PCR mix composed of 10 mM Tris (pH 9.2), 50 mM KCl, 1.5 mM MgCl2, 0.2 mM dNTP, 15 pmol of LV4922 primer (5′-CAC GGC CCA GCA TTC TAC A), and 2.5 U of Taq polymerase. After an initial incubation at 94°C for 3 min, 25 reaction cycles were carried out with 10 s at 94°C, 30 s at 45°C, and 2 min at 72°C, followed by a final incubation at 72°C for 7 min. Positive PCR amplicons were purified from gels and polished by incubating DNA in polishing buffer, 0.5 mM dNTP, and 0.5 U of Pfu DNA polymerase (Stratagene) at 72°C for 30 min. The polished DNA fragment was subsequently inserted into the pCR-Script cloning vector (Stratagene). Based on the sequence of sample 2004/00, new primers, DK1 (5′-CTA AAG GAA GGT GGC ATG GA) and DK2 (5′-GTC ACC AGC CAA CCC TG), were designed that were used in the subsequent RT-PCR amplification of the ORF2 sequence of selected isolates.
Sequencing of ORF1 and ORF2.
RT-PCR amplicons obtained from ORF1 by using the JV12 and JV13 primers targeting a region of the RNA polymerase and sequences containing the entire ORF2 cloned into pCR-Script (Stratagene) were sequenced. After RT-PCR, ORF1 amplification products were purified by using a Stratagene PCR purification kit (Stratagene). Positive clones containing ORF2 were purified by using a Qiagen plasmid purification kit. The DNA sequences were determined by using the ABI Prism ready reaction BigDye sequencing kit (Applied Biosystems) and an ABI automated sequencer (Applied Biosystems). RT-PCR amplicons from ORF1 were sequenced on both strands, whereas three ORF2 clones of each isolate were sequenced on both strands.
Host gene polymorphism characterization.
DNA was extracted from saliva samples (100 μl), and the FUT2 (Secretor), FUT3 (Lewis), and ABO genotypes were determined by using sequence-specific primers and PCR (PCR-SSP) as previously described (2, 20).
Sequence analysis.
Overlapping nucleic acid sequences were combined for analysis and edited with a lasergene software package (DNASTAR, Inc., Madison, Wis). Amino acid secondary structure predictions were made by using the PSIPRED secondary structural prediction program, based on position-specific scoring matrices (9).
Nucleotide sequence accession numbers.
The nucleotide sequences determined in the present study have been deposited in the GenBank sequence database. In the text below, the designations for the partial ORF1 sequences and the corresponding sequence accession numbers are as follows: July 00, AY247424; Aug 00, AY247425; Sep 00, AY247426; Oct 00, AY247427; Nov 00, AY247428; Dec 00, AY247429; Jan 01, AY247423; Feb 01, AY247430; Apr 01, AY247419; May 01, AY247420; June 01, AY247421; and July 01, AY247422.The ORF2 sequences from July 00 to July 01 have accession numbers in ascending order from AY247431 to AY247442.
RESULTS
Clinical findings.
After visiting a local restaurant in June 2000, the patient (born in 1935), along with other visitors to the restaurant, developed extensive vomiting and diarrhea resulting in moderate dehydration. He was admitted to the hospital for intravenous rehydration. Excretion of calicivirus was demonstrated by EM and PCR. The acute symptoms turned into chronic diarrhea with four to eight episodes per day, and severe weight loss developed over the next several weeks.
Immunoglobulin treatment.
Due to the severity of the symptoms and prolonged excretion of virus, it was decided to try available treatments. Initially, breast milk treatment (125 ml given three times per day) was evaluated for 3 weeks starting 12 December 2000, followed by oral immunoglobulin treatment (10 g/day) for 15 days beginning 11 January 2001 and then intravenous immunoglobulin treatment (0.4 g/kg) for 5 days beginning 13 February 2001. However, none of these treatments reduced the severity of diarrhea or the secretion of calicivirus, as determined by the number of diarrheal episodes and virus excretion.
To reduce the immunosuppression, the azathioprine dosage was reduced from 125 to 75 mg daily and the cyclosporine dosage was reduced to ca. 120 μg/liter. None of these treatments had any effect on the number of diarrheal episodes or on virus excretion, as determined by EM and PCR.
