Abstract
Papillomaviruses replicate in stratified epithelia of skin and mucosa. Infection with certain human papillomavirus (HPV) types is the main cause of anogenital neoplasia, in particular cervical cancer. Early events of papillomavirus infectivity are poorly understood. While heparan sulfate proteoglycans (HSPGs) mediate initial binding to the cell surface, the class of proteins carrying heparan sulfates has not been defined. Here we examined two processes of papillomavirus infection, attachment of virus-like particles (VLP) to cells and infection with authentic HPV type 11 (HPV11) virions. Of the HSPGs, syndecan-1 is the major epithelial form and is strongly upregulated in wound edge keratinocytes. We employed K562 cells, which lack HSPGs except minor amounts of endogenous betaglycan, and stable clones that express cDNAs of syndecan-1, syndecan-4, or glypican-1. Binding of VLP correlated with levels of heparan sulfate on the cell surface. Parental K562 bound HPV16 VLP weakly, whereas all three K562 transfectants demonstrated enhanced binding, with the highest binding capacity observed for syndecan-1-transfected cells, which also expressed the most HSPG. For HPV11 infectivity assays, a high virion inoculum was required to infect K562 cells, whereas ectopic expression of syndecan-1 increased permissiveness eightfold and expression of syndecan-4 or glypican-1 fourfold. Infection of keratinocytes was eliminated by treatment with heparitinase, but not phospholipase C, further implicating the syndecan family of integral membrane proteins as receptor proteins. Human keratinocytes with a homozygous deletion of α6 integrin are permissive for HPV11 infection. These results indicate that several HSPGs can serve as HPV receptors and support a putative role for syndecan-1, rather than α6 integrin, as a primary receptor protein in natural HPV infection of keratinocytes.
Papillomaviruses comprise a large group of species- and tissue-specific DNA tumor viruses found in higher vertebrates from chaffinches to humans and include more than 90 known human papillomavirus (HPV) genotypes. Papillomaviruses cause mainly benign epithelial papillomas or warts on skin and mucosa (condylomata acuminata). Several high-risk HPV types, most often HPV type 16 (HPV16), are the primary etiologic agents for anogenital malignancy, in particular cervical cancer, which is the second most common cause of cancer-related deaths in women worldwide (41, 57). The papillomavirus virion consists of a nonenveloped capsid comprised of the L1 major and L2 minor structural proteins surrounding a minichromosome of ∼8 kb of double-stranded closed circular and histone-associated DNA. Even in the absence of other viral proteins, the L1 protein self-assembles into empty capsids or virus-like particles (VLP) (24). Subunit vaccines based on VLP have been developed, and prophylactic immunizations have demonstrated safety and efficacy in preventing papillomavirus infection and associated neoplasia (18, 23, 25, 28, 44).
Papillomaviruses do not complete their life cycle leading to productive infection without concomitant differentiation of the epithelial cell and thus there is no efficient system to propagate virions in tissue culture. At least in part due to these difficulties, the search for the cellular receptor for papillomaviruses has so far been unsuccessful and the mechanisms of viral entry into the host cell are unclear. Experimental models to study early aspects of HPV infection include transient infection of keratinocytes or epithelial cell lines with authentic virions (50). Limited amounts of HPV virions can be isolated from natural lesions, from foreskin chips propagated in immunocompromised animals or from keratinocyte raft cultures that have been used to analyze papillomavirus entry and neutralization (9, 32). More recently, infectivity assays have been developed by using pseudovirions that consist of papillomavirus capsids enclosing a marker genome (36, 53). In addition, VLP have been employed to demonstrate that papillomaviruses bind to cells across many types and species, indicating that the attachment moiety is proteinaceous and highly conserved (39, 56). One model suggests that cell attachment is initiated by interaction of the viral capsid with a primary receptor of relatively low specificity and proceeds by binding to a secondary receptor that results in internalization and infection (21). Infection with native papillomaviruses or pseudovirions or VLP uptake into intracellular vesicles has been reported to occur via either clathrin- or caveolae-mediated endocytosis, with delayed uptake kinetics (4, 11, 13, 33, 39, 56). α6 integrin was proposed as a putative papillomavirus receptor based on the requirement for HPV6 VLP binding to cells, although this has been questioned (15, 48). However, many models suffer from the mismatch of virus and host cells and the difficulties in distinguishing protein uptake from authentic papillomavirus infection.
Many enveloped and nonenveloped viruses use heparan sulfate proteoglycans (HSPGs) as primary attachment molecules to enter cells (6, 17, 20, 21, 47). HSPGs also serve as coreceptor molecules mediating binding for numerous viruses, bacteria, and protozoa, e.g., herpes simplex virus (HSV), Neisseria, and Plasmodium. However, the particular HSPG required for infectivity has not been examined for any viruses, with the exception of human immunodeficiency virus type 1 (43).
Cell surface heparan sulfates are mostly members of two major gene families of membrane-bound proteoglycans, the syndecans and glypicans (reviewed in reference 2). Syndecans and glypicans bind proteins of the extracellular environment via their heparan sulfate chains, regulating a wide spectrum of biological activities, including cell proliferation and differentiation, morphogenesis, wound repair, and host defense. The syndecans comprise a family of four distinct genes encoding integral membrane proteins. The ectodomains are extended proteins that carry heparan sulfate chains distal from and chondroitin sulfates close to the plasma membrane. The glypicans are a family of at least six different gene products that are linked to the cell membrane via a glycosylphosphatidylinositol (GPI) anchor. The glypican core proteins are likely to be globular and place heparan sulfate chains adjacent to the plasma membrane. Other part-time proteoglycans such as CD44 or betaglycan may bear heparan sulfate chains under certain conditions only. Syndecans and glypicans are expressed in a cell-, tissue-, and development-specific fashion. Syndecan-1 is the predominant HSPG in mammary epithelia and its expression is increased during epithelial differentiation and wound healing and decreased during malignant transformation, whereas syndecan-4 is expressed in focal adhesions of adherent cells. The ectodomains of both syndecan-1 and -4 are shed from the cell surface by proteases and released into wound fluids. The glypicans are expressed predominantly in the central nervous system, but glypican-1 is widely expressed in tissues including the epidermis and hair follicles (12, 29).
Heparan sulfate glucosaminoglycans (GAG) have also been proposed as the primary cell surface moieties mediating interaction with papillomaviruses (17, 21, 46). However, the identity of the specific core protein(s) carrying heparan sulfate GAG and its contribution to cellular interaction with papillomaviruses have not yet been identified. Therefore, we wanted to examine these questions for papillomaviruses, in particular which HSPG(s) supports attachment and infection of keratinocytes, the natural host cells for HPV infection.
MATERIALS AND METHODS
Cell lines.
Keratinocytes deficient for α6 integrin expression (BOUA-SV) were derived from a patient with junctional epidermolysis bullosa carrying a homozygous mutation in the integrin α6 gene (16), and KH-SV keratinocytes were derived from a healthy donor. Both cell lines were immortalized by simian virus 40. Cells were maintained at 37°C as monolayers in serum-free, low-Ca2+ (0.15 M) keratinocyte growth medium (KGM) supplemented with bovine pituitary extract (Clonetics/BioWhittaker). To express α6 integrin ectopically, BOUA-SV keratinocytes were infected with retroviral vectors encoding either human α6 integrin (BOUA-SV-α6) or a neomycin resistance gene only (BOUA-SV-neo) (54). Cell lines were selected and passaged in neomycin-containing KGM, and expression of α6 and β4 integrins was verified by flow cytometry. The erythroleukemia cell line K562, either wild type (wt) or stably transfected with expression vectors coding for syndecan-1, syndecan-4, glypican-1, or empty hygromycin B vector control (pRep4), was generously provided by Guido David, Louvain, Belgium (51). Cells were grown as a suspension culture in RPMI 1640 medium (Gibco) supplemented with 10% fetal calf serum (FCS) (Gibco) and 200 μg of hygromycin B (Gibco) per ml. Sf-9 insect cells were grown at 27°C as suspension cultures in Grace's insect medium supplemented with 5% FCS.
Monoclonal antibodies.
For flow cytometry analysis, monoclonal antibodies (MAb) against human α6 integrin (CD 49f-FITC; PharMingen) and anti-human β4 integrin (CD 104-PE; PharMingen) were used at a 1:20 dilution. Antibodies directed against betaglycan (TGF-β RIII; R&D Systems), heparan sulfate (F58-10E4; Seikagaku), syndecan-1 (B-B4; HybriDomus), syndecan-4 (F94-8G3), or glypican-1 (F48-S1) (the last MAb was kindly provided by G. David [51]) were used at 2 μg/μl. The nonneutralizing MAb Camvir-1 (Pharmingen) is directed against a linear epitope of HPV16 L1 (31). The neutralizing mouse MAbs H11.B2 and H16.E70 are directed against conformational L1 epitopes of the HPV11 or HPV16 capsid surface, respectively, and are capable of preventing VLP from binding to cells (8, 38, 49).
