Abstract
The iminodiacetic acid component of the title salt, C4H8NO4 +·C7H7SO3 −, is protonated at the N atom. The cation uses the ammonium group to form hydrogen bonds to the O atoms of two adjacent sulfonate groups. In addition, the carboxylic acid portions of the cation form hydrogen bonds to the sulfonate groups. The hydrogen-bonding interactions give rise to a layer structure.
Related literature
For the crystal structures of iminodiacetic acid hydrohalides, see: Oskarsson (1973 ▶, 1974a
▶,b
▶, 1976 ▶).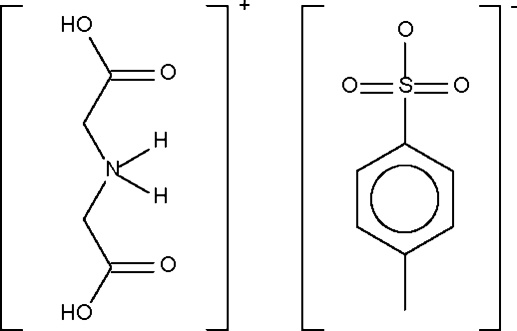
Experimental
Crystal data
C4H8NO4 +·C7H7O3S−
M r = 305.30
Orthorhombic,

a = 9.9291 (2) Å
b = 10.3636 (2) Å
c = 25.8862 (5) Å
V = 2663.72 (9) Å3
Z = 8
Mo Kα radiation
μ = 0.28 mm−1
T = 100 (2) K
0.27 × 0.27 × 0.27 mm
Data collection
Bruker SMART APEX diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.929, T max = 0.929
20842 measured reflections
3059 independent reflections
2560 reflections with I > 2σ(I)
R int = 0.048
Refinement
R[F 2 > 2σ(F 2)] = 0.038
wR(F 2) = 0.119
S = 1.15
3059 reflections
198 parameters
4 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.42 e Å−3
Δρmin = −0.50 e Å−3
Data collection: APEX2 (Bruker, 2007 ▶); cell refinement: SAINT (Bruker, 2007 ▶); data reduction: SAINT); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: publCIF (Westrip, 2008 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808026214/bh2187sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808026214/bh2187Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1N1⋯O2i | 0.88 (1) | 2.02 (1) | 2.885 (2) | 167 (2) |
| N1—H1N2⋯O3ii | 0.88 (1) | 2.06 (2) | 2.792 (2) | 140 (2) |
| O5—H5O⋯O1 | 0.84 (1) | 1.79 (1) | 2.607 (2) | 164 (3) |
| O7—H7O⋯O2iii | 0.84 (1) | 1.85 (1) | 2.659 (2) | 160 (3) |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
Acknowledgments
We thank the University of Malaya for funding this study (SF022/2007 A, FS339/2008 A) and also for the purchase of the diffractometer.
supplementary crystallographic information
Comment
(type here to add)
Experimental
Iminodiacetic acid (0.55 g, 4 mmol) and p-toluenesulfonic acid (0.65 g, 4 mmol) were heated in toluene (100 ml) for 1 h. Crystals were isolated from the cool solution after several days.
Refinement
Carbon-bound H-atoms were placed in calculated positions (C—H 0.95 to 0.99 Å) and were included in the refinement in the riding model approximation, with Uiso(H) set to 1.2 to 1.5Ueq(carrier C). The acid and ammonium H atoms were refined with distance restraints of O—H = 0.84 (1) and N—H = 0.88 (1) Å; their isotropic displacement parameters were freely refined.
Figures
Fig. 1.
Thermal ellipsoid plot (Barbour, 2001) of (C4H8NO4)+(C7H7O3S)- at the 70% probability level. Hydrogen atoms are drawn as spheres of arbitrary radius.