Persistent viral shedding and modification of clinical symptoms.
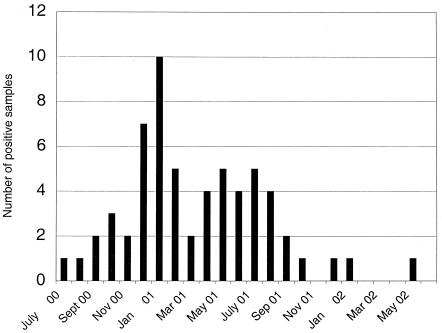
During the period from July 2000 to July 2001, 51 fecal samples were collected from the patient, with an average of four samples collected per month (Fig. 1). All samples were initially analyzed by EM, and in 49 of 51 fecal samples calicivirus was detected. The two EM-negative samples were shown to be positive for calicivirus RNA when retested by RT-PCR. In addition, 22 other EM-positive samples were confirmed to contain Norwalk-like viruses by PCR. In addition to the stool samples 12 urine and 1 serum sample were collected and examined by EM and RT-PCR, but none of these samples were positive for norovirus.
FIG. 1.
Chronicexcretion of norovirus. Monthly distribution of norovirus positive specimens.
At present (May 2003), the patient still has more than four diarrheal episodes/day, and symptomatic treatment with parenteral nutrition 5 days per week, loperamidhydrochlorid (2 mg given three times per day), and tinctura opii are used to maintain body weight and fluid balance. The patient suffered from diarrhea, vomiting, and nausea early in the course of the infection but, although the severity of the diarrhea remains, the vomiting and nausea have disappeared.
Sequence analysis of the polymerase gene (ORF1).
Nucleotide sequences of ORF1 were determined from 12 isolates collected in approximately 1-month intervals from July 2000 to July 2001. Sequences with a length of 254 bp were obtained and compared. During the 12-month study period, the individual isolates displayed sequence identities of between 97.6 and 100% with the two last collected isolates (June/01 and July/01) being most divergent from each other (2.4%). Over the entire study period nine sporadic nucleotide changes were demonstrated. Two of these nucleotide changes occurred in the last isolate collected in July 2001. The changes were scattered throughout the sequence, but four of the substitutions occurred in isolate June/01. However, repeated RT-PCR and sequencing of this isolate did not reveal any differences from the initially determined sequence. One of the sporadic changes appearing in isolate April/01 reappeared in the same position 2 months later in the last collected isolate (July/01). Only one nucleotide substitution, introduced in the second collected isolate (Aug/00), accumulated throughout the study period.
All of the changes were in the third codon position and were nucleotide transitions in eight of nine cases. Of the eight possible transitions, four were U→C, two were C→U, one was A→G, and one was G→A.Two of the changes resulted in amino acid changes. The sporadic nucleotide change in isolate Nov 00 resulted in a shift from Glu to Asp. Furthermore, a permanent single nucleotide shift introduced in isolate Aug/00 resulted in a change from Thr to Ile.
Nucleotide sequence analyses of the capsid gene (ORF2).
The complete nucleotide sequences of ORF2 from the 12 isolates described above were obtained and compared. Sequences were determined from both strands on three clones from each of the 12 isolates. Sequence analysis showed that the RNA was 1,647 nucleotides long and coded for a protein of 548 amino acids with a deduced molecular mass of 59.6 kDa. Sequence comparison showed a 93% nucleotide identity between the first isolate, July/00, and Norwalk-like human calicivirus/Oberhausen 455/01/DE and 92 and 87% identity with Arg320 and the Mx strain, respectively. Pairwise comparative nucleotide sequence analysis demonstrated that during the 12-month study period all sequences were different compared to the original sequence. The identity between the individual isolates varied between 97.1 to 99.1%, and the differences were due to a single nucleotide changes. No deletions or insertions were detected. Not surprisingly, the majority of the changes were transitions rather than transversions. However, of the four possible transitions, one was predominant: 41% (23 of 56) were C→U, 14% were U→C, and 14% were G→A. In addition to these changes, 10 of 12 sequences contained nucleotide microheterogeneities, which are defined as the presence of different nucleotides at the same position in different cDNA clones. There was a range of 4 to 19 microheterogeneities found in each isolate, except in isolates Aug/00 and April/01, where no such changes were found. The vast majority of these nucleotide heterogeneities were transitions, with most changes being from C to U and from U to C. Furthermore, most of the microheterogeneities appeared to be independent from isolate to isolate and were in most cases sporadic, but in 10 cases the microheterogeneity reoccurred at a specific position in one or more sequential isolates.
