Abstract
We report here that fusions of single-chain antibodies (scFvs) to the autotransporter β domain of the IgA protease of Neisseria gonorrhoeae are instrumental in locating virus-neutralizing activity on the cell surface of Escherichia coli. E. coli cells displaying scFvs against the transmissible gastroenteritis coronavirus on their surface blocked in vivo the access of the infectious agent to cultured epithelial cells. This result raises prospects for antiviral strategies aimed at hindering the entry into target cells by bacteria that naturally colonize the same intestinal niches.
Secretory immunoglobulins present on mucosal surfaces (e.g., immunoglobulins A [IgAs]) provide an early barrier against most virulent agents that invade their hosts through that port of entry (14, 16). Such Igs are as efficient when provided by the host organism itself as when acquired from an external source, typically, the Igs present in the maternal milk (19). The barriers built by secretory Igs in the mucosa are particularly important in naturally immunocompromised hosts, such as newborn animals and infants, which are more susceptible to a fatal outcome after an initial infection (6). Typically, Igs neutralize viruses in the mucosa by preventing their adherence to epithelial cells (14). Unfortunately, this mechanism is not efficacious in all cases and disease does occur.
The transmissible gastroenteritis coronavirus (TGEV), which infects respiratory and enteric tissues, is an important porcine disease that causes nearly 100% mortality in infected newborn animals (7). Previous studies have identified a mouse monoclonal antibody (MAb), named 6A.C3, which fully neutralizes TGEV and TGEV-related coronaviruses infecting pigs, cats, and dogs (3). The outstanding neutralizing ability of 6A.C3 is maintained in various contexts in vivo. Transgenic mice engineered to secrete 6A.C3 in milk (2, 18) produced an antibody which maintained in full its intrinsic neutralizing activity. This finding suggested a plausible approach for developing a sort of passive immunity against TGEV in young animals who feed on such a milk.
In this work, we explored a different approach for creating scenarios of passive immunity, e.g., the use of live bacteria as the vehicle to deliver the TGEV-neutralizing activity at the required sites. The rationale is that the locations of entry of the infectious agent (the mucosal epithelia) are also the natural niches of enteric bacteria (i.e., Escherichia coli) that can be programmed genetically to provide neutralizing antibodies. Construction of such bacteria requires the expression and secretion of active antibodies in E. coli. This process needs (i) the expression of the heavy (VH) and light (VL) variable domains that assemble the antigen-binding site of the antibody, (ii) the formation of disulfide bonds in the V domains for correct folding, and (iii) the selection of suitable vector systems to target active antibodies to the cell surface and the external medium. As shown below, we have successfully met these needs by exploiting some key features of the mechanism of secretion of the IgA protease (IgAP) from Neisseria gonorrhoeae.
Expression of the anti-TGEV antibody 6A.C3 in E. coli.
The antigen-binding site of the original 6A.C3 MAb was recreated as a single-chain Fv protein (scFv) by employing the corresponding VH and VL domains from the 6A.C3 hybridoma (8). Although the apparent affinity of the resulting scFv was reduced 50-fold (8), probably due to the conversion of the bivalent MAb into a monovalent scFv molecule (5, 11, 17), the new scFv (i.e., 6AC3-scFv) retained the TGEV-neutralizing activity of the full-size 6A.C3 MAb. The 6AC3-scFv protein was, therefore, still helpful in validating the in situ neutralization concept. To this end, we next attempted to fuse the 6AC3-scFv to a carrier protein able to translocate the antibody moiety to the E. coli surface. The vehicle of choice was the transporter domain of the IgAP from N. gonorrhoeae. IgAP belongs to the autotransporter family of secreted proteins (10). All members of this family, which are described as present in an increasing number of pathogenic bacteria (9), share the same modular structure, with an N-passenger domain that is exposed to the external medium and a C-terminal transporter β domain driving the translocation of the passenger across the outer membrane (OM). The N-passenger module can be replaced by heterologous domains, which may become exposed to the medium provided that the hydrodynamic radius of the folded passenger protein is not longer than ∼2 nm (4, 13, 15, 20; unpublished results). Such a secretion system tolerates the folding and the passage of disulfide-bond-containing proteins (1, 21; unpublished results). Furthermore, when fused to the β domain of an autotransporter, active scFvs can be targeted to the external medium of E. coli (21). On this basis, we set out to produce a hybrid protein that fused in frame the sequence of the 6AC3-scFv protein to the C-terminal transporter module of the IgAP (Fig. 1A).