Crystal data
| C4H8NO4+·C7H7O3S– | F000 = 1280 |
| Mr = 305.30 | Dx = 1.523 Mg m−3 |
| Orthorhombic, Pbca | Mo Kα radiation λ = 0.71073 Å |
| Hall symbol: -P 2ac 2ab | Cell parameters from 4293 reflections |
| a = 9.9291 (2) Å | θ = 2.9–27.2º |
| b = 10.3636 (2) Å | µ = 0.28 mm−1 |
| c = 25.8862 (5) Å | T = 100 (2) K |
| V = 2663.72 (9) Å3 | Triangular block, colorless |
| Z = 8 | 0.27 × 0.27 × 0.27 mm |
Data collection
| Bruker SMART APEX diffractometer | 3059 independent reflections |
| Radiation source: fine-focus sealed tube | 2560 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.048 |
| T = 100(2) K | θmax = 27.5º |
| ω scans | θmin = 1.6º |
| Absorption correction: multi-scan(SADABS; Sheldrick, 1996) | h = −12→7 |
| Tmin = 0.930, Tmax = 0.930 | k = −13→13 |
| 20842 measured reflections | l = −33→33 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.038 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.119 | w = 1/[σ2(Fo2) + (0.0644P)2 + 0.8206P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.15 | (Δ/σ)max = 0.001 |
| 3059 reflections | Δρmax = 0.42 e Å−3 |
| 198 parameters | Δρmin = −0.50 e Å−3 |
| 4 restraints | Extinction correction: none |
| Primary atom site location: structure-invariant direct methods |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.49041 (5) | 0.84948 (4) | 0.358654 (17) | 0.01248 (14) | |
| O1 | 0.55419 (15) | 0.74172 (13) | 0.38508 (5) | 0.0195 (3) | |
| O2 | 0.56209 (14) | 0.88382 (13) | 0.31102 (5) | 0.0163 (3) | |
| O3 | 0.34727 (14) | 0.83294 (13) | 0.35029 (5) | 0.0190 (3) | |
| O4 | 0.40223 (14) | 0.52637 (13) | 0.30853 (5) | 0.0171 (3) | |
| O5 | 0.51261 (16) | 0.49339 (13) | 0.38301 (5) | 0.0199 (3) | |
| H5O | 0.510 (3) | 0.5747 (10) | 0.3841 (10) | 0.034 (7)* | |
| O6 | 0.20627 (14) | 0.17528 (13) | 0.21695 (5) | 0.0176 (3) | |
| O7 | 0.29656 (14) | −0.02406 (13) | 0.22217 (5) | 0.0163 (3) | |
| H7O | 0.229 (2) | −0.047 (3) | 0.2048 (10) | 0.051 (9)* | |
| N1 | 0.37466 (17) | 0.27383 (15) | 0.28845 (6) | 0.0129 (3) | |
| H1N1 | 0.402 (3) | 0.316 (2) | 0.2610 (7) | 0.031 (7)* | |
| H1N2 | 0.2899 (11) | 0.296 (2) | 0.2924 (8) | 0.014 (5)* | |
| C1 | 0.50864 (19) | 0.98391 (18) | 0.40013 (7) | 0.0141 (4) | |
| C2 | 0.3995 (2) | 1.02980 (19) | 0.42797 (8) | 0.0187 (4) | |
| H2 | 0.3146 | 0.9879 | 0.4256 | 0.022* | |
| C3 | 0.4152 (2) | 1.1376 (2) | 0.45941 (8) | 0.0212 (4) | |
| H3 | 0.3404 | 1.1681 | 0.4788 | 0.025* | |
| C4 | 0.5374 (2) | 1.20164 (19) | 0.46306 (7) | 0.0183 (4) | |
| C5 | 0.6471 (2) | 1.15329 (19) | 0.43556 (8) | 0.0191 (4) | |
| H5 | 0.7319 | 1.1952 | 0.4380 | 0.023* | |
| C6 | 0.6335 (2) | 1.04440 (19) | 0.40462 (7) | 0.0171 (4) | |
| H6 | 0.7093 | 1.0112 | 0.3866 | 0.021* | |
| C7 | 0.5517 (2) | 1.3217 (2) | 0.49545 (8) | 0.0247 (5) | |
| H7A | 0.4636 | 1.3631 | 0.4993 | 0.037* | |
| H7B | 0.6142 | 1.3815 | 0.4785 | 0.037* | |
| H7C | 0.5868 | 1.2985 | 0.5296 | 0.037* | |
| C8 | 0.45172 (19) | 0.45546 (18) | 0.34029 (7) | 0.0137 (4) | |