Accumulation of amino acid changes in the immunodominant protruding P2 domain of the capsid results in structural changes.
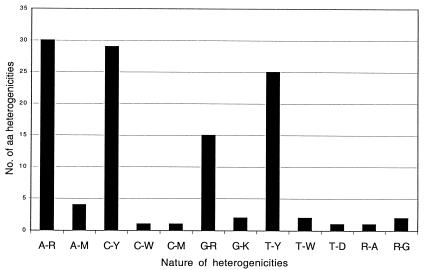
Nucleotide substitutions and microheterogeneities were found in 94 of 548 codons (17%). These changes resulted in sporadic or permanent amino acid changes in 32 of the affected codons (Fig. 2). Figure 3 illustrates the sporadic and permanent heterogeneity found in ORF2 and shows that A→R, C→Y, and T→Y were the most frequent alterations. Thirteen of the amino acid changes were sporadic and occurred only once, whereas another four point mutations were detected in the last isolate. At the four amino acid positions 255, 291, 294, and 515, the mutated amino acid varied from among two or three different residues.
FIG. 2.
Amino acid sequence and substitutions in ORF2 of 12 isolates. Underlined sequence refer to the protruding P2 subdomain of the capsid.
FIG. 3.
Sporadic and permanent amino acid microheterogenicities in ORF2.
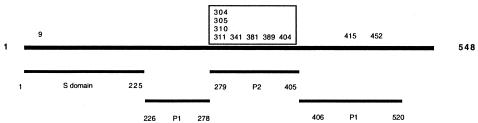
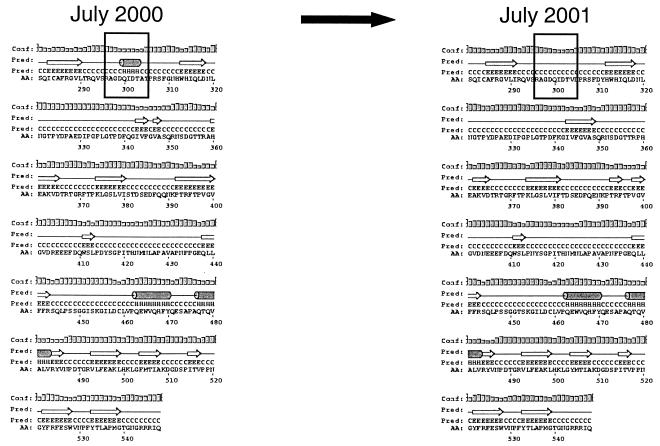
Except for sporadic amino acid changes, 11amino acids were cumulative; i.e., they remained once they had occurred (Fig. 2 and 4). The most notable finding was that 8 of 11 amino acids accumulated within the P2 region of the capsid (Fig. 4), which is located on the exterior part of the capsid (17, 25) and thus could be subjected to immune-response-mediated mutations. Of the three amino acids located outside the P2 region, one was located in the extreme N terminus of the S domain and the two other changes were located as positions 415 and 452 in the P1 region (Fig. 4). Interestingly, a secondary structure prediction of the mutant sequences revealed the disappearance of a helix structure in the P2 region, where the helix structure was present in the first isolate but absent 1 year later (Fig. 5).
FIG. 4.
Schematic illustration of norovirus capsid protein. The numbers refer to amino acids. The S domain represents the most conserved region of the sequence. P refers to the protruding domain, with P2 being the most variable. The numbers on top of the line refer to 11 accumulated amino acid changes, with 8 of 11 (boxed) accumulating within the P2 domain.
FIG. 5.