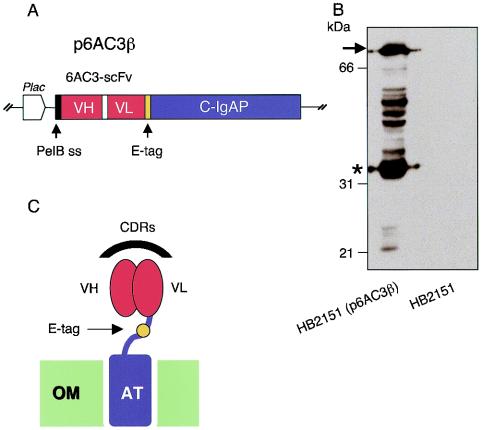
FIG. 1.
(A) Organization of the relevant insert of plasmid p6AC3β, encoding the 6AC3β fusion. The sequences corresponding to the pelB leader (ss), the scFv, and the C-IgAP segments are indicated along with the lac promoter (Plac) and the fragment encoding the E-tag epitope. (B) Expression of the 6AC3β hybrid in E. coli. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels of crude extracts of induced E. coli HB2151(p6AC3β) cells expressing 6AC3β were probed by immunoblotting with an anti E-tag MAb. The location of the full-size 80-kDa 6AC3β protein is indicated with an arrow. The 35-kDa major proteolytic band corresponding to 6AC3-scFv is indicated with an asterisk. (C) Simplified sketch of the localization and predicted domain structure of the 6AC3β protein. Although the autotransporters (ATs) are structured as oligomeric complexes (22), only a monomer is shown. The illustration includes the AT domain of the IgA protease of Neisseria inserted into the bacterial OM and bound to the VH and VL modules of the scFv by a linker which incorporates the E-tag. This assembly allows the presentation of the complementarity determinant regions (CDRs) of the scFv to the cell exterior.
For generating the constructs expressing the desired scFv-β domain hybrid (hereafter named 6AC3β protein), the sequence encoding 6AC3 was excised from plasmid p6AC3g3 (8) as a 0.7-kb SfiI-NotI fragment, which was then cloned in vector pF11β (21). The resulting plasmid (named p6AC3β; Fig. 1A) fused the 6AC3-scFv protein to the transporter C domain of the IgAP (C-IgAP). To ensure secretion, the hybrid sequence was placed in frame to the N-terminal signal peptide of the pelB gene (Fig. 1A). Furthermore, expression of the hybrid protein 6AC3β is controlled by the lac promoter and can be induced by adding isopropyl-β-d-thiogalactopyranoside (IPTG). Production of protein 6AC3β is easily detected with a MAb that recognizes the E-tag epitope engineered in the region between the scFv module and the C-IgAP transporter domain (21, 22).
To test expression of the hybrid protein, E. coli HB2151 cells (8) were transformed with plasmid p6AC3β and induced with 0.1 mM IPTG for 3 h at 30°C in Luria-Bertani medium. As shown in Fig. 1B, a major band of the expected size (∼80 kDa) along with a series of smaller extra products corresponding to proteolysis of the hybrid protein was observed. The presence of such degradation products has been noticed before in other scFv-C-IgAP fusions targeted to the OM (21, 22). The major ∼35-kDa band was proven to correspond to the scFv that remained trapped in the hydrophilic pore formed by the autotransporter complex (21, 22). This proteolysis can be prevented if other scFv types that are less prone to aggregation are fused with C-IgAP (23; unpublished results). Figure 1C depicts the putative topology on the bacterial surface of the scFv passenger of a 6AC3β monomer.
TGEV-neutralizing activity of E. coli cells expressing 6AC3β.
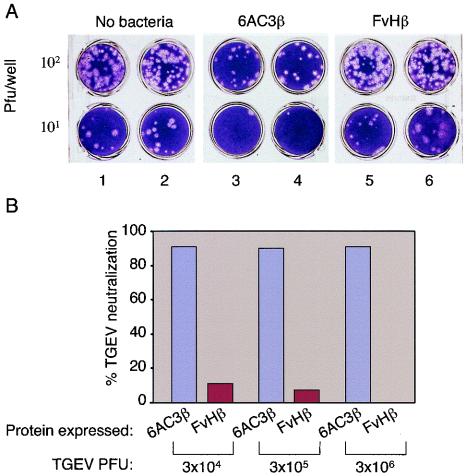
In order to test whether the E. coli cells expressing the 6AC3β hybrid showed TGEV-neutralizing activity, we used a viral infection assay (8). To this end, 3 × 106 PFU of the TGEV strain PUR46-MAD (3) were incubated in 200 μl of PBS buffer with 108 E. coli HB2151 cells expressing the 6AC3β hybrid (Fig. 2A, lanes 3 and 4). Controls (Fig. 2A, lanes 5 and 6 and lanes 1 and 2) included E. coli HB2151 cells expressing the control scFv-C-IgAP fusion FvHβ (bearing an antibody raised against C-terminal His tags [21]) as well as buffer without bacteria. After 30 min, samples were centrifuged to remove bacteria and adsorbed TGEV particles. Supernatants containing the free viruses were added in 10-fold serial dilutions to duplicate monolayers of swine testis (ST) cells grown in tissue culture plates. After a further 48 h of incubation, the ST cell monolayers were stained with crystal violet to visualize the plaques formed by TGEV replication. As shown in Fig. 2A, a distinct and specific neutralization of TGEV became evident in samples in which the virus had been preincubated with the bacteria expressing 6AC3β. No neutralization was seen in the samples treated with bacteria expressing the control FvHβ protein (Fig. 2A). TGEV-neutralizing activity was also not detected in the culture supernatants of induced E. coli HB2151 (p6AC3β) cells (data not shown), thus indicating that the neutralizing scFv remained attached to the E. coli cell surface.
