Abstract
An essential step in human immunodeficiency virus type 1 (HIV-1) replication is the movement of the viral preintegration complex from the cytoplasm into the nucleus. The pathway(s) and timing for HIV-1 DNA nuclear entry in cycling cells have not been established. Here, we show that if cycling cells are infected before S phase, viral DNA can be integrated prior to passage of the host DNA replication fork through the integration site, as indicated by stable inheritance in both daughter cells. We conclude that efficient nuclear entry can occur independently of mitotic nuclear disassembly in cycling cells.
Integration of human immunodeficiency virus type 1 (HIV-1) DNA into host cell DNA is an essential step in viral replication, as it allows efficient expression and stable inheritance of the viral DNA (the provirus) (5, 8). Integration is catalyzed by the virus-encoded integrase protein (IN), which is assembled with viral DNA in the “preintegration complex.” HIV-1 (a lentivirus) can infect noncycling cells, and this requires movement of the preintegration complex into the nucleus. Although several viral determinants have been implicated in facilitating nuclear import (6, 9), the process is poorly understood. It has generally been assumed that defects in HIV-1 nuclear import would not be manifested in cycling cells, as access to host DNA could occur during nuclear disassembly at mitosis (as discussed in references 1 and 19). To define precisely the early events in HIV-1 infection, we asked if nuclear import occurred during interphase or was restricted to the short mitotic window in cycling cells.
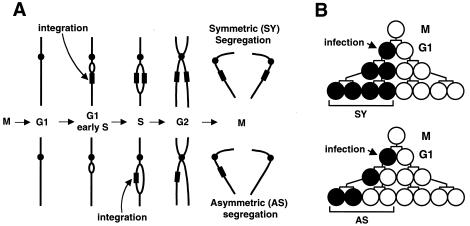
Experimental design: timing of HIV integration in cycling cells as measured by segregation of proviruses to daughter cells.
As an unequivocal indicator of when in the cell cycle HIV-1 DNA nuclear import and integration occur, we monitored integration with respect to passage of the host DNA replication fork through the integrated DNA (10). If integration occurs at a site in unreplicated host DNA, the provirus will be duplicated when that site is replicated during S phase and will be inherited by both daughter cells after mitosis (symmetric [SY] segregation) (Fig. 1A). If integration occurs at a site that has already been replicated (e.g., in late S, G2, or postmitosis), only one sister chromatid will carry the provirus and, accordingly, it will be inherited by only one daughter cell (asymmetric [AS] segregation). We established an experimental system whereby access to unreplicated host DNA would require nuclear import during interphase. Segregation was monitored by following viral green fluorescent protein (GFP) reporter expression during colony outgrowth of singly infected cells (Fig. 1B), as described in detail below.
FIG. 1.
Experimental design and interpretation. (A) Diagram showing the outcome of retroviral DNA integration into unreplicated (top) or replicated host DNA (bottom) in cells progressing through the cell cycle. Host DNA is depicted as a single acrocentric chromosome. Integration into replicated DNA is depicted during S but could occur during G2 or post-mitosis. If mitosis is required for nuclear entry and integration, only AS segregation would be observed. See text for a further description. (B) Experimental design. Mitotic cells (M) were prepared by shake-off. After entry into G1, cells were infected with HIV-1, ASV, or MLV GFP vectors. Pedigrees show outgrowth of the colony after synchronous cell division. Open and closed circles indicate uninfected and infected cells, respectively. Diagram shows predicted outcomes if integration occurs in unreplicated (top) or replicated (bottom) host DNA, resulting in SY or AS segregation, respectively.