Schematic representations of secondary structure predictions of the deduced proteins from isolates showing the disappearance of the helix structure in the P2 region. Both panels depict the amino-terminal region (amino acids 281 to 548) of the proteins, including the variable P2 region. Boxed regions indicate the disappearance of a helix structure within the P2 region.
Host gene polymorphism characterization revealed a Secretor+ status.
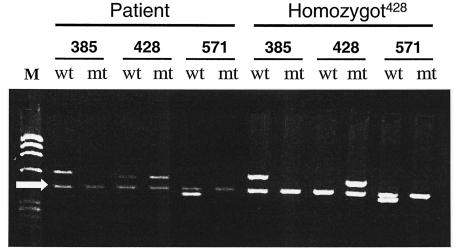
Certain individuals appear to be resistant to calicivirus infections. Recent studies have shown an association between histo-blood group antigens and virus binding to blood cells, saliva, and gastrointestinal cells (6, 7, 13). To determine whether this individual belongs to the “susceptible” group, we performed polymorphism characterization at three loci on the FUT2 gene located on chromosome 19. As illustrated in Fig. 6, the individual was genotyped as Secretor+, thus being heterozygous (+/−) mutated at nucleotide 428 (10) and homozygous (+/+) at positions 385 and 571 of the FUT2 gene (4, 5). This individual was characterized at five loci of the FUT3 gene and typed as Lewis positive with two wild-type alleles. His ABO genotype was O1O1. Phenotypically, he would thus be typed as O Le(a−b+) by the hemagglutination technique.
FIG. 6.
FUT2 gene polymorphism characterization. The PCR-SSP pattern from saliva shows that the patient is Secretor+, since the pattern indicates heterozygous mutation at nucleotide 428 and no mutations at positions 385 and 571. For allele characterization the following primers were used: allele 385 (primers 385wt [wt] and 385A→T [mt]), allele 428 (primers 428wt [wt] and 428G→A [mt]), and allele 571 (primers 571wt [wt] and 571C→T [mt]), as indicated in the figure. M, Molecular weight markers (φX174). The arrow indicates an internal PCR gene control (human growth hormone) of 428 bp. The right side of the panel (homozygot428) shows a non-Secretor control being homozygously mutated at nucleotide 428 of FUT2. PCR products were separated on agarose gels (1%) and stained with ethidium bromide.
DISCUSSION
In the present study we analyzed the clinical, virological, and genetic characteristics of an immunosuppressed individual with persistent diarrhea and calicivirus excretion. This study has not only provided new clinical information about the course of the infection but also provided novel genetic information about susceptibility and evolution mechanisms of calicivirus.
Nucleotide sequencing of the polymerase gene revealed nine sporadic and scattered nucleotide changes, with one nucleotide substitution being accumulated. In total these alterations resulted in two amino acid changes. Since the polymerase gene most likely is not subjected to immunological pressure, it is reasonable to believe that most, if not all, of these changes were the result of lack of proofreading by the RNA polymerase. However, since four of the substitutions occurred in the same isolate, questions were raised about PCR amplification errors. Repeated RT-PCR and subsequent sequencing revealed, however, a sequence identical to the original sequence, suggesting that substitutions were not a result of errors introduced by the DNA polymerase used in the PCR but rather a result of lack of proofreading of the viral polymerase.
In contrast to the polymerase gene, the mutations that occurred in the capsid were more interesting. The Norwalk capsid protein contains two distinct domains: the shell (S) domain and a protruding (P) domain, including two subdomains with a distal globular domain called P2 and a more central stem domain called P1 (17-19, 25). The N-terminal 225 residues of the capsid contain the S domain, and residues from 225 to the end of the C-terminal contain the P domain, consisting of two subdomains (P1 and P2). The P1 domain is located between the S and P2 domains and is formed by residues 226 to 278 and the C terminus. Whereas the P1 domain is moderately conserved (17, 25), the P2 domain is highly variable in particular between residues 279 and 405 (17), which is the most exposed region of the structure.