FIG. 2.
Neutralization of TGEV infection by the E. coli cells expressing the 6AC3β hybrid. (A) TGEV (3 × 106 PFU) was incubated for 30 min at 37°C with 108 E. coli HB2151 cells expressing the proteins indicated (6AC3β and FvHβ) in each case. After centrifugation, serial dilutions of the supernatant were added to monolayers of ST cells grown in vitro. The plaques caused by TGEV replication were visualized after 48 h by fixing and staining the ST cells. (B) Quantification of TGEV neutralization. The numbers of plaques produced by TGEV infection in samples incubated with E. coli cells expressing the proteins indicated or without this incubation were compared. Neutralization rates are shown as percentage ratios of samples with various amounts of PFU treated with bacteria to those lacking any treatment. The data are the average of results for at least three independent experiments.
Next, the neutralization brought about by E. coli populations expressing 6AC3β preset at 108 bacteria/200 μl by using various titers of TGEV (3 × 104 to 3 ×106) was measured (Fig. 2A). It is noteworthy that the percentage of virus neutralization by E. coli producing 6AC3β was always around 93% ± 2% (mean ± standard deviation),regardless of the number of TGEV PFU employed in the assay. This result was not due to the escape of viral mutants lacking the target antigen. Instead, the percentage of nonneutralized viruses may reflect the lower affinity of monomeric 6AC3-scFv relative to that of the bivalent 6AC3 MAb, which provides >99.99% neutralization (8). Consistent with this notion, an increase of purified 6AC3-scFv from 0.5 to 5 μg/ml in TGEV neutralization assays elicited a neutralization of ∼99% (8). A minor decrease in the titer of active viruses (<10%) was caused by E. coli cells expressing the unrelated FvHβ on viral populations of <106 PFU. This was probably due to some residual nonspecific adhesion of viroids to the charged surface of bacterial cells. This effect was not observed when higher TGEV titers (e.g., 106 PFU) were used.
Together, the data presented above indicate that bacteria expressing fusions of autotransporter domains to antiviral scFvs are capable of placing a neutralizing activity on the bacterial cell surface. These observations expand and generalize our previous efforts to design E. coli strains that are able to produce functional antibodies in various cellular compartments as well as our efforts to exploit in situ their target-binding abilities(8, 12, 21).
In conclusion, our data show that the anti-TGEV activity of 6AC3-scFv is significantly preserved when it is fused to C-IgAP and that this hybrid protein is displayed on the surface of E. coli cells in a form able to reach out and neutralize the infectious agent prior to their binding to epithelial cells. These results put forward the enticing possibility of engineering antibody-producing E. coli strains that are able to colonize the very same intestinal niches engaged by TGEV and other pathogens and so create a protective barrier to infection in vivo.
Acknowledgments
The excellent technical work of Sofía Fraile is greatly appreciated. We also appreciate the assistance of Luis Enjuanes and his group from the Centro Nacional de Biotecnología (Madrid, Spain).
This work was supported by grants BIO2001-2274 and BMC2002-03024 from the Spanish Ministerio de Ciencia y Tecnología (MCyT), by grant COLIRED-O157 (G03/025) of the Spanish Fondo de Investigaciones Sanitarias (FIS), and by the Programa de Grupos Estratégicos of the Autonomous Community of Madrid.