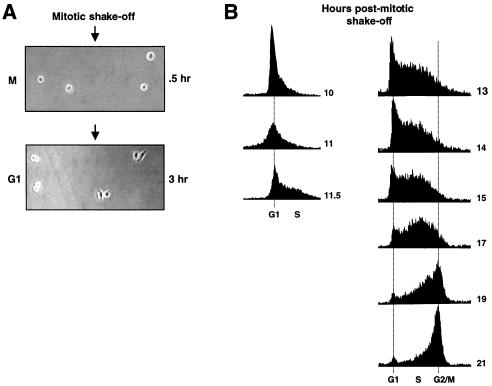
Initially, synchronized cells were infected early in the cell cycle, allowing maximum time for viral reverse transcription and integration to occur prior to completion of S phase. G1-synchronized HeLa cells were prepared by mitotic shake-off. To increase the percentage of mitotic cells, cultures were treated with nocodazole (16 ng/ml) for 2 to 4 h. Mitotic cells were released by tapping and collected by centrifugation. After washing in fresh medium, cells were resuspended and plated (ca. 1 × 103 to 2 × 103 cells per 150 mm culture dish). Synchronization was monitored in parallel cultures by FACScan analyses for DNA content (Fig. 2B). Plating efficiency was ca. 30%, and ca. 80% of the plated cells entered G1 as indicated by the appearance of cell doublets (Fig. 2A). In most experiments, G1 cell doublets were marked manually prior to infection to ensure that all cells observed during GFP readout had entered G1 at the time of infection. A majority of cell doublets continued synchronous growth up to the 8- and 16-cell stages. To achieve this high efficiency of synchronization, we had to test HeLa cells from several sources. The culture chosen (obtained from Tim Yen, Fox Chase Cancer Center, Philadelphia, Pa.) showed efficient synchronous outgrowth from single cells and also produced compact colonies, which aided in scoring GFP segregation. GFP expression was typically recorded at 48 to 72 h postinfection. Colonies of 8 cells, and occasionally of 4 and 16 cells, were scored. The majority of GFP-positive colonies gave informative readout; that is, the cell number was as expected from synchronous outgrowth. Approximately 10 to 50 GFP-positive colonies were analyzed per experiment, and each point represents at least three independent experiments. Digital micrographs were acquired as described previously (12).
FIG. 2.
Synchronization of HeLa cells and delineation of S phase. (A) Mitotic HeLa cells were prepared by brief nocodazole treatment, shake-off, and replating. Phase-contrast micrographs of cells after replating and attachment (top, 0.5 h after plating) or after G1 entry (bottom, 3 h after plating) are shown. Different fields are shown in the two micrographs. Cell doublets that are flat, clearly indicating G1 entry (e.g., two colonies on the right), were typically marked prior to infection such that subsequent GFP segregation could be attributed to infection of synchronized cells. (B) HeLa cells were prepared by mitotic shake-off as in panel A and were analyzed for DNA content by FACScan analysis with standard techniques. Series on the left shows entry into S phase, and series on the right shows exit from S phase (from separate synchronization experiments). Time, in hours post-mitotic shake-off, is indicated to the right of each graph.
HIV-1 infection during early G1 results in frequent SY segregation of the provirus to both daughter cells, indicating nuclear import and integration prior to mitosis.
Cultures were infected at 3 h after G1 entry (Fig. 1B) with a replication-defective HIV-1 vector encoding the EGFP gene under control of the CMV-IE promoter-enhancer, pPCW-eGFP (4). This vector was prepared by cotransfecting 293T cells with the packaging plasmids described previously (16). This HIV-1 vector encodes the DNA flap, which was shown to be important for nuclear import (19). The HIV-1 vector packaging system provides Gag and Pol proteins, Vpr, and the VSV-G surface protein to mediate cellular entry. As the vector genome is devoid of all replicative genes, no virus spread can occur within the colony. For preparation of vector stocks, virus-containing cell supernatants were passed through a 0.45-μm-pore-size filter to eliminate transfer of GFP-expressing producer cells during the infection. HeLa cells were infected by exposure to virus for 2 h. To interpret the GFP segregation patterns, it is important that the primary infected cell contain a single integrated provirus. Viral vector stocks were therefore diluted to the point where only 10 to 20% of colonies were GFP positive, corresponding to an effective multiplicity of infection (MOI) of less than 0.05 to 0.1 (correcting for two cells per colony at the time of infection). By Poisson distribution analyses, under these conditions the fraction of cells that experienced two integration events is negligible (0.0045 to 0.0012).
Integration and segregation of proviral DNA were monitored by following GFP reporter expression in daughters and granddaughters of the infected cell by using fluorescence microscopy. At a low MOI, only one of the two G1 cells per doublet will be infected in the vast majority of colonies (Fig. 1B). The uninfected bystander cell served as an internal control to monitor synchronous outgrowth of the colony and to confirm that there is no unusual cell-to-cell transmission of GFP. Due to outgrowth of one uninfected and one infected cell, SY provirus segregation would result in 50% GFP-positive cells per colony, while AS segregation would produce 25% GFP-positive cells (Fig. 1B). Under these experimental conditions, significant GFP expression was not detected after infection with an HIV-1 vector carrying an inactivating mutation in integrase (16) (data not shown), confirming that the observed GFP segregation required vector DNA integration. We also confirmed that GFP readout directly correlated with integrated DNA by first passaging parallel infected cultures to eliminate unintegrated DNA, followed by sorting of GFP-positive and -negative cells and measurement of viral DNA by quantitative real-time PCR (data not shown). As demonstrated below, SY segregation was dependent on the infection time with respect to S phase, essentially ruling out the possibility that SY colonies could result from multiple integration events (i.e., in different chromosomes).
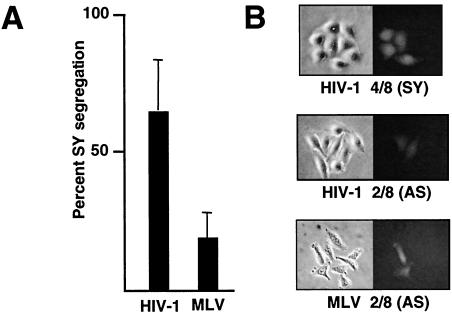
HIV-1 vector infection at 3 h into G1 resulted in 64% ± 19% GFP-positive SY colonies (Fig. 3A and B). The remaining GFP-positive colonies scored as AS type, as expected (Fig. 3B). The high percentage of SY segregation is consistent with efficient integration into unreplicated host DNA and duplication to both sister chromatids when the host DNA replication fork passed through the newly integrated provirus. Integration into unreplicated host DNA requires that nuclear import of the preintegration complex occur prior to the end of S phase. We also infected HeLa cells synchronized 3 h post-entry into G1 with a similar murine leukemia virus (MLV)-based GFP vector (pLEGFP-C1; BD Biosciences) that was prepared by transfecting the AmphoPack-293 cell line (BD Biosciences). MLV is believed to be more dependent on mitosis for nuclear import (17), and the percentage of SY segregants was lower, 19% ± 9% (Fig. 3A). However, as described below, this level of SY segregation is significantly above the background of the assay. We also note that the infection time (early in G1) strongly favors detection of mitosis-independent integration into unreplicated DNA. These results indicate that, under these conditions, MLV may not be strictly dependent on mitosis for nuclear entry in cycling cells. Recently, we (12) and others (11) have demonstrated that an alpharetrovirus, avian sarcoma virus (ASV), can infect noncycling cells, implying the existence of a mitosis-independent nuclear import pathway for this virus. We found that early-G1 infection with an ASV-GFP vector (12) resulted in a lower percentage of SY segregation (35% ± 7%) than was observed with the HIV-1 vector. However, the readout may be an underestimate of SY segregation due to rapid variegation of GFP expression within a subset of colonies (not apparent with HIV-1 or MLV vectors), which we confirmed to be due to gene silencing (unpublished data).
FIG. 3.
Frequency of SY segregation after infection early in G1. (A) Percentage of SY GFP colony patterns after infection of HeLa cell doublets, 3 h into G1 with HIV-1 or MLV GFP vectors. Standard deviations (error bars) are shown. (B) Representative patterns of GFP expression after infection of G1 cell doublets with the HIV-1 and MLV GFP vectors and subsequent synchronous outgrowth. Examples of AS and SY patterns are shown.
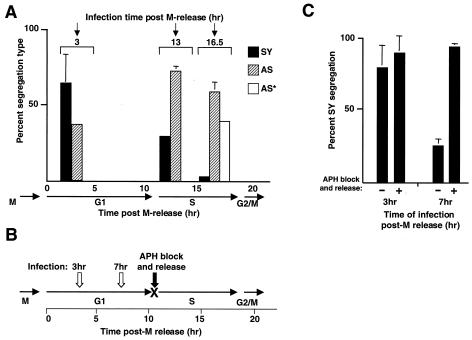
The simplest interpretation of the segregation pattern observed after HIV-1 vector infection predicts that, as more of the host DNA is replicated, the probability of integration into unreplicated DNA will decrease. To test this prediction, HeLa cells were synchronized as described in the Fig. 2 legend and were infected with the HIV-1 GFP vector at 13 and 16.5 h post-release into G1. As shown in Fig. 4A, there was a decrease in the percentage of SY colonies after infection at 13 h (within S phase) compared to 3 h, with a concomitant increase in AS colonies, and these results support our interpretations of the segregation patterns. To provide biochemical support for our interpretations, we measured the timing of integration with the Alu-PCR method. To facilitate detection, scaled-up monolayer cultures were infected at a high MOI 3 h post-release into G1. HIV-1 DNA integration could be detected prior to the end of S phase, as expected (data not shown).
FIG. 4.
Effect of infection time and cell cycle delay on frequencies of SY and AS segregation. (A) Effect of infection time on frequencies of SY and AS segregation. Cells were prepared by mitotic shake-off as described in the legend to Fig. 2 and were infected with the HIV-1 GFP vector at 13 or 16.5 h post-M phase, as indicated at the top. As a comparison, data for the 3-h time point are reproduced from Fig. 3A. The timing of cell cycle stages (determined in Fig. 2B) is shown below the graph. Colonies were scored according to SY, AS, or AS* patterns, and the results are expressed as a percentage of total informative GFP colonies for each time point. The sum of columns at each time point is 100%. Standard deviations (error bars) are shown for the most prominent colony type. (B) Effect of cell cycle delay on frequencies of SY and AS segregation. Diagram of experimental design is shown. Cells were infected at 3 or 7 h after G1 entry (open arrows) and were treated with aphidicolin (APH) for 18 h and released (filled arrow) or were left untreated as a control. The transient drug-induced G1/S arrest is indicated (X). (C) Results of experiment diagrammed in panel B. Quantitation of SY segregation as a percentage of total of informative GFP-positive colonies is shown.
After infection at 16.5 h into the cell cycle (late S phase), SY colonies were observed only rarely (Fig. 4A), consistent with the depletion of unreplicated host DNA sites. This low percentage of SY colonies establishes the background of the assay.
Provirus segregation patterns after HIV infection late in the cell cycle suggest that mitosis may cause a delay in integration.
Infection at 16.5 h resulted in loss of the SY pattern and concomitant increase in the AS pattern (2/8) as well as a novel 1/8 pattern (marked as AS* in Fig. 4A). We believe that the AS* pattern is informative, as follows: infection near the end of S phase (16.5 h) increases the probability that cells will pass through mitosis (ca. 21 h) prior to viral DNA integration. If mitosis were the primary route of nuclear entry after infection late in the cell cycle and if integration occurred during G1 in one of four daughter cells, only the AS pattern would be expected. The AS* pattern (1/8) indicates that integration did take place after mitosis but was delayed, as it must have occurred in a replicated site (i.e., after entry into S phase) in the DNA of one of the four daughter cells. Furthermore, we note that the SY/AS ratio produced from infection at 3 h is similar to the AS/AS* ratio from the 16.5-h infection. Infection at 16.5 h led therefore to the pattern expected if the cells had already divided, with one of the four daughters being infected early in the next G1. The delay in integration indicated by the AS* pattern is inconsistent with rapid integration after mitosis but rather suggests a requirement for reestablishment of an interphase-dependent import pathway after mitosis. We also found that the overall efficiency of GFP transduction was not enhanced by infecting just prior to mitosis (i.e., 16.5 h) (data not shown), implying that mitosis did not promote access to host DNA. Although other interpretations are possible, these results suggest that, for HIV-1, nuclear entry during mitosis may not be a significant pathway and that integration may be tightly coupled with the import pathways used during interphase or in noncycling cells. We note that AS* (or AS) segregation could also reflect a delay in integration due to a requirement for S-phase-specific factors, rather than a delay in nuclear import.
A transient delay in S-phase entry confirms that SY segregation of proviruses is the result of nuclear import and integration during interphase.
As a further test of these interpretations, we asked if a block in the cell cycle at the start of S phase would increase the frequency of SY segregation by “forcing” integration into unreplicated host DNA. HeLa cells were synchronized by mitotic shake-off as described in the Fig. 2 legend and were infected with the HIV-1 GFP vector at 3 h or 7 h into G1 and then were either treated with aphidicolin (2 μg/ml) or were left untreated as a control (Fig. 4B). The effectiveness of the aphidicolin-induced G1/S block was confirmed by the persistence of two-cell colonies compared to the continued cell division observed in the untreated control cultures. After 18 h of treatment, the aphidicolin was removed, colony outgrowth resumed, and GFP expression was monitored. In the untreated control, infection at 3 h resulted primarily in SY segregation (79%) as expected, and introduction of the transient G1/S arrest enhanced the percentage of this pattern slightly (90%) (Fig. 4C). In the 7-h untreated control infection, we observed a reduction in SY colonies relative to the 3-h infection, as would be predicted from the data in Fig. 4A. Introduction of the transient G1/S arrest after infection at 7 h led to a dramatic shift from 25 to 94% SY colonies (Fig. 4C). These results show that the transient delay in S-phase entry promotes SY segregation, as would be predicted if SY colonies were produced by integration into unreplicated host DNA.
Summary and conclusions.
The experiments described here were designed to measure the timing of HIV-1 DNA integration with respect to the S phase and mitosis in synchronized, cycling cells. Typically, retroviral DNA metabolism is monitored biochemically by using DNA blotting or PCR-based methods. However, it is clear that such methods vary widely in sensitivity, possibly leading to significant discrepancies with respect to the timing of HIV-1 integration (18). Furthermore, a significant portion of HIV-1 DNA does not become integrated (3, 18) and the rare circular forms of viral DNA, commonly followed as markers for nuclear entry, are dead-end products. Here we implemented a system that allowed us to follow single integration events within synchronized cell populations. We measured the relative frequencies of integration events that occur either before (SY segregation), or after (AS segregation), the host DNA replication fork has passed through the integration site during S phase. We therefore could monitor the timing of nuclear import of only those viral DNAs that ultimately become integrated. We confirmed that SY and AS segregation reflected the timing of nuclear import and integration, as the SY/AS ratio was affected by the timing of infection, as well as a delay in S-phase entry (Fig. 4). Our results are consistent with fairly rapid nuclear import and integration (3, 18), as infection during early G1 resulted in a significant percentage of integration into unreplicated DNA (i.e., during G1 or early S phase), and this may reflect a tight coupling between nuclear import and integration. In this regard, our results also provide some evidence that a mitosis-based pathway is not a significant alternative nuclear entry route for HIV-1 in cycling cells and that nuclear import and/or integration may actually be delayed by passage through mitosis.
It should be noted that the timing of integration measured here reflects the net efficiency of all upstream steps, including viral entry, cytoplasmic trafficking, and reverse transcription. Therefore, the differences in the SY/AS ratio noted among HIV-1, ASV, and MLV vectors do not necessarily reflect differences in the efficiencies of nuclear import. Pseudotyping of the HIV-1 and ASV vectors with the VSV-G and murine amphotropic Env proteins, respectively, provides unnatural entry routes for these viruses that may influence the timing of nuclear access. Therefore, our results formally describe the behavior of these pseudotyped vectors rather than of the natural viral counterparts. Despite this caveat, our results indicate that mitosis-independent nuclear import of the HIV-1 preintegration complex is quite efficient in cycling cells.
The results described here provide important new insights into the mechanism(s) and timing by which lentiviral DNA gains access to and integrates in the host nuclear DNA in cycling cells. Previous studies by Roe et al. (17) and others (10, 13) have indicated that the nuclear entry pathway for the prototypic retrovirus MLV, a gammaretrovirus, is primarily mitosis dependent, with integration of viral DNA occurring soon after nuclear reassembly. Here we provide evidence that MLV nuclear entry is not strictly dependent on mitosis in cycling cells. However, this requirement for mitosis is proposed to account, in part, for the dependency on cell cycling for efficient infection by MLV-based vectors. In contrast to MLV, HIV-1 can infect noncycling cells efficiently. Several viral determinants have been implicated in promotion of HIV nuclear import, including three viral proteins (MA, IN, and Vpr), as well as a DNA flap structure (6, 9, 19). However, roles for the DNA flap and IN nuclear localization signal (NLS) have recently been questioned (7, 14, 15). One early report presented evidence that a mutation in the HIV-1 MA nuclear localization signal affected replication in noncycling, but not in cycling, cells (2). Although a role for this MA NLS has also been controversial, these results have been interpreted to indicate that, in cycling cells, HIV-1 uses a mitosis-dependent nuclear import pathway similar to that described for MLV (discussed in references 1 and 19). Here we show that, after infection early in the cell cycle, efficient HIV-1 integration can occur in unreplicated host DNA, implicating a mitosis-independent pathway in cycling cells. The HIV-1 determinants that are required for this interphase import pathway remain to be identified. Preliminary experiments indicate that use of an HIV-1 packaging system deficient in Vpr, Nef, and Vif results in a ca. 2-fold decrease in SY segregation (data not shown). Although the determinants remain to be fully characterized, our results raise the prospect that the HIV-1 nuclear import pathway may be a rational target for therapeutic intervention in both noncycling and cycling cells.
Acknowledgments
We thank Peter Adams, Vincent Guacci, Bill Mason, and Tim Yen for critical comments on the manuscript and Sam Litwin (Fox Chase Cancer Center Biostatistics Facility) for helpful discussions. We are also grateful to John Kappes for providing the HIV-1 GFP vector and to Didier Trono for providing the HIV vector packaging plasmids.
This work was supported by National Institutes of Health grants AI40385, CA71515, and CA06927 and also by an appropriation from the Commonwealth of Pennsylvania.
The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or any other sponsoring organization.
REFERENCES
- 1.Bouyac-Bertoia, M., J. D. Dvorin, R. A. Fouchier, Y. Jenkins, B. E. Meyer, L. I. Wu, M. Emerman, and M. H. Malim. 2001. HIV-1 infection requires a functional integrase NLS. Mol. Cell 7:1025-1035. [DOI] [PubMed] [Google Scholar]
- 2.Bukrinsky, M. I., S. Haggerty, M. P. Dempsey, N. Sharova, A. Adzhubel, L. Spitz, P. Lewis, D. Goldfarb, M. Emerman, and M. Stevenson. 1993. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature 365:666-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler, S. L., M. S. Hansen, and F. D. Bushman. 2001. A quantitative assay for HIV DNA integration in vivo. Nat. Med. 7:631-634. [DOI] [PubMed] [Google Scholar]
- 4.Chen, W., X. Wu, D. N. Levasseur, H. Liu, L. Lai, J. C. Kappes, and T. M. Townes. 2000. Lentiviral vector transduction of hematopoietic stem cells that mediate long-term reconstitution of lethally irradiated mice. Stem Cells 18:352-359. [DOI] [PubMed] [Google Scholar]
- 5.Coffin, J. M., S. H. Hughes, and H. Varmus. 1997. Retroviruses. Cold Spring Harbor Laboratory Press, Plainview, N.Y. [PubMed]
- 6.Cullen, B. R. 2001. Journey to the center of the cell. Cell 105:697-700. [DOI] [PubMed] [Google Scholar]
- 7.Dvorin, J. D., P. Bell, G. G. Maul, M. Yamashita, M. Emerman, and M. H. Malim. 2002. Reassessment of the roles of integrase and the central DNA flap in human immunodeficiency virus type 1 nuclear import. J. Virol. 76:12087-12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flint, S. J., L. W. Enquist, R. M. Krug, V. R. Racaniello, and A. M. Skalka. 2000. Principles of virology: molecular biology, pathogenesis, and control. ASM Press, Washington, D.C.
- 9.Fouchier, R. A., and M. H. Malim. 1999. Nuclear import of human immunodeficiency virus type-1 preintegration complexes. Adv. Virus Res. 52:275-299. [DOI] [PubMed] [Google Scholar]
- 10.Hajihosseini, M., L. Iavachev, and J. Price. 1993. Evidence that retroviruses integrate into post-replication host DNA. EMBO J. 12:4969-4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatziioannou, T., and S. P. Goff. 2001. Infection of nondividing cells by Rous sarcoma virus. J. Virol. 75:9526-9531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz, R. A., J. G. Greger, K. Darby, P. Boimel, G. F. Rall, and A. M. Skalka. 2002. Transduction of interphase cells by avian sarcoma virus. J. Virol. 76:5422-5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis, P. F., and M. Emerman. 1994. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J. Virol. 68:510-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Limon, A., E. Devroe, R. Lu, H. Z. Ghory, P. A. Silver, and A. Engelman. 2002. Nuclear localization of human immunodeficiency virus type 1 preintegration complexes (PICs): V165A and R166A are pleiotropic integrase mutants primarily defective for integration, not PIC nuclear import. J. Virol. 76:10598-10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Limon, A., N. Nakajima, R. Lu, H. Z. Ghory, and A. Engelman. 2002. Wild-type levels of nuclear localization and human immunodeficiency virus type 1 replication in the absence of the central DNA flap. J. Virol. 76:12078-12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naldini, L., U. Blömer, P. Gallay, D. Ory, R. Mulligan, F. H. Gage, I. M. Verma, and D. Trono. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272:263-267. [DOI] [PubMed] [Google Scholar]
- 17.Roe, T.-Y., T. C. Reynolds, G. Yu, and P. O. Brown. 1993. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 12:2099-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandegraaff, N., R. Kumar, C. J. Burrell, and P. Li. 2001. Kinetics of human immunodeficiency virus type 1 (HIV) DNA integration in acutely infected cells as determined using a novel assay for detection of integrated HIV DNA. J. Virol. 75:11253-11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zennou, V., C. Petit, D. Guetard, U. Nerhbass, L. Montagnier, and P. Charneau. 2000. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell 101:173-185. [DOI] [PubMed] [Google Scholar]