Except for the sporadic amino acid changes, 11 amino acids were accumulated in the capsid (Fig. 4). It is interesting that 10 of 11 amino acids accumulated in the protruding P domain and only 1 amino acid accumulated in the more conserved S domain. A more novel observation was that 8 of 11 amino acids accumulated within the hypervariable P2 domain (Fig. 4), suggesting an immune-response-driven mutation event. Support for this suggestion is that P2 is a domain that previously has been proposed to contain determinants for strain specificity (17) and that a monoclonal antibody that recognizes a region between the residues 300 and 384 in the P2 domain inhibits the binding of Norwalk virus capsid to cells (28).
Secondary structure predictions revealed, as a consequence of the accumulated mutations in P2, the disappearance of a helix structure. To our knowledge, this is the first description of such an event for calicivirus and, by putting all current information together, we speculate that the structural changes of the capsid may have led to the possible emergence of a new phenotype. Although we have no conclusive data that prove that the virus has changed phenotype characteristics during the course of infection, our suggestion is nevertheless supported by a previous prediction that structural variations in the P2 domain could alter host or phenotype characteristics (16, 17).
Our structural comparisons were based on the structure of Norwalk virus(16), a virus that belongs to genogroup I, in contrast to the virus described in the present study, which belongs to genogroup II. Although structural differences might occur between the genogroups, the overall architecture of the Norwalk virus capsid is similar to that of animal caliciviruses, such as rabbit hemorrhagic disease virus (25).
An interesting observation was that clinical symptoms varied over time, with diarrhea, vomiting, and nausea occurring in the early phase of the illness, followed by the disappearance of vomiting and nausea later in the course of the illness. Although several studies have shown that the relative frequencies of these symptoms vary among different outbreaks, ages, and settings (15, 21, 22), it has not to our knowledge been previously reported that calicivirus-associated diarrhea can last for years. The disappearance of vomiting and nausea appeared not to be related to reduced excretion of virus or diarrheal episodes. During the chronic excretion, the virus was transmitted to the partner and nursing staff, showing that infectious virus and not merely noninfectious particles were excreted. Furthermore, no pathogenic bacteria or virus other than norovirus was found in the samples, which strongly suggests that the clinical symptoms were the result of an ongoing norovirus infection and not merely that norovirus was asymptomatically excreted concomitant with a pathogenic microorganism.
The patient was in a state of severe immunosuppression, as illustrated by a CD4/CD8 ratio of 0.37. However, his immunoglobulin concentrations were normal for immunoglobulin G (IgG), IgA and IgM, suggesting that his cellular immunity was impaired but that his humoral immunity was intact. With an intact B-cell immunity, antibody-mediated mutations might have been the most reasonable explanation for the cause of the structural changes of the capsid. Further support for this view is the observation that antibodies do recognize this domain and can prevent virus binding (29).
To investigate whether the patient was Secretor+ and thus expressed the native H antigen on mucosa and saliva, polymorphism studies of three different FUT2 loci were undertaken. Particular attention was given to the FUT2 gene 428G→A mutation since this is the most common inactivating Secretor gene mutation among Caucasians (10, 11).
This individual was found to carry one functional and one nonfunctional FUT2 gene mutated at nucleotide 428. He is thus heterozygous (+/−) (Secretor+) and can fucosylate the Galβ1-3GlcNAc core into the H type 1 structure. This is the only transferase cloned to date which can synthesize the H type 1 and is thus the necessary prerequisite for secretion of ABH antigens in saliva. However, since this individual also carries functional Lewis alleles, the H type 1 structure is probably fucosylated further to the Lewis b structure. Whether this antigen, also typically found in secretions or on epithelial cells, is a potential receptor for the virus has not yet been carefully tested.
In conclusion, the present study is the first report of RNA evolution of a norovirus and suggests an immune-response-driven mechanism for virus evolution with, in this case, predicted structural changes of the viral capsid possibly resulting in a new emerging phenotype. Furthermore, our FUT2 polymorphism characterization further supports the hypothesis of host gene determinants in norovirus susceptibility.
Acknowledgments
This study was partly supported by the European Union (grants QLRT-1999-00634 and QLRT-1999-00594) and grant 8266 from the Swedish Research Council.
REFERENCES
- 1.Boom, R., C. Sol, M. Salimans, C. Jansen, P. Wertheim-van Dillen, and J. Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grahn, A., A. Elmgren, L. Aberg, L. Svensson, P. A. Jansson, P. Lonnroth, and G. Larson. 2001. Determination of Lewis FUT3 gene mutations by PCR using sequence-specific primers enables efficient genotyping of clinical samples. Hum. Mutat. 18:358-359. [DOI] [PubMed] [Google Scholar]
- 3.Hedlund, K. O., E. Rubilar-Abreu, and L. Svensson. 2000. Epidemiology of calicivirus infections in Sweden, 1994-1998. J. Infect. Dis. 181(Suppl. 2):S275-S280. [DOI] [PubMed] [Google Scholar]
- 4.Henry, S., R. Mollicone, P. Fernandez, B. Samuelsson, R. Oriol, and G. Larson. 1996. Molecular basis for erythrocyte Le(a+b+) and salivary ABH partial-Secretor phenotypes: expression of a FUT2 Secretor allele with an A→T mutation at nucleotide 385 correlates with reduced α(1,2)fucosyltransferase activity. Glycoconj. J. 13:985-993. [DOI] [PubMed] [Google Scholar]
- 5.Henry, S., R. Mollicone, J. B. Lowe, B. Samuelsson, and G. Larson. 1996. A second nonsecretor allele of the blood group α(1,2)fucosyl-transferase gene (FUT2). Vox Sang 70:21-25. [DOI] [PubMed] [Google Scholar]
- 6.Hutson, A. M., R. L. Atmar, D. Y. Graham, and M. K. Estes. 2002. Norwalk virus infection and disease is associated with ABO histo-blood group type. J. Infect. Dis. 185:1335-1337. [DOI] [PubMed] [Google Scholar]
- 7.Hutson, A. M., R. L. Atmar, D. M. Marcus, and M. K. Estes. 2003. Norwalk virus-like particle hemagglutination by binding to h histo-blood group antigens. J. Virol. 77:405-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansson, P. J., M. Torven, A. C. Hammarlund, U. Bjorne, K. O. Hedlund, and L. Svensson. 2002. Food-borne outbreak of gastroenteritis associated with genogroup I calicivirus. J. Clin. Microbiol. 40:794-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones, D. T. 1999. Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 292:195-202. [DOI] [PubMed] [Google Scholar]
- 10.Kelly, R. J., S. Rouquier, D. Giorgi, G. G. Lennon, and J. B. Lowe. 1995. Sequence and expression of a candidate for the human Secretor blood group α(1,2)fucosyltransferase gene (FUT2): homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-Secretor phenotype. J. Biol. Chem. 270:4640-4649. [DOI] [PubMed] [Google Scholar]
- 11.Larson, G., L. Svensson, L. Hynsjo, A. Elmgren, and L. Rydberg. 1999. Typing for the human Lewis blood group system by quantitative fluorescence-activated flow cytometry: large differences in antigen presentation on erythrocytes between A(1), A(2), B, O phenotypes. Vox Sang 77:227-236. [DOI] [PubMed] [Google Scholar]
- 12.Lopman, B. A., D. W. Brown, and M. Koopmans. 2002. Human caliciviruses in Europe. J. Clin. Virol. 24:137-160. [DOI] [PubMed] [Google Scholar]
- 13.Marionneau, S., N. Ruvoen, B. Le Moullac-Vaidye, M. Clement, A. Cailleau-Thomas, G. Ruiz-Palacois, P. Huang, X. Jiang, and J. Le Pendu. 2002. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of Secretor individuals. Gastroenterology 122:1967-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pang, X. L., S. Honma, S. Nakata, and T. Vesikari. 2000. Human caliciviruses in acute gastroenteritis of young children in the community. J. Infect. Dis. 181(Suppl. 2):S288-S294. [DOI] [PubMed] [Google Scholar]
- 15.Pang, X. L., J. Joensuu, and T. Vesikari. 1999. Human calicivirus-associated sporadic gastroenteritis in Finnish children less than two years of age followed prospectively during a rotavirus vaccine trial. Pediatr. Infect. Dis. J. 18:420-426. [DOI] [PubMed] [Google Scholar]
- 16.Prasad, B. V., S. Crawford, J. A. Lawton, J. Pesavento, M. Hardy, and M. K. Estes. 2001. Structural studies on gastroenteritis viruses. Novartis Found. Symp. 238:26-46. [DOI] [PubMed] [Google Scholar]
- 17.Prasad, B. V., M. E. Hardy, T. Dokland, J. Bella, M. G. Rossmann, and M. K. Estes. 1999. X-ray crystallographic structure of the Norwalk virus capsid. Science 286:287-290. [DOI] [PubMed] [Google Scholar]
- 18.Prasad, B. V., M. E. Hardy, X. Jiang, and M. K. Estes. 1996. Structure of Norwalk virus. Arch. Virol. Suppl. 12:237-242. [DOI] [PubMed] [Google Scholar]
- 19.Prasad, B. V., R. Rothnagel, X. Jiang, and M. K. Estes. 1994. Three-dimensional structure of baculovirus-expressed Norwalk virus capsids. J. Virol. 68:5117-5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Procter, J., J. Crawford, M. Bunce, and K. I. Welsh. 1997. A rapid molecular method (polymerase chain reaction with sequence-specific primers) to genotype for ABO blood group and secretor status and its potential for organ transplants. Tissue Antigens 50:475-483. [DOI] [PubMed] [Google Scholar]
- 21.Rockx, B., M. De Wit, H. Vennema, J. Vinje, E. De Bruin, Y. Van Duynhoven, and M. Koopmans. 2002. Natural history of human calicivirus infection: a prospective cohort study. Clin. Infect. Dis. 35:246-253. [DOI] [PubMed] [Google Scholar]
- 22.Sakai, Y., S. Nakata, S. Honma, M. Tatsumi, K. Numata-Kinoshita, and S. Chiba. 2001. Clinical severity of Norwalk virus and Sapporo virus gastroenteritis in children in Hokkaido, Japan. Pediatr. Infect. Dis. J. 20:849-853. [DOI] [PubMed] [Google Scholar]
- 23.Serpa, J., R. Almeida, C. Oliveira, F. S. Silva, E. Silva, C. Reis, J. Le Pendu, G. Oliveira, L. M. Ribeiro, and L. David. 2003. Lewis enzyme (α1-3/4 fucosyltransferase) polymorphisms do not explain the Lewis phenotype in the gastric mucosa of a Portuguese population. J. Hum. Genet. 48:183-189. [DOI] [PubMed] [Google Scholar]
- 24.Thornhill, T., E. Kalica, R. G. Wyatt, A. Z. Kapikian, and R. M. Chanock. 1975. Pattern of shedding of the Norwalk particles in stools during experimentally induced gastroenteritis in volunteers as demonstrated by immune electron microscopy. J. Infect. Dis. 132:28-34. [DOI] [PubMed] [Google Scholar]
- 25.Venkataram Prasad, B. V., M. E. Hardy, and M. K. Estes. 2000. Structural studies of recombinant Norwalk capsids. J. Infect. Dis. 181(Suppl. 2):S317-S321. [DOI] [PubMed] [Google Scholar]
- 26.Vinje, J., S. Altena, and M. Koopmans. 1997. The incidence and genetic variability of small round-structured viruses in outbreaks of gastroenteritis in The Netherlands. J. Infect. Dis. 176:1374-1378. [DOI] [PubMed] [Google Scholar]
- 27.Vinje, J., and M. Koopmans. 1996. Molecular detection and epidemiology of small round-structured viruses in outbreaks of gastroenteritis in The Netherlands. J. Infect. Dis. 174:610-615. [DOI] [PubMed] [Google Scholar]
- 28.White, L. J., J. M. Ball, M. E. Hardy, T. N. Tanaka, N. Kitamoto, and M. K. Estes. 1996. Attachment and entry of recombinant Norwalk virus capsids to cultured human and animal cell lines. J. Virol. 70:6589-6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White, L. J., M. E. Hardy, and M. K. Estes. 1997. Biochemical characterization of a smaller form of recombinant Norwalk virus capsids assembled in insect cells. J. Virol. 71:8066-8072. [DOI] [PMC free article] [PubMed] [Google Scholar]