REFERENCES
- 1.Brandon, L. D., and M. B. Goldberg. 2001. Periplasmic transit and disulfide bond formation of the autotransported Shigella protein IcsA. J. Bacteriol. 183:951-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castilla, J., B. Pintado, I. Sola, J. M. Sanchez-Morgado, and L. Enjuanes. 1998. Engineering passive immunity in transgenic mice secreting virus-neutralizing antibodies in milk. Nat. Biotechnol. 16:349-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castilla, J., I. Sola, and L. Enjuanes. 1997. Interference of coronavirus infection by expression of immunoglobulin G (IgG) or IgA virus-neutralizing antibodies. J. Virol. 71:5251-5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornelis, P. 2000. Expressing genes in different Escherichia coli compartments. Curr. Opin. Biotechnol. 11:450-454. [DOI] [PubMed] [Google Scholar]
- 5.Crothers, D. M., and H. Metzger. 1972. The influence of polyvalency on the binding properties of antibodies. Immunochemistry 9:341-357. [DOI] [PubMed] [Google Scholar]
- 6.Crowe, J. E., Jr. 2001. Influence of maternal antibodies on neonatal immunization against respiratory viruses. Clin. Infect. Dis. 33:1720-1727. [DOI] [PubMed] [Google Scholar]
- 7.Enjuanes, L., and B. A. M. Van der Zeijst. 1995. Molecular basis of transmissible gastroenteritis coronavirus epidemiology, p. 337-376. In S. G. Siddell (ed.), The coronaviridae. Plenum Press, New York, N.Y.
- 8.Fernandez, L. A., I. Sola, L. Enjuanes, and V. de Lorenzo. 2000. Specific secretion of active single-chain Fv antibodies into the supernatants of Escherichia coli cultures by use of the hemolysin system. Appl. Environ. Microbiol. 66:5024-5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henderson, I. R., and J. P. Nataro. 2001. Virulence functions of autotransporter proteins. Infect. Immun. 69:1231-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson, I. R., F. Navarro-Garcia, and J. P. Nataro. 1998. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 6:370-378. [DOI] [PubMed] [Google Scholar]
- 11.Hornick, C. L., and F. Karush. 1972. Antibody affinity. 3. The role of multivalence. Immunochemistry 9:325-340. [DOI] [PubMed] [Google Scholar]
- 12.Jurado, P., D. Ritz, J. Beckwith, V. de Lorenzo, and L. A. Fernandez. 2002. Production of functional single-chain Fv antibodies in the cytoplasm of Escherichia coli. J. Mol. Biol. 320:1-10. [DOI] [PubMed] [Google Scholar]
- 13.Kjaergaard, K., H. Hasman, M. A. Schembri, and P. Klemm. 2002. Antigen 43-mediated autotransporter display, a versatile bacterial cell surface presentation system. J. Bacteriol. 184:4197-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamm, M. E. 1997. Interaction of antigens and antibodies at mucosal surfaces. Annu. Rev. Microbiol. 51:311-340. [DOI] [PubMed] [Google Scholar]
- 15.Lattemann, C. T., J. Maurer, E. Gerland, and T. F. Meyer. 2000. Autodisplay: functional display of active beta-lactamase on the surface of Escherichia coli by the AIDA-I autotransporter. J. Bacteriol. 182:3726-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazanec, M. B., C. S. Kaetzel, M. E. Lamm, D. Fletcher, and J. G. Nedrud. 1992. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc. Natl. Acad. Sci. USA 89:6901-6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pack, P., K. Müller, R. Zahn, and A. Plückthun. 1995. Tetravalent miniantibodies with high avidity assembling in Escherichia coli. J. Mol. Biol. 246:28-34. [DOI] [PubMed] [Google Scholar]
- 18.Sola, I., J. Castilla, B. Pintado, J. M. Sanchez-Morgado, B. Whitelaw, J. Clark, and L. Enjuanes. 1998. Transgenic mice secreting coronavirus neutralizing antibodies into the milk. J. Virol. 72:3762-3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staats, H. F., R. J. Jackson, M. Marinaro, I. Takahashi, H. Kiyono, and J. R. McGhee. 1994. Mucosal immunity to infection with implications for vaccine development. Curr. Opin. Immunol. 6:572-583. [DOI] [PubMed] [Google Scholar]
- 20.Valls, M., S. Atrian, V. de Lorenzo, and L. A. Fernandez. 2000. Engineering a mouse metallothionein on the cell surface of Ralstonia eutropha CH34 for immobilization of heavy metals in soil. Nat. Biotechnol. 18:661-665. [DOI] [PubMed] [Google Scholar]
- 21.Veiga, E., V. de Lorenzo, and L. A. Fernandez. 1999. Probing secretion and translocation of a beta-autotransporter using a reporter single-chain Fv as a cognate passenger domain. Mol. Microbiol. 33:1232-1243. [DOI] [PubMed] [Google Scholar]
- 22.Veiga, E., E. Sugawara, H. Nikaido, V. de Lorenzo, and L. A. Fernandez. 2002. Export of autotransported proteins proceeds through an oligomeric ring shaped by C-terminal domains. EMBO J. 21:2122-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Worn, A., and A. Plückthun. 2001. Stability engineering of antibody single-chain Fv fragments. J. Mol. Biol. 305:989-1010. [DOI] [PubMed] [Google Scholar]