| C9 | 0.45326 (19) | 0.31071 (18) | 0.33494 (7) | 0.0137 (4) | |
| H9A | 0.5472 | 0.2798 | 0.3315 | 0.016* | |
| H9B | 0.4132 | 0.2705 | 0.3660 | 0.016* | |
| C10 | 0.3811 (2) | 0.13288 (17) | 0.27813 (7) | 0.0142 (4) | |
| H10A | 0.3570 | 0.0845 | 0.3098 | 0.017* | |
| H10B | 0.4738 | 0.1085 | 0.2680 | 0.017* | |
| C11 | 0.28461 (19) | 0.09925 (18) | 0.23534 (7) | 0.0132 (4) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0104 (2) | 0.0118 (2) | 0.0153 (2) | −0.00084 (17) | −0.00072 (16) | 0.00057 (16) |
| O1 | 0.0240 (8) | 0.0125 (7) | 0.0221 (7) | −0.0013 (6) | −0.0072 (6) | 0.0031 (5) |
| O2 | 0.0164 (7) | 0.0158 (7) | 0.0168 (7) | −0.0021 (6) | 0.0028 (5) | −0.0016 (5) |
| O3 | 0.0109 (7) | 0.0226 (8) | 0.0233 (7) | −0.0016 (6) | −0.0006 (6) | −0.0024 (5) |
| O4 | 0.0169 (7) | 0.0148 (7) | 0.0197 (7) | 0.0007 (6) | −0.0025 (5) | 0.0007 (5) |
| O5 | 0.0293 (9) | 0.0111 (7) | 0.0192 (7) | −0.0007 (6) | −0.0092 (6) | −0.0014 (5) |
| O6 | 0.0156 (7) | 0.0155 (7) | 0.0217 (7) | −0.0016 (6) | −0.0030 (5) | 0.0033 (5) |
| O7 | 0.0134 (7) | 0.0143 (7) | 0.0211 (7) | −0.0023 (6) | −0.0006 (6) | −0.0043 (5) |
| N1 | 0.0123 (8) | 0.0109 (8) | 0.0154 (8) | 0.0011 (6) | −0.0002 (6) | 0.0000 (6) |
| C1 | 0.0144 (9) | 0.0131 (9) | 0.0149 (8) | −0.0001 (7) | −0.0003 (7) | 0.0010 (7) |
| C2 | 0.0148 (10) | 0.0198 (10) | 0.0215 (9) | −0.0019 (8) | 0.0032 (8) | 0.0004 (8) |
| C3 | 0.0190 (11) | 0.0217 (10) | 0.0228 (10) | 0.0025 (8) | 0.0066 (8) | −0.0020 (8) |
| C4 | 0.0237 (11) | 0.0162 (10) | 0.0150 (9) | 0.0007 (8) | −0.0001 (8) | 0.0004 (7) |
| C5 | 0.0145 (10) | 0.0225 (10) | 0.0202 (9) | −0.0045 (8) | 0.0002 (8) | −0.0023 (8) |
| C6 | 0.0130 (9) | 0.0199 (10) | 0.0183 (9) | 0.0004 (8) | 0.0014 (7) | −0.0027 (7) |
| C7 | 0.0276 (12) | 0.0219 (11) | 0.0247 (10) | −0.0011 (9) | 0.0010 (9) | −0.0067 (8) |
| C8 | 0.0102 (9) | 0.0150 (9) | 0.0161 (8) | −0.0005 (7) | 0.0005 (7) | 0.0004 (7) |
| C9 | 0.0125 (9) | 0.0133 (9) | 0.0152 (9) | 0.0002 (7) | −0.0026 (7) | 0.0000 (7) |
| C10 | 0.0132 (9) | 0.0095 (8) | 0.0200 (9) | 0.0004 (7) | −0.0017 (7) | −0.0009 (7) |
| C11 | 0.0108 (9) | 0.0132 (9) | 0.0157 (8) | −0.0019 (7) | 0.0028 (7) | 0.0012 (7) |
Geometric parameters (Å, °)
| S1—O3 | 1.4478 (14) | C2—H2 | 0.9500 |
| S1—O1 | 1.4547 (14) | C3—C4 | 1.386 (3) |
| S1—O2 | 1.4675 (13) | C3—H3 | 0.9500 |
| S1—C1 | 1.768 (2) | C4—C5 | 1.394 (3) |
| O4—C8 | 1.207 (2) | C4—C7 | 1.507 (3) |
| O5—C8 | 1.320 (2) | C5—C6 | 1.390 (3) |
| O5—H5o | 0.84 (1) | C5—H5 | 0.9500 |
| O6—C11 | 1.205 (2) | C6—H6 | 0.9500 |
| O7—C11 | 1.328 (2) | C7—H7A | 0.9800 |
| O7—H7o | 0.84 (1) | C7—H7B | 0.9800 |
| N1—C9 | 1.484 (2) | C7—H7C | 0.9800 |
| N1—C10 | 1.486 (2) | C8—C9 | 1.507 (3) |
| N1—H1n1 | 0.88 (1) | C9—H9A | 0.9900 |
| N1—H1n2 | 0.88 (1) | C9—H9B | 0.9900 |
| C1—C2 | 1.385 (3) | C10—C11 | 1.505 (3) |
| C1—C6 | 1.394 (3) | C10—H10A | 0.9900 |
| C2—C3 | 1.391 (3) | C10—H10B | 0.9900 |
| O3—S1—O1 | 114.00 (9) | C4—C5—H5 | 119.7 |