Baculovirus expression and purification of HPV16 L1 VLP.
Recombinant baculoviruses were generated as described previously (24). Sf-9 insect cells were infected at a high multiplicity of infection (MOI) of ∼10 and harvested after 3 days. High-molecular-mass structures were separated from cell lysates by use of a 35% sucrose cushion followed by cesium chloride (CsCl) density gradient equilibrium centrifugation. Visible bands were collected and extensively dialyzed against phosphate-buffered saline (PBS)-0.5 M NaCl-0.05% NaN3, and aliquots were examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie staining (26). Purified VLP were adsorbed on glow-discharged carbon-coated copper grids for 10 min, dried briefly on filter paper, floated on a drop of glutaraldehyde (2.5% in 0.1 M sodium cacodylate buffer, pH 7.0), and negatively stained with uranyl acetate (1% in distilled water). Finally, grids were dried on filter paper and examined in a JEOL EM 1010 or a Philips CM 100 transmission electron microscope at 80 kV, with an objective aperture of 30 μm (49).
Flow cytometry analysis.
Keratinocyte cell lines were washed with PBS and incubated for 10 min with 5 mM EDTA-PBS at 37°C for detachment. K562 cells and keratinocytes were washed twice with cold 2 mM EDTA-PBS (Gibco) containing 0.5% bovine serum albumin (MACS buffer). MAbs were used at saturating concentrations of 20 μg/μl. Cells (105) were incubated on ice for 1 h with antibodies in a total volume of 100 μl, washed twice with MACS buffer, incubated with fluorescein isothiocyanate-labeled goat anti-mouse immunoglobulin G (Alexa Fluor 488; Molecular Probes), and analyzed on a Becton Dickinson FACScan. Results are expressed in fluorescence units (log scale; experimental result minus isotype control antibody result); experiments were repeated twice independently.
Digestion of cells with heparitinase and PLC.
Cells (105) were washed twice with cold PBS, resuspended in digestion buffer (20 mM Tris-HCl, 50 mM NaCl, 4 mM CaCl2, 0.01% bovine serum albumin; pH 7.5), and incubated in a total volume of 100 μl at 37°C for 1 h with (or without) 10 μl of heparitinase (1.9 U/ml) (Seikagaku) or phosphatidylinositol-phospholipase C (PI-PLC) (13 U/ml) (Sigma). Cells were washed with MACS buffer and analyzed.
VLP binding assays.
KH-SV or BOUA-SV keratinocyte lines were grown as adherent monolayers and, prior to reaching confluence, were washed once with PBS, detached with 0.25% trypsin-0.02% EDTA (Gibco), and held in suspension for 120 min at 37°C. K562 cells were grown in suspension culture. Cells (105) in 50-μl volumes were washed twice with cold PBS and incubated for 60 min on ice with a 1:10 dilution of antibody (anti-α6 [CD 49f] or anti-β4 [CD 104]; 0.5 mg/ml), 30-U/ml heparitinase or 13-U/ml PI-PLC (Sigma) (27), or no treatment for 2 h at 37°C. Cells were washed twice with cold PBS, and HPV16 L1 VLP (125 ng) were added. Following incubation for 60 min on ice, cells were washed extensively with PBS, resuspended in loading buffer, boiled for 5 min, and analyzed by SDS-PAGE and Western blotting. When indicated, HPV16 L1 VLP (125 ng) were incubated for 60 min with a neutralizing mouse anti-HPV16 L1 MAb (H16.E70) (8) at a dilution of 1:10 before they were added to cells. Cell-VLP complexes were washed three times with PBS, resuspended in loading buffer, boiled for 5 min, and run on SDS-PAGE gels. Binding of L1 VLP to cells was detected by immunoblotting using the L1-specific mouse MAb Camvir-1.
Saturation of HPV16 L1 VLP binding to KH-SV or BOUA-SV cells.
Cells (105) were incubated for 1 h on ice with increasing concentrations of VLP (31 to 2,000 ng) in a total volume of 50 μl. Supernatants were separated by centrifugation and cell pellets were washed three times with PBS. Supernatants or cell lysates were loaded onto separate gels, and cell-bound or free VLP were detected with MAb Camvir-1.
Isolation of HPV11 virions from condylomata acuminata.
A surgically removed large human genital wart (wet weight, ∼4 g) was cut into small pieces, washed twice with PBS and once with extraction buffer (50 mM HEPES [pH 8], 100 mM NaCl, 0.1 mM CaCl2), and harvested by centrifugation at 500 × g for 2 min. Warts were resuspended in 20 ml of extraction buffer, homogenized twice for 1 min each on ice with an Ultra Turax T25, and centrifuged for 10 min at 10,000 × g at 4°C, and the supernatants were saved. The remaining pellet was sequentially extracted with 10 ml of extraction buffer containing 1 M NaCl and finally with buffer containing 0.05% (wt/vol) sodium deoxycholate, incubated at 4°C for 2 h, and then rehomogenized. After a final centrifugation, the supernatants were combined and run through a 0.2-μm-pore-size filter to remove contaminants, and aliquots were stored at −70°C. A Hybrid capture II test (Digene) and DNA sequencing revealed that the virus was HPV11. The concentration of HPV11 particles in the condylomata extract was calculated from the number of viral genomes analyzed by quantitative PCR in comparison to a dilution series of a known amount of cloned HPV11 genomes and was determined to be 4.5 × 106 particles per ml.
HPV11 transient infectivity assay (RT-PCR).
Keratinocytes (3 × 105 cells) were seeded into 60-mm-diameter tissue culture plates. The next day the culture medium was aspirated and cells were infected with 2 to 4 μl (unless stated differently) of HPV11 stock solution (corresponding to 9 × 103 to 18 × 103 particles) in 1 ml of KBM (Clonetics/BioWhittaker). As a specificity control, virions were incubated with neutralizing MAb H11.B2 before being added to cells as indicated previously (10). Similarly, 3 × 105 K562 cells were suspended in 1 ml of RPMI, seeded into 60-mm-diameter dishes, and infected. Cells and virus were incubated for 1 h at 37°C with gentle rocking every 15 min and then fed with 3 ml of fresh KGM or RPMI-10% FCS, respectively. After 3 days of incubation, total cellular RNA was harvested by using Tri Reagent (Molecular Research Center, Cincinnati, Ohio). For first-strand cDNA synthesis, oligo-p(dT)15 primers were used (Roche). Spliced HPV11 E1-E4 mRNA was detected by two rounds of 30-cycle nested PCR as described previously (50). The expected final sizes of the PCR amplicons were 628 bp for spliced HPV11 mRNA and 429 bp for spliced β-actin mRNA.
RESULTS
Influence of ectopic expression of syndecan-1, syndecan-4, or glypican-1 in K562 cells upon HPV16 VLP binding.
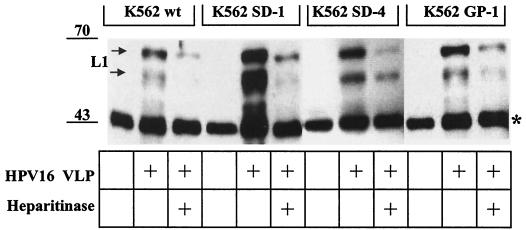
Because the heparan sulfate-carrying core protein(s) that mediates papillomavirus binding has not been defined, we investigated K562 cells transfected with members of two major families of HSPG and tested them for the capacity to bind HPV16 VLP. The erythroleukemia cell line K562 does not express syndecans and glypicans at the cell surface but expresses small amounts of betaglycan-transforming growth factor beta receptor III (TGFβR-III) (1). K562 transfectants stably expressing either syndecan-1, syndecan-4, or glypican-1 (51) were analyzed by use of in vitro attachment assays for the capacity to bind HPV16 VLP (Fig. 1). The amount of HPV16 VLP that bound K562 cells (K562 SD-1) expressing syndecan-1 was strongly enhanced compared to parental K562 cells (K562 wt). Increased binding to syndecan-4 (K562 SD-4)- and glypican-1 (K562 GP-1)-expressing cells was also observed, although at lower levels than K562 SD-1 transfectants. The amounts of bound VLP correlated with the levels of heparan sulfate at the cell surface, as determined by flow cytometry (see Fig. 3A). As expected, binding of VLP was strongly reduced by heparitinase treatment of cells (Fig. 1), which removes heparan sulfate from the cell surface (data not shown). These results suggest that prototypic members of syndecan, glypican, and betaglycan families of HSPG receptors expressed on the surfaces of K562 cells support HPV16 VLP binding. Thus, it appears that the quantity of heparan sulfate moieties rather than a specific core protein determines cell-VLP interactions.
FIG. 1.
Binding of HPV16 VLP to K562 cells and K562 transfectants expressing syndecan-1, syndecan-4, or glypican-1. Cell lines were incubated in the presence of HPV16 VLP or PBS for 1 h and washed extensively, and VLP in complex with cells were detected by immunoblotting using MAb Camvir-1. When indicated, cells were digested with heparitinase for 1 h to remove heparan sulfate from the cell surface prior to incubation with VLP. The double band at ∼50 to 55 kDa corresponds to posttranslational modification of L1. The band at ∼45 kDa (indicated by a star) represents a cellular protein cross-reacting with MAb Camvir-1 (in the absence of added VLP) that serves as an internal control for equal loading.
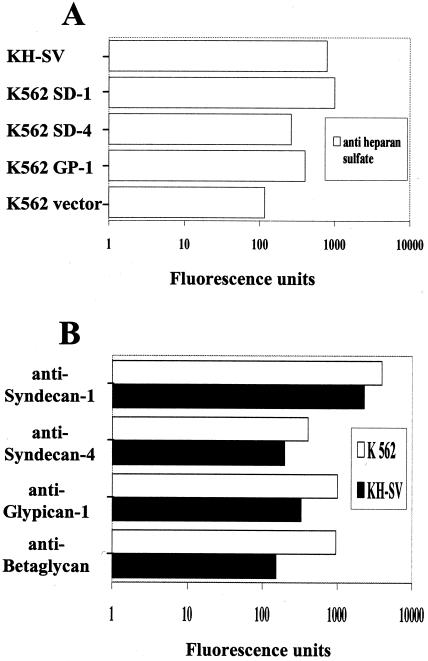
FIG. 3.
Flow cytometry analysis of KH-SV keratinocytes and K562 cell lines expressing syndecan-1 (K562 SD-1), syndecan-4 (K562 SD-4), glypican-1 (K562 GP-1), or empty eukaryotic vector (K562 vector) for the expression of heparan sulfate (A) or syndecan-1, syndecan-4, glypican-1, or betaglycan (B). Staining was performed as described in Materials and Methods using MAbs against heparan sulfate, syndecan-1, syndecan-4, glypican-1, and betaglycan-TGF-β RIII as indicated. Results are expressed in fluorescence units (log scale; experimental results minus isotype control antibody results) and are representative of at least two independent experiments.
Expression of syndecan-1, syndecan-4, or glypican-1 enhances infection of K562 cells by HPV11.
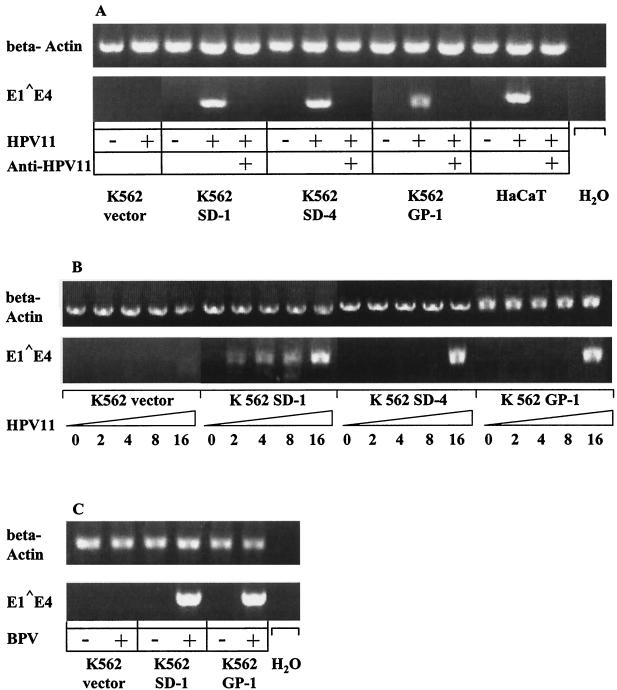
To determine whether binding of viral capsids translates into later events of infection, K562 transfectants were examined for their susceptibility to infection by HPV11 virions isolated from genital warts. A transient infectivity assay was first established in the HaCaT keratinocyte line (Fig. 2A) and then applied to K562 cells as described previously (50). Cells were incubated with HPV11 virions (18 × 103 particles) for 3 days, total RNA was then extracted, and the presence of E1-E4 viral mRNA was assessed by nested RT-PCR. The size of the cDNA amplification products was consistent with the expected size of 628 bp (Fig. 2A). To distinguish authentic HPV11 infection from possible transfection of viral DNA into the cell, an HPV11-neutralizing antibody was used (10). As expected, preincubation of HPV11 with the neutralizing mouse MAb H11.B2 abolished infection of HaCaT keratinocytes (and K562 cells), as shown by the absence of detectable spliced viral mRNA (Fig. 2A). Early E1-E4 transcripts were detected both in K562 cells that stably overexpressed syndecan-1, syndecan-4, or glypican-1 and in control HaCaT keratinocytes. Although addition of HPV11 virions at a similar MOI to K562 cells transfected with the empty eukaryotic vector pRep4 did not lead to detectable viral mRNA expression (Fig. 2A), transcription occurred following infection with a higher HPV11 inoculum (Fig. 2B; see below). Similar data were obtained by use of bovine papillomavirus (BPV) virions (isolated from a bovine skin wart) in an analogous infectivity-nested RT-PCR assay. Transfection with syndecan-1 or glypican-1 enhances susceptibility of K562 cells to infection with 0.2 μl of BPV virion solution (5 focus-forming units) (Fig. 2C; not determined for syndecan-4-transfected cells), whereas 1 μl of BPV virions (25 focus-forming units) were required for detectable infection of K562 vector cells (data not shown). These data indicate that infectivity results are not limited to the genetically nonrelated HPV11 and BPV but may be representative for all papillomaviruses as well.
FIG. 2.
Papillomavirus infectivity assays (RT-PCR) of K562 transfectants expressing an empty eukaryotic vector (K562 vector), syndecan-1 (K562 SD-1), syndecan-4 (K562 SD-4), or glypican-1 (K562 GP-1) or of HaCaT cells. Cells were infected with the indicated amounts of HPV11 (A and B) or 0.2 μl (5 focus-forming units) of BPV (C) virions for 1 h, incubated for 3 days, and analyzed for E1-E4 spliced viral RNA expression by RT-PCR. Amplicons were separated by gel electrophoresis and visualized by ethidium bromide staining. Neutralization of virions with anti-HPV11 MAb (H11.B2), infection of HaCaT keratinocytes, PCR in the absence of cDNA (H2O), and amplification of β-actin mRNA served as appropriate controls as indicated.
Susceptibility to HPV11 infection correlates with levels of heparan sulfate expression by K562 lines.
Binding of VLP to syndecan-1-transfected cells is greater than that to syndecan-4- and glypican-1-transfected or wt K562 cells and correlates with heparan sulfate overexpression at the cell surface (Fig. 1 and 3). To determine whether susceptibility to HPV11 infection also correlates with heparan sulfate expression levels, we performed infectivity assays using titrations of the HPV11 stock solution, starting with a relative excess (input of 16 μl, corresponding to 7.2 × 104 particles) of virions and using twofold dilutions down to 2 μl (9 × 103 particles). In a representative experiment (Fig. 2B), incubation of cells with 2 μl of HPV11 particles was sufficient for detection of viral RNA expression for syndecan-1 transfectants, 16 μl of HPV11 virion solution was required for both syndecan-4 and glypican-1 cell lines, and ≥16 μl of HPV11 solution was required for wt K562 cells to have an infection that was detectable by RT-PCR (Fig. 2B; note weak band for K562 vector cells infected with 16 μl of HPV11 and data not shown). These data are consistent with the K562 attachment assays described above and suggest that (i) various proteoglycans, i.e., syndecans, glypicans, and betaglycan, can confer susceptibility to HPV11 infection upon K562 cells, while the core protein per se may not play a decisive role, and (ii) a threshold level of cell surface heparan sulfate may exist that determines permissiveness for infection, at least for a low virus inoculum.
Expression of heparan sulfate, syndecan-1, syndecan-4, glypican-1, and betaglycan in human keratinocyte (KH-SV) and K562 cell lines.
Basal keratinocytes of stratified epithelia are the assumed target cells in natural papillomavirus infections (35). The simian virus 40-immortalized human cell line KH-SV maintains the phenotype of primary keratinocytes and was used as a convenient surrogate for primary cells. Cells were grown under low-calcium conditions to inhibit differentiation and were analyzed for surface expression of prototype members of the HSPG family by quantitative immunofluorescence flow cytometry, using MAbs against syndecan-1, syndecan-4, glypican-1, and betaglycan-TGF-β RIII under saturating conditions (20 μg/ml). As shown in Fig. 3B, syndecan-1 was expressed at high levels, indicating that it is the major HSPG of human keratinocytes (3, 51), whereas syndecan-4, glypican-1, and betaglycan are expressed at considerably lower levels. Keratinocyte expression levels of syndecan-1 and syndecan-4 were about 50% compared to the respective transfected K562 cells, whereas glypican-1 and betaglycan expression appeared even lower than that by glypican-1-transfected or wt K562 cell lines, respectively (Fig. 3B). Importantly, heparan sulfate expression of K562 transfectants correlated with the relative expression levels of transfected HSPGs (Fig. 3). These results and previous observations suggest that the high levels of heparan sulfate expression on keratinocytes are predominantly due to syndecan-1 (3, 34).
Human keratinocytes bind HPV16 VLP via HSPGs.
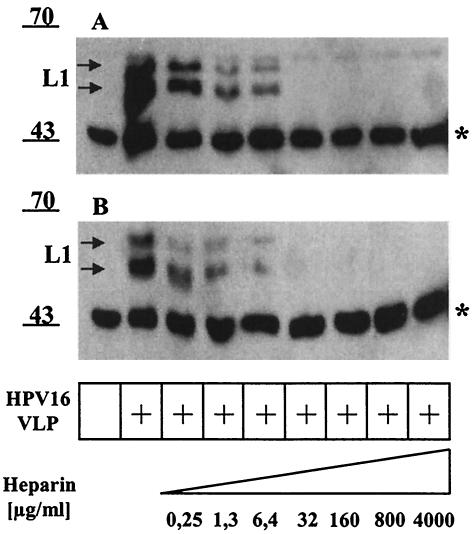
Previous studies have identified HSPGs as primary attachment structures on HaCaT keratinocytes by using binding of VLP derived from HPV11 or cottontail rabbit papillomavirus or HPV33 pseudovirion infection as the readout (11, 17, 21). Conversely, the cellular adhesion receptor α6 integrin was implicated in binding of HPV6 VLP. To examine if high-risk HPV16 capsids bind human keratinocytes via HSPGs, HPV16 VLP were incubated with KH-SV wt keratinocytes in the absence or presence of high-molecular-mass (5 to 28 kDa) heparin. Following a wash step, cell-bound VLP were visualized by Western blotting using the MAb Camvir-1. Increasing concentrations of heparin (0.25 to 4,000 μg/ml) decreased VLP binding in a dose-dependent manner, with ∼50% inhibition observed at the lowest concentration (0.25 μg/ml) of heparin employed and complete inhibition detectable at ≥32 μg/ml (Fig. 4A). The two L1 species (doublet), at 50 to 55 kDa, correspond to posttranslational modification of L1 expressed in insect cells. The band at ∼45 kDa (indicated with a star) represents a cellular protein cross-reacting with Camvir-1 that serves as a convenient internal control for equal loading. Extending previous results, these data implicate HSPGs as primary attachment receptors for HPV16 on human keratinocytes. Interestingly, binding inhibition occurred at similar heparin concentrations in the case of the α6 integrin null keratinocyte line BOUA-SV, which indicates that HPV16 VLP interaction with human keratinocytes occurs independently from α6 integrin expression.
FIG. 4.
Inhibition of HPV16 VLP binding to wt (KH-SV) or α6 integrin-deficient (BOUA-SV) keratinocytes with heparin. KH-SV (A) and BOUA-SV (B) keratinocytes were incubated with HPV16 VLP in the absence or presence of increasing concentrations of heparin (0.25 to 4,000 μg/ml). Following extensive washing, HPV16 L1 bound to the cells was detected by immunoblotting using MAb Camvir-1. The band at ∼45 kDa (indicated by a star) represents a cellular protein cross-reacting with MAb Camvir-1.
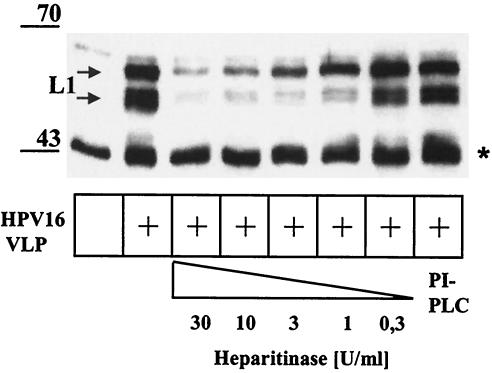
Heparitinase, but not PLC, pretreatment of human keratinocytes inhibits binding by HPV16 VLP.
Syndecans and betaglycans are integral membrane proteins, whereas glypicans are linked to the cell surface via a GPI anchor (3). To further analyze which heparan sulfated protein(s) mediates HPV capsid binding to human keratinocytes, KH-SV cells were incubated with increasing concentrations of heparitinase to remove heparan sulfates or with PI-PLC to remove GPI-anchored proteins, including glypicans. Enzyme activities were monitored by flow cytometry for the ability to remove heparan sulfate GAG or GPI-anchored proteins (data not shown). Following enzyme digestion, the cells were shifted to 4°C to prevent protein reexpression, incubated with VLP, and analyzed for the ability of capsid binding. As shown in Fig. 5, heparitinase digestion reduced VLP binding to keratinocytes in a dose-dependent manner. Treatment with 13 U of PI-PLC per ml eliminated glypican from the cell surface yet had no significant inhibitory effect on VLP binding. These results suggest that GPI-anchored glypicans are not the primary receptors for VLP binding to keratinocytes.
FIG. 5.
Inhibition of HPV16 VLP binding to human keratinocytes (KH-SV) by treatment with heparitinase, but not by PLC. Keratinocytes (KH-SV) were incubated with increasing concentrations of heparitinase or PI-PLC (13 U/ml) or left untreated, as indicated. Following a wash step with PBS, cells were incubated with (or without) VLP for 1 h and washed extensively, and cell-VLP complexes were analyzed by SDS-PAGE and Western blotting using MAb Camvir-1. The band at ∼45 kDa (indicated by a star) represents a cellular protein cross-reacting with MAb Camvir-1.
Using MAb B-B4 directed against syndecan-1, we also attempted to directly interfere with VLP binding to syndecan-1, the major HSPG of keratinocytes and therefore the primary HPV receptor candidate. Preincubation of KH-SV cells with MAb B-B4 did not reduce VLP-cell binding (data not shown). However, the existing antibodies to syndecan-1 are directed against the protein core, and therefore VLP binding to cells via extended heparan sulfate side chains may not be inhibited.
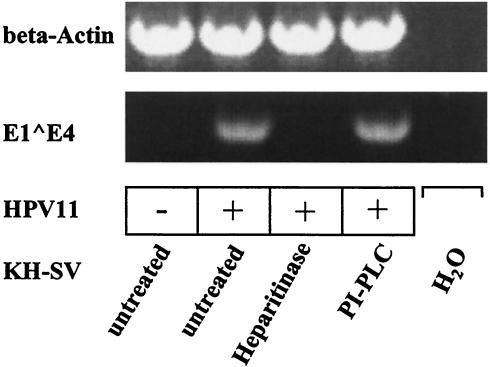
To relate results from infectivity assays obtained with K562 cells to the natural host cells of papillomaviruses, we analyzed susceptibility of keratinocytes to HPV11 virion infection. Because human keratinocytes deficient in syndecans or glypicans do not exist, KH-SV keratinocytes were exposed to heparitinase to remove heparan sulfate GAG or to PI-PLC to remove GPI-anchored glypicans from the cell surfaces. The removal of heparan sulfate or glypican-1, respectively, from the cell surface was verified by flow cytometry (data not shown). Cells were infected with HPV11 virions and cell extracts were analyzed by RT-PCR. Spliced E1-E4 viral mRNA was detected in untreated cells and in KH-SV cells treated with PI-PLC, but not in heparitinase-predigested cells (Fig. 6). Amplicons were absent from cell extracts following mock infection or from PCR control reactions performed in the absence of DNA template, demonstrating specificity. These results imply that syndecans or betaglycans are required for HPV11 infection of human keratinocytes, whereas glypicans do not appear to play a major role.
FIG. 6.
Inhibition of HPV11 infection of human keratinocytes by treatment with heparitinase, but not by PLC. KH-SV cells were treated with heparitinase (1.9 U/ml) or PI-PLC (13 U/ml) or left untreated, as indicated. Subsequently, cells were incubated with HPV11 virions, incubated for 3 days, and analyzed for E1-E4 spliced viral RNA expression by RT-PCR. Amplicons were separated by gel electrophoresis and visualized by ethidium bromide staining. PCR in the absence of cDNA (H2O) and β-actin amplification served as specificity controls.
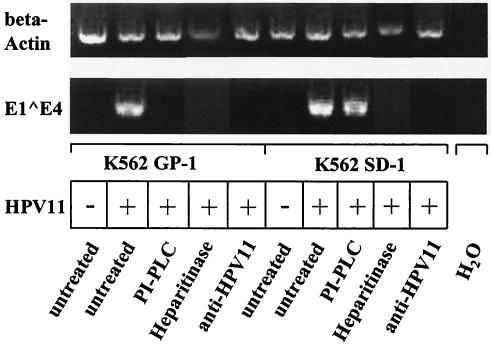
To corroborate the significance of the infectivity result obtained with PI-PLC treatment of KH-SV cells, glypican-1- and syndecan-1-transfected K562 cells were digested with PI-PLC under similar conditions (removal of glypican-1 was verified by flow cytometry; data not shown) and incubated with HPV11 virions, and RT-PCR was performed after 3 days. As shown in Fig. 7, removal of GPI-anchored proteins rendered glypican-1-transfected K562 nonpermissive for HPV11 infection, whereas PI-PLC treatment of syndecan-1-transfected K562 cells did not affect the permissiveness for infection. As expected, heparitinase digestion of K562 GP-1 and K562 SD-1 transfectants abolished HPV11 infection of both cell lines. In addition, several control experiments verified the specificity of the results (Fig. 7). These data indicate that glypican-1 overexpression in K562 cells (comprising the majority of cell surface HSPGs) enhances HPV11 infectivity, whereas low-level expression of glypican-1 in keratinocytes (expressing high levels of endogenous syndecan-1) does not appear to be essential for susceptibility to HPV11 infection.
FIG. 7.
Transient infection of K562 cell lines expressing glypican-1 (K562 GP-1) or syndecan-1 (K562 SD-1) digested with PLC or heparitinase with native HPV11 virions (RT-PCR infectivity assay). Cells were treated with PI-PLC (13 U/ml) or heparitinase (1.9 U/ml) for 1 h or were left untreated, washed with PBS, infected with native HPV11 virions or mock infected, as indicated, and incubated for 3 days. Total RNA was isolated and E1-E4 transcripts were amplified by RT-PCR. Amplicons were separated by gel electrophoresis and visualized by ethidium bromide staining. Neutralization with an anti-HPV11 MAb (H11.B2) and amplification of β-actin served as controls, as indicated.
Similar binding efficacy of HPV16 VLP to α6 integrin-deficient (BOUA-SV) or wt (KH-SV) human keratinocytes.
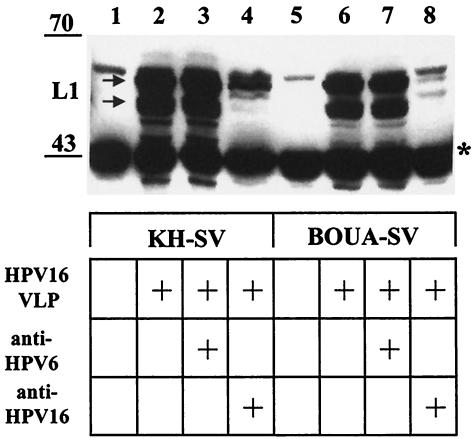
α6 integrin has been proposed as the binding receptor for HPV6 VLP (15). However, further analysis has revealed that α6 integrin is not required for HPV11 VLP binding, HPV33 pseudoinfection, or BPV type 4 (BPV4) infection (17, 21, 48). To further address this issue, we performed attachment assays using VLP of high-risk HPV16 and human epidermal keratinocytes as appropriate target cells. We took advantage of keratinocytes (BOUA-SV) isolated from a patient with a blistering skin disease that lacked α6 integrin expression due to a homozygous deletion (16). For comparison, human keratinocytes (KH-SV) isolated from healthy donor skin were used. Keratinocytes grown to subconfluence were detached by trypsin-EDTA treatment and held in suspension for 2 h to allow reexpression of cell surface proteins. Following incubation with 125 ng of VLP for 1 h, cell-VLP complexes were extensively washed, lysed in SDS sample buffer, and analyzed by immunoblotting for the presence of L1, using the MAb Camvir-1 directed against a linear epitope of HPV16 L1 (15).
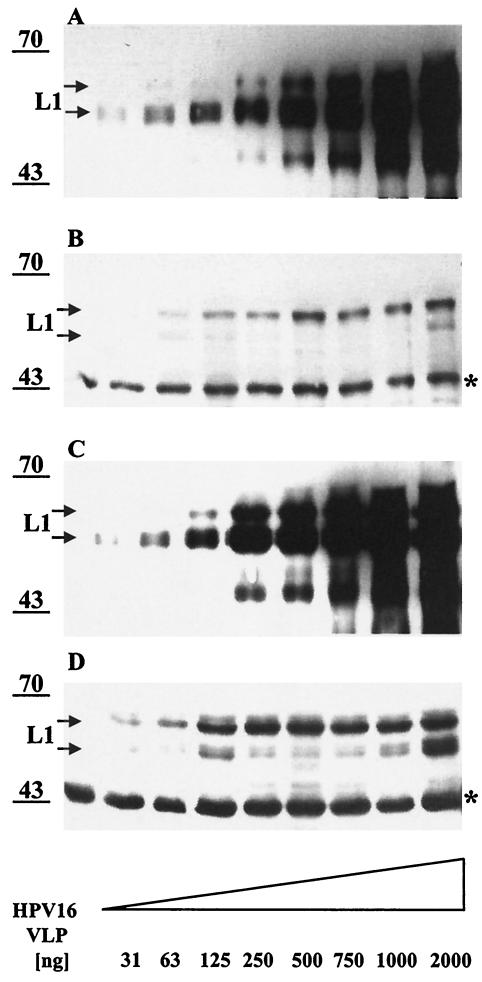
The amount of HPV16 VLP that attached to the integrin α6 null keratinocyte line BOUA-SV (105 cells) appeared similar to that binding KH-SV keratinocytes (105 cells) (Fig. 8, compare lanes 2 and 6 [arrow]), suggesting that α6 integrin expression on the keratinocyte surface is not required for initial HPV16 L1 capsid binding. MAb H16.E70 blocks both surface binding and infection by HPV16 (39). Preincubation of VLP with H16.E70 prevents binding to keratinocytes to a large extent when compared to VLP binding in the presence of a control MAb directed against heterologous type HPV6 L1 VLP (≥90% reduction in L1 immunoreactivity) (Fig. 8, lanes 3, 4, 7, and 8), indicating that the assay specifically detects cell binding of assembled particles. In addition, when increasing amounts (31 to 2,000 ng) of HPV16 VLP were incubated with a constant number (105) of cells, the amount of cell-bound VLP increased initially and remained constant at ≥500 ng for KH-SV cells and ≥250 ng for BOUA-SV cells (Fig. 9B and D). Concomitantly, the amount of unbound VLP increased in the supernatant fraction (Fig. 9A and C), indicating that the number of cell surface receptors promoting VLP attachment are limited (56). The amount of L1 capsids bound to BOUA-SV cells appeared comparable over the whole range of input VLP to that in complex with KH-SV cells, suggesting that α6 integrin expression by keratinocytes does not play a major role in attachment of HPV16 VLP to their natural host cells (compare Fig. 9B and D; note that the signal for the 45-kDa cellular control protein is stronger for BOUA-SV than for KH-SV cells).
FIG. 8.
Attachment of HPV16 L1 VLP to KH-SV (wt) or BOUA-SV (α6 integrin-deficient) human keratinocytes. KH-SV (lanes 1 to 4) or BOUA-SV (lanes 5 to 8) cells were incubated with HPV16 VLP for 1 h and washed, and capsid binding was analyzed by immunoblotting using MAb Camvir-1. Specificity of binding is demonstrated by incubation of VLP with HPV16-neutralizing MAb H16.E70 (lanes 4 and 8) or HPV6-neutralizing MAb H6.C6 (lanes 3 and 7) as controls. The cross-reacting cellular protein at ∼45 kDa (indicated by a star) serves as an internal control for equal protein loading.
FIG. 9.
Binding of HPV16 L1 VLP to human KH-SV (wt) and BOUA-SV (α6 integrin-deficient) keratinocytes is saturable. KH-SV (A and B) and BOUA-SV (C and D) cells were incubated without (lane 1) or with increasing amounts (31 to 2,000 ng) of HPV16 L1 VLP and incubated for 1 h before centrifugation. Supernatants and lysates of washed VLP-cell complexes were separated by SDS-PAGE, and cell-bound (B and D) or free (A and C) VLP were detected with MAb Camvir-1 by immunoblotting. The band at ∼45 kDa (indicated by a star) represents a cellular protein cross-reacting with MAb Camvir-1.
In addition, we generated revertant lines from α6 integrin-deficient keratinocytes that overexpressed α6-integrin (BOUA-SV-α6) from a retroviral expression vector or the neomycin resistance gene alone as a control (BOUA-SV-neo). The level of HPV16 VLP binding to BOUA-SV-α6 cells was similar to that for the BOUA-SV-neo line (data not shown). These results confirm that under our conditions α6 integrin expression does not contribute to HPV16 VLP binding to their natural host cells.
α6 integrin-deficient (BOUA-SV) keratinocytes are susceptible to infection with authentic HPV11 virions.
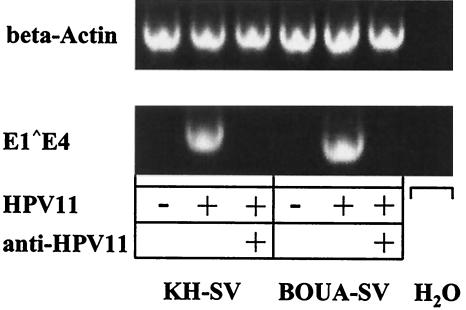
For early events of papillomavirus infection, attachment of virions to the cells is necessary but may not be sufficient for subsequent steps such as internalization, uncoating, and early gene transcription that precede productive infection. To further examine a possible postattachment role for α6 integrin in HPV infectivity pathways, we used authentic HPV11 virions in an in vitro infectivity assay to directly determine whether capsid binding translates into infectious events. With viral early transcription as the readout, we examined whether α6 integrin-deficient keratinocytes were resistant to HPV11 infection compared to wt keratinocytes. Importantly, spliced viral RNA was detectable in extracts from HPV11-infected BOUA-SV and KH-SV cells, but not from mock-infected cells (Fig. 10).
FIG. 10.
Transient infection of wt (KH-SV) or α6 integrin-deficient (BOUA-SV) keratinocytes with native HPV11 virions (RT-PCR infectivity assay). Cells were infected with native HPV11 virions or left untreated and incubated for 3 days. Total RNA was isolated and E1-E4 transcripts were amplified by RT-PCR. Amplicons were separated by gel electrophoresis and visualized by ethidium bromide staining. Neutralization with an anti-HPV11 MAb (H11.B2) and amplification of β-actin served as controls, as indicated.
Infection of KH-SV cells with titrations of virions demonstrated that similar to K562 SD-1 transfectants, 1 to 2 μl of HPV11 inoculum (4.5 × 103 to 9 × 103 particles) was sufficient for detection of expression of viral RNA (Fig. 6 and 10; data not shown). These results indicated that high-level expression of heparan sulfate, predominantly by syndecan-1, appears sufficient for viral infectivity. Preincubation of HPV11 with the neutralizing MAb H11.B2 abolished infectivity, as shown by the absence of detectable spliced RNA. Similar results were obtained using BPV virions isolated from bovine skin warts in analogous RT-PCR infectivity assays (data not shown). These results establish that infection of human keratinocytes with authentic genital-mucosal type HPV11 virions is not dependent on α6 integrin surface expression. These data are in agreement with previous results from HPV33 pseudoinfection and BPV4 infection (48, 53) and strongly suggest that independence of papillomavirus infection from keratinocyte α6 integrin expression is not restricted to a limited number of animal or human types.
DISCUSSION
For this study we examined the role of prototypic members of the syndecan and glypican families of HSPGs because the heparan sulfate-carrying protein(s) necessary for papillomavirus binding and infection has not been identified. In addition, we attempted to resolve the controversial issue of whether α6 integrin is required for HPV binding to and infection of human keratinocytes. To evaluate attachment of the viral capsid to cells, we used baculovirus-expressed VLP of HPV16, the type most commonly associated with genital cancer. Although VLP share many immunologic and structural aspects with infectious HPV in initial cell binding, they may not accurately mimic later aspects of HPV infection. Thus, we examined subsequent steps of papillomavirus infection by using authentic infection with native HPV11 virions, as this mucosal type can be readily isolated from human genital warts.
In vitro attachment assays using α6 integrin-deficient human keratinocytes or revertants ectopically overexpressing the integrin from a retroviral vector demonstrated that α6 integrin expression is not required for HPV16 VLP binding. The reasons for the divergent results in binding requirements for HPV6 (15) and HPV16 L1 (this report) VLP are unclear, as both types have similar tropism for genital tissues, but they may be explained if HPV16 follows a different entry pathway and/or use of human keratinocytes herein as appropriate natural target cells (4). More importantly, following infection with authentic HPV11 virions, E1-E4 spliced viral transcripts are detected in keratinocyte lines that either express or do not express α6 integrin. These results unambiguously establish that expression of α6 integrin is not essential for authentic HPV11 infection of human keratinocytes in vitro. These findings are in agreement with previous reports demonstrating that α6 integrin is not a necessary receptor for infection by BPV4 virions or HPV33 pseudovirions. These results also argue against a major role of α6 integrin in papillomavirus infection in vivo, although more subtle effects, e.g., on the kinetics of virion uptake and/or transcription, have not been ruled out by the experimental protocols we used.
Because the specific heparan sulfate-carrying cellular receptor protein(s) has not yet been identified, we examined the role of prototype members of two major families of HSPGs, syndecans and glypicans, in papillomavirus infection. Two processes were studied: (i) initial VLP-cell binding and (ii) authentic HPV11 virion infectivity as measured by expression of viral spliced transcripts. We took advantage of K562 erythroleukemia cells stably transfected with syndecan-1, syndecan-4, or glypican-1 cDNA. Transgene expression results in increased levels of heparan sulfate GAG moieties on their cell surfaces in addition to the low levels of HSPG on their parental or vector-transfected cells that are primarily due to endogenous betaglycan expression.
K562 cells that expressed either of the transfected HSPGs promoted HPV16 VLP binding, as measured by the increase in immunoreactive L1 in complex with the cells. Binding was strongest to syndecan-1-transfected K562 cells compared to syndecan-4- and glypican-1-transfected or vector-transfected lines and correlated with expression levels of heparan sulfate. In comparison to vector-transfected cells, all HSPG transfectants demonstrated increased susceptibility to transient HPV11 infection, as determined by the appearance of spliced viral mRNA. Interestingly, in viral titration experiments HPV11 infection of wt K562 cells was observed when the viral inoculum was increased 16-fold over the dose used in typical experiments. In addition, the dosage of virions required to detect infection in syndecan-1 lines was eightfold lower than that for syndecan-4 and glypican-1 lines. This indicates that the level of cell surface heparan sulfate rather than the specific core protein of the transfected HSPG determines the susceptibility of K562 lines to infection. These results differ from those reported for human immunodeficiency virus infection, in which the syndecans as a single family of HSPGs mediate virus attachment to macrophages (43).
Binding was strongly reduced when heparan sulfates were removed from the K562 cell surfaces by heparitinase treatment. Similarly, inhibition of capsid binding and loss of HPV11 infectivity were observed when keratinocytes were treated with heparitinase or when VLP or virions were exposed to heparin. Interestingly, human keratinocytes treated with PI-PLC did not show reduced VLP binding or elimination of infectivity. These results indicate that the integral membrane proteins of the syndecan and/or betaglycan families, rather than GPI-linked glypicans, mediate papillomavirus infection of natural host cells.
The initial interaction of HPV with cells appears to occur by binding to cell surface heparan sulfate GAG. Several viruses, including HSV, use multiple receptors sequentially. Indeed, binding to HSPGs on the cell surface is often only the first step in a series of events between the virus and the cell that is required for virus entry and the initiation of infection (47). For papillomaviruses, these steps may involve other cell surface proteins, i.e., a secondary receptor, and interaction with the minor capsid protein L2 in addition to L1 (22, 40).
Syndecan-1 is the most abundant heparan-sulfated protein expressed on epithelial keratinocytes, whereas syndecan-4, glypican-1, and betaglycan expression levels are an order of magnitude lower (Fig. 3A) (2, 34). As the magnitude of VLP binding and the particle-to-infectivity ratio tend to correlate with heparan sulfate expression levels on the cell surface, it appears plausible that syndecan-1 serves as a primary HPV receptor on human keratinocytes. Several criteria are fulfilled in support of this hypothesis. The absence of syndecan-1 reduces VLP binding and renders K562 cells resistant to a low virus inoculum, whereas its stable expression increases binding and allows permissiveness for low levels of HPV11, similar to the case for keratinocytes. Importantly, removal of GPI-anchored glypicans abolished infection of glypican-1-transfected K562 lines, but not permissiveness of syndecan-1-transfected K562 or KH-SV keratinocytes expressing high levels of endogenous syndecan-1.
However, because of the absence of suitable experimental systems (i.e., specific HSPG knockout human keratinocytes or production of functionally blocking antibodies), we cannot exclude that HPV infects keratinocytes via other syndecan heparan sulfates. Other members of the syndecan family, e.g., syndecan-4, or other HSPGs that are expressed at lower levels, e.g., betaglycan, may play a minor role and synergize with or complement syndecan-1. This possibility is raised by the observations that syndecan-4-transfected K562 cells are susceptible to transient HPV11 infection (Fig. 2A and B) and that K562 wt cells (expressing betaglycan) can bind low levels of VLP (Fig. 1) and are permissive for infection with a high virus inoculum. Nevertheless, taken together these results, albeit indirectly, argue that quantitatively syndecan-1 may serve as a primary attachment receptor for HPV in natural infection.
In contrast to many other viruses, HPVs do not induce lytic infection, and the mechanisms for release of HPV virions from the cornified cell envelopes of terminally differentiated keratinocytes (corneocytes) are unknown. It has been suggested that HPV11 infection may occur with cell-free as well as cell-associated virions and that their mechanisms of cellular uptake and receptor usage may differ (5). Because we have prepared wart extracts by differential centrifugation and a final 0.2-μm-pore-size filter sterilization step to remove contaminants, the presence of subcellular components associated with virions is unlikely but cannot be ruled out. However, VLP attachment and HPV infectivity were inhibited by neutralizing antibodies (37), strongly suggesting that primary cell interaction occurred via L1 capsid determinants. Neutralizing antibodies that inhibit virion-cell interaction recognize nonlinear epitopes on L1 capsid surface loops, and footprints of such antibodies bound to the capsid surface have been determined by cryo-electron microscopy and mutational analysis (7, 30, 38, 49, 52). However, the heparan sulfate binding site of the capsid is not necessarily identical to or even overlapping with neutralization determinants, since antibodies decorating the capsid surface may sterically hinder interaction to an adjacent receptor binding structure (that may even be inaccessible to antibodies).
Heparan sulfates are widely expressed on cells, extracellular matrices, and basement membranes. In addition, HSPGs are shed from the cell surface and are detectable in wound fluids (3). Thus, papillomavirus binding of heparan sulfate or free heparin can either enable the virus to bind cells bearing entry receptors or prevent the virus from reaching the target cell. Interestingly, skin lesions produce a high number of infectious virions, which may be necessary to accomplish transmission, whereas fewer virions present in genital warts appear sufficient for spread by intimate contact.
Papillomaviruses need to infect basal (stem) cells exposed by minor abrasion to establish latent or long-lasting infection, whereas infection of suprabasal or transient amplifying cells may lead to transient infection that resolves when cells are shed from the surface by epithelial turnover (55). Under normal conditions, syndecan-1 expression is modest in basal keratinocytes and induced in suprabasal layers during differentiation (19, 42). Importantly, syndecan-1 is strongly upregulated during wound healing and widely expressed by migrating and proliferating keratinocytes and on adjacent hair follicles (14). Interestingly, hair follicle stem cells have been implicated as primary target cells of the high-risk cottontail rabbit papillomavirus (45). Thus, basal keratinocytes exposed by minor trauma or abrasion, in addition to suprabasal (transient amplifying) cells, overexpress syndecan-1 and thus may strongly upregulate the ability to attach and internalize papillomaviruses in vivo. Permissiveness for HPV infection by epithelial stem cells appears crucial for establishing a persistent infection, a requirement for efficient viral propagation and carcinogenesis.
Acknowledgments
We gratefully acknowledge the generous gifts of cells and antibodies by Guido David and Norbert Fusenig and of bovine wart extracts by Richard Roden. We also thank Doug Lowy and Richard Roden for critical reading of the manuscript and Dieter Maurer for helpful discussions.
This study was supported by a grant to R.K. from the Fonds zur Förderung der wissenschaftlichen Forschung (FWF; P13365-B01).
REFERENCES
- 1.Attisano, L., J. L. Wrana, F. Lopez-Casillas, and J. Massague. 1994. TGF-beta receptors and actions. Biochim. Biophys. Acta 1222:71-80. [DOI] [PubMed] [Google Scholar]
- 2.Bernfield, M., M. Gotte, P. W. Park, O. Reizes, M. L. Fitzgerald, J. Lincecum, and M. Zako. 1999. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68:729-777. [DOI] [PubMed] [Google Scholar]
- 3.Bernfield, M., M. T. Hinkes, and R. L. Gallo. 1993. Developmental expression of the syndecans: possible function and regulation. Development 1993(Suppl.):205-212. [PubMed] [Google Scholar]
- 4.Bousarghin, L., A. Touze, P. Y. Sizaret, and P. Coursaget. 2003. Human papillomavirus types 16, 31, and 58 use different endocytosis pathways to enter cells. J. Virol. 77:3846-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryan, J. T., and D. R. Brown. 2001. Transmission of human papillomavirus type 11 infection by desquamated cornified cells. Virology 281:35-42. [DOI] [PubMed] [Google Scholar]
- 6.Byrnes, A. P., and D. E. Griffin. 1998. Binding of Sindbis virus to cell surface heparan sulfate. J. Virol. 72:7349-7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, X. S., R. L. Garcea, I. Goldberg, G. Casini, and S. C. Harrison. 2000. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol. Cell 5:557-567. [DOI] [PubMed] [Google Scholar]
- 8.Christensen, N., J. Dillner, C. Eklund, J. Carter, G. Wipf, C. Reed, N. Cladel, and D. Galloway. 1996. Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology 223:174-184. [DOI] [PubMed] [Google Scholar]
- 9.Christensen, N. D., and J. W. Kreider. 1990. Antibody-mediated neutralization in vivo of infectious papillomaviruses. J. Virol. 64:3151-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen, N. D., J. W. Kreider, N. M. Cladel, S. D. Patrick, and P. A. Welsh. 1990. Monoclonal antibody-mediated neutralization of infectious human papillomavirus type 11. J. Virol. 64:5678-5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen, N. D., C. A. Reed, T. D. Culp, P. L. Hermonat, M. K. Howett, R. A. Anderson, and L. J. Zaneveld. 2001. Papillomavirus microbicidal activities of high-molecular-weight cellulose sulfate, dextran sulfate, and polystyrene sulfonate. Antimicrob. Agents Chemother. 45:3427-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.David, G., B. van der Schueren, P. Marynen, J. J. Cassiman, and H. van den Berghe. 1992. Molecular cloning of amphiglycan, a novel integral membrane heparan sulfate proteoglycan expressed by epithelial and fibroblastic cells. J. Cell Biol. 118:961-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Day, P. M., D. R. Lowy, and J. T. Schiller. 2003. Papillomaviruses infect cells via a clathrin-dependent pathway. Virology 307:1-11. [DOI] [PubMed] [Google Scholar]
- 14.Elenius, K., S. Vainio, M. Laato, M. Salmivirta, I. Thesleff, and M. Jalkanen. 1991. Induced expression of syndecan in healing wounds. J. Cell Biol. 114:585-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evander, M., I. H. Frazer, E. Payne, Y. M. Qi, K. Hengst, and N. A. McMillan. 1997. Identification of the alpha6 integrin as a candidate receptor for papillomaviruses. J. Virol. 71:2449-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gache, Y., C. Romero-Graillet, A. Spadafora, C. Lepinard, P. Descamps, C. B. Bardon, J. P. Ortonne, and G. Meneguzzi. 1998. A novel homozygous mutation affecting integrin alpha6 in a case of junctional epidermolysis bullosa with pyloric atresia detected in utero by ultrasound examination. J. Investig. Dermatol. 111:914-916. [DOI] [PubMed] [Google Scholar]
- 17.Giroglou, T., L. Florin, F. Schafer, R. E. Streeck, and M. Sapp. 2001. Human papillomavirus infection requires cell surface heparan sulfate J. Virol. 75:1565-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harro, C. D., Y. Y. Pang, R. B. Roden, A. Hildesheim, Z. Wang, M. J. Reynolds, T. C. Mast, R. Robinson, B. R. Murphy, R. A. Karron, J. Dillner, J. T. Schiller, and D. R. Lowy. 2001. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J. Natl. Cancer Inst. 93:284-292. [DOI] [PubMed] [Google Scholar]
- 19.Inki, P., H. Larjava, K. Haapasalmi, H. M. Miettinen, R. Grenman, and M. Jalkanen. 1994. Expression of syndecan-1 is induced by differentiation and suppressed by malignant transformation of human keratinocytes. Eur. J. Cell Biol. 63:43-51. [PubMed] [Google Scholar]
- 20.Jackson, T., F. M. Ellard, R. A. Ghazaleh, S. M. Brookes, W. E. Blakemore, A. H. Corteyn, D. I. Stuart, J. W. Newman, and A. M. King. 1996. Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J. Virol. 70:5282-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joyce, J. G., J. S. Tung, C. T. Przysiecki, J. C. Cook, E. D. Lehman, J. A. Sands, K. U. Jansen, and P. M. Keller. 1999. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J. Biol. Chem. 274:5810-5822. [DOI] [PubMed] [Google Scholar]
- 22.Kawana, K., K. Matsumoto, H. Yoshikawa, Y. Taketani, T. Kawana, K. Yoshiike, and T. Kanda. 1998. A surface immunodeterminant of human papillomavirus type 16 minor capsid protein L2. Virology 245:353-359. [DOI] [PubMed] [Google Scholar]
- 23.Kirnbauer, R. 1996. Papillomavirus-like particles for serology and vaccine development. Intervirology 39:54-61. [DOI] [PubMed] [Google Scholar]
- 24.Kirnbauer, R., F. Booy, N. Cheng, D. R. Lowy, and J. T. Schiller. 1992. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 89:12180-12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirnbauer, R., L. Chandrachud, B. O'Neil, E. Wagner, G. Grindlay, A. Armstrong, G. McGarvie, J. Schiller, D. Lowy, and M. Campo. 1996. Virus-like particles of bovine papillomavirus type-4 in prophylactic and therapeutic immunization. Virology 219:37-44. [DOI] [PubMed] [Google Scholar]
- 26.Kirnbauer, R., J. Taub, H. Greenstone, R. Roden, M. Dürst, L. Gissmann, D. R. Lowy, and J. T. Schiller. 1993. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles J. Virol. 67:6929-6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kost, T. A., J. A. Kessler, I. R. Patel, J. G. Gray, L. K. Overton, and S. G. Carter. 1991. Human immunodeficiency virus infection and syncytium formation in HeLa cells expressing glycophospholipid-anchored CD4. J. Virol. 65:3276-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koutsky, L. A., K. A. Ault, C. M. Wheeler, D. R. Brown, E. Barr, F. B. Alvarez, L. M. Chiacchierini, and K. U. Jansen. 2002. A controlled trial of a human papillomavirus type 16 vaccine. N. Engl. J. Med. 347:1645-1651. [DOI] [PubMed] [Google Scholar]
- 29.Litwack, E. D., J. K. Ivins, A. Kumbasar, S. Paine-Saunders, C. S. Stipp, and A. D. Lander. 1998. Expression of the heparan sulfate proteoglycan glypican-1 in the developing rodent. Dev. Dyn. 211:72-87. [DOI] [PubMed] [Google Scholar]
- 30.Ludmerer, S. W., D. Benincasa, G. E. Mark, and N. D. Christensen. 1997. A neutralizing epitope of human papillomavirus type 11 is principally described by a continuous set of residues which overlap a distinct linear, surface-exposed epitope. J. Virol. 71:3834-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLean, C. S., M. J. Churcher, J. Meinke, G. L. Smith, G. Higgins, M. Stanley, and A. C. Minson. 1990. Production and characterisation of a monoclonal antibody to human papillomavirus type 16 using recombinant vaccinia virus. J. Clin. Pathol. 43:488-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyers, C., M. G. Frattini, J. B. Hudson, and L. A. Laimins. 1992. Biosynthesis of human papillomavirus from a continuous cell line upon epithelial differentiation. Science 257:971-973. [DOI] [PubMed] [Google Scholar]
- 33.Müller, M., L. Gissmann, R. J. Cristiano, X. Y. Sun, I. H. Frazer, A. B. Jenson, A. Alonso, H. Zentgraf, and J. Zhou. 1995. Papillomavirus capsid binding and uptake by cells from different tissues and species. J. Virol. 69:948-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oksala, O., T. Salo, R. Tammi, L. Hakkinen, M. Jalkanen, P. Inki, and H. Larjava. 1995. Expression of proteoglycans and hyaluronan during wound healing. J. Histochem. Cytochem. 43:125-135. [DOI] [PubMed] [Google Scholar]
- 35.Orth, G. 1987. Epidermodysplasia verruciformis, p. 199-243. In N. P. Salzman and P. M. Howley (ed.), The papovaviridae, vol. 2. The papillomaviruses. Plenum Press, New York, N.Y.
- 36.Roden, R., H. Greenstone, R. Kirnbauer, F. Booy, J. Joel, D. Lowy, and J. Schiller. 1996. In vitro generation and type-specific neutralization of a human papillomavirus type 16 virion pseudotype. J. Virol. 70:5875-5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roden, R., N. Hubbert, R. Kirnbauer, N. Christensen, D. Lowy, and J. Schiller. 1996. Assessment of the serological relatedness of genital human papillomaviruses by hemagglutination inhibition. J. Virol. 70:3298-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roden, R. B., A. Armstrong, P. Haderer, N. D. Christensen, N. L. Hubbert, D. R. Lowy, J. T. Schiller, and R. Kirnbauer. 1997. Characterization of a human papillomavirus type 16 variant-dependent neutralizing epitope. J. Virol. 71:6247-6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roden, R. B., R. Kirnbauer, A. B. Jenson, D. R. Lowy, and J. T. Schiller. 1994. Interaction of papillomaviruses with the cell surface. J. Virol. 68:7260-7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roden, R. B., E. M. Weissinger, D. W. Henderson, F. Booy, R. Kirnbauer, J. F. Mushinski, D. R. Lowy, and J. T. Schiller. 1994. Neutralization of bovine papillomavirus by antibodies to L1 and L2 capsid proteins. J. Virol. 68:7570-7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salzman, N. P., P. M. Howley, et al. (ed.). 1987. The papovaviridae, vol. 2. The papillomaviruses. Plenum Press, New York, N.Y.
- 42.Sanderson, R. D., M. T. Hinkes, and M. Bernfield. 1992. Syndecan-1, a cell-surface proteoglycan, changes in size and abundance when keratinocytes stratify. J. Investig. Dermatol. 99:390-396. [DOI] [PubMed] [Google Scholar]
- 43.Saphire, A. C., M. D. Bobardt, Z. Zhang, G. David, and P. A. Gallay. 2001. Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J. Virol. 75:9187-9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schiller, J. T., and M. Okun. 1996. Papillomavirus vaccines: current status and future prospects. Adv. Dermatol. 11:355-380. [PubMed] [Google Scholar]
- 45.Schmitt, A., A. Rochat, R. Zeltner, L. Borenstein, Y. Barrandon, F. O. Wettstein, and T. Iftner. 1996. The primary target cells of the high-risk cottontail rabbit papillomavirus colocalize with hair follicle stem cells. J. Virol. 70:1912-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Selinka, H., T. Giroglou, and M. Sapp. 2002. Analysis of the infectious entry pathway of human papillomavirus type 33 pseudovirions. Virology 299:279-287. [DOI] [PubMed] [Google Scholar]
- 47.Shukla, D., and P. Spear. 2001. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J. Clin. Investig. 108:503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sibbet, G., C. Romero-Graillet, G. Meneguzzi, and M. Saveria Campo. 2000. Alpha6 integrin is not the obligatory cell receptor for bovine papillomavirus type 4. J. Gen. Virol. 81:1629. [DOI] [PubMed] [Google Scholar]
- 49.Slupetzky, K., S. Shafti-Keramat, P. Lenz, S. Brandt, A. Grassauer, M. Sara, and R. Kirnbauer. 2001. Chimeric papillomavirus-like particles expressing a foreign epitope on capsid surface loops. J. Gen. Virol. 82:2799-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith, L. H., C. Foster, M. E. Hitchcock, G. S. Leiserowitz, K. Hall, R. Isseroff, N. D. Christensen, and J. W. Kreider. 1995. Titration of HPV-11 infectivity and antibody neutralization can be measured in vitro. J. Investig. Dermatol. 105:438-444. [DOI] [PubMed] [Google Scholar]
- 51.Steinfeld, R., H. Van Den Berghe, and G. David. 1996. Stimulation of fibroblast growth factor receptor-1 occupancy and signaling by cell surface-associated syndecans and glypican. J. Cell Biol. 133:405-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trus, B. L., R. B. Roden, H. L. Greenstone, M. Vrhel, J. T. Schiller, and F. P. Booy. 1997. Novel structural features of bovine papillomavirus capsid revealed by a three-dimensional reconstruction to 9 Å resolution. Nat. Struct. Biol. 4:413-420. [DOI] [PubMed] [Google Scholar]
- 53.Unckell, F., R. E. Streeck, and M. Sapp. 1997. Generation and neutralization of pseudovirions of human papillomavirus type 33. J. Virol. 71:2934-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vailly, J., L. Gagnoux-Palacios, E. Dell-Ambra, C. Romero, M. Pinola, G. Zambruno, M. De-Luca, J. P. Ortonne, and G. Meneguzzi. 1998. Corrective gene transfer of keratinocytes from patients with junctional epidermolysis bullosa restores assembly of hemidesmosomes in reconstructed epithelia. Gene Ther. 5:1322-1332. [DOI] [PubMed] [Google Scholar]
- 55.Vinther, J., and B. Norrild. 2003. Clearance of cervical human papillomavirus infections. Int. J. Cancer 104:255-256. [DOI] [PubMed] [Google Scholar]
- 56.Volpers, C., F. Unckell, P. Schirmacher, R. E. Streeck, and M. Sapp. 1995. Binding and internalization of human papillomavirus type 33 virus-like particles by eukaryotic cells. J. Virol. 69:3258-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.zur Hausen, H. 1996. Papillomavirus infection—a major cause of human cancers. Biochim. Biophys. Acta 1288:F55-F78. [DOI] [PubMed] [Google Scholar]