Abstract
The title compound, C15H20O5S, is an intermediate in the synthesis of novel aminocarboxylic acid derivatives. The cyclohexane ring exhibits a chair conformation. In the crystal structure, adjacent molecules form dimers via O—H⋯O hydrogen bonds.
Related literature
For the use of aminocarboxylic acid derivatives as anti-ulcer agents, see: Hoshina et al. (1984 ▶). For related structures, see: Qi et al. (2008 ▶); van Koningsveld et al. (1972 ▶).
Experimental
Crystal data
C15H20O5S
M r = 312.37
Monoclinic,

a = 12.545 (4) Å
b = 10.085 (3) Å
c = 12.654 (6) Å
β = 98.05 (3)°
V = 1585.1 (10) Å3
Z = 4
Mo Kα radiation
μ = 0.22 mm−1
T = 291 (2) K
0.45 × 0.40 × 0.38 mm
Data collection
Enraf–Nonius CAD-4 diffractometer
Absorption correction: none
4142 measured reflections
2931 independent reflections
1794 reflections with I > 2σ(I)
R int = 0.004
3 standard reflections every 250 reflections intensity decay: 0.8%
Refinement
R[F 2 > 2σ(F 2)] = 0.044
wR(F 2) = 0.130
S = 1.03
2931 reflections
197 parameters
H-atom parameters constrained
Δρmax = 0.24 e Å−3
Δρmin = −0.26 e Å−3
Data collection: DIFRAC (Gabe et al., 1993 ▶); cell refinement: DIFRAC; data reduction: NRCVAX (Gabe et al., 1989 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808003176/zl2098sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808003176/zl2098Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O5—H5⋯O4i | 0.82 | 1.83 | 2.642 (3) | 173 |
Symmetry code: (i)  .
.
supplementary crystallographic information
Comment
Some aminocarboxylic acid derivatives are used as anti-ulcer agents (Hoshina et al., 1984). To find new anti-ulcer agents, a series of trans/cis-cyclohexanecarboxylic acid derivatives were designed and synthesized.
In this paper, we want to report the synthesis and structure of the title compound, cis-4-(tosyloxymethyl)cyclohexanecarboxylic acid.
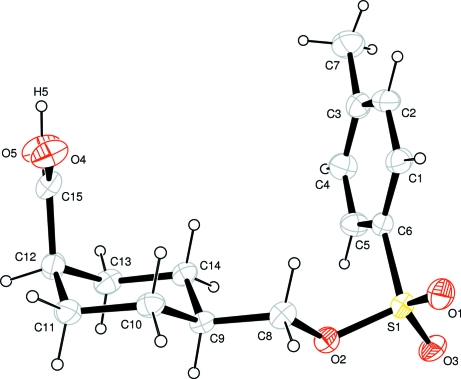
The cyclohexane ring exhibits a chair conformation and the cyclohexane C—C bond lengths and C—C—C endocyclic angles are in the range found for similar compounds (van Koningsveld, 1972) (Fig.1). They agree well with those of trans-4-(tosyloxymethyl)cyclohexanecarboxylic acid (Qi et al., 2008).
In the crystal structure, two