| O3—S1—O2 | 112.28 (8) | C5—C6—C1 | 119.93 (18) |
| O1—S1—O2 | 111.72 (8) | C5—C6—H6 | 120.0 |
| O3—S1—C1 | 106.53 (9) | C1—C6—H6 | 120.0 |
| O1—S1—C1 | 105.95 (9) | C4—C7—H7A | 109.5 |
| O2—S1—C1 | 105.63 (8) | C4—C7—H7B | 109.5 |
| C8—O5—H5O | 108.2 (18) | H7A—C7—H7B | 109.5 |
| C11—O7—H7O | 110 (2) | C4—C7—H7C | 109.5 |
| C9—N1—C10 | 112.10 (14) | H7A—C7—H7C | 109.5 |
| C9—N1—H1N1 | 111.7 (17) | H7B—C7—H7C | 109.5 |
| C10—N1—H1N1 | 109.4 (16) | O4—C8—O5 | 125.13 (18) |
| C9—N1—H1N2 | 110.2 (14) | O4—C8—C9 | 123.20 (17) |
| C10—N1—H1N2 | 108.3 (15) | O5—C8—C9 | 111.66 (15) |
| H1N1—N1—H1N2 | 105 (2) | N1—C9—C8 | 109.01 (15) |
| C2—C1—C6 | 119.85 (18) | N1—C9—H9A | 109.9 |
| C2—C1—S1 | 120.41 (15) | C8—C9—H9A | 109.9 |
| C6—C1—S1 | 119.73 (15) | N1—C9—H9B | 109.9 |
| C1—C2—C3 | 119.53 (19) | C8—C9—H9B | 109.9 |
| C1—C2—H2 | 120.2 | H9A—C9—H9B | 108.3 |
| C3—C2—H2 | 120.2 | N1—C10—C11 | 109.43 (15) |
| C4—C3—C2 | 121.46 (18) | N1—C10—H10A | 109.8 |
| C4—C3—H3 | 119.3 | C11—C10—H10A | 109.8 |
| C2—C3—H3 | 119.3 | N1—C10—H10B | 109.8 |
| C3—C4—C5 | 118.49 (18) | C11—C10—H10B | 109.8 |
| C3—C4—C7 | 121.06 (19) | H10A—C10—H10B | 108.2 |
| C5—C4—C7 | 120.4 (2) | O6—C11—O7 | 125.83 (17) |
| C6—C5—C4 | 120.68 (19) | O6—C11—C10 | 123.36 (17) |
| C6—C5—H5 | 119.7 | O7—C11—C10 | 110.79 (16) |
| O3—S1—C1—C2 | 16.35 (19) | C3—C4—C5—C6 | 0.9 (3) |
| O1—S1—C1—C2 | −105.40 (17) | C7—C4—C5—C6 | −178.42 (18) |
| O2—S1—C1—C2 | 135.94 (16) | C4—C5—C6—C1 | 1.2 (3) |
| O3—S1—C1—C6 | −163.36 (15) | C2—C1—C6—C5 | −2.3 (3) |
| O1—S1—C1—C6 | 74.89 (17) | S1—C1—C6—C5 | 177.39 (15) |
| O2—S1—C1—C6 | −43.77 (18) | C10—N1—C9—C8 | 175.26 (15) |
| C6—C1—C2—C3 | 1.3 (3) | O4—C8—C9—N1 | −5.4 (3) |
| S1—C1—C2—C3 | −178.42 (15) | O5—C8—C9—N1 | 175.76 (15) |
| C1—C2—C3—C4 | 0.9 (3) | C9—N1—C10—C11 | 172.17 (15) |
| C2—C3—C4—C5 | −1.9 (3) | N1—C10—C11—O6 | −6.8 (3) |
| C2—C3—C4—C7 | 177.37 (19) | N1—C10—C11—O7 | 174.56 (14) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1N1···O2i | 0.88 (1) | 2.02 (1) | 2.885 (2) | 167 (2) |
| N1—H1N2···O3ii | 0.88 (1) | 2.06 (2) | 2.792 (2) | 140 (2) |
| O5—H5O···O1 | 0.84 (1) | 1.79 (1) | 2.607 (2) | 164 (3) |
| O7—H7O···O2iii | 0.84 (1) | 1.85 (1) | 2.659 (2) | 160 (3) |
Symmetry codes: (i) −x+1, y−1/2, −z+1/2; (ii) −x+1/2, y−1/2, z; (iii) x−1/2, y−1, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BH2187).
References
- Barbour, L. J. (2001). J. Supramol. Chem.1, 189–191.
- Bruker (2007). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Oskarsson, Å. (1973). Acta Cryst. B29, 1747–1751.
- Oskarsson, Å. (1974a). Acta Cryst. B30, 780–783.
- Oskarsson, Å. (1974b). Acta Cryst. B30, 1184–1188.
- Oskarsson, A. (1976). Acta Cryst. B32, 2163–2170.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2008). publCIF In preparation.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808026214/bh2187sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808026214/bh2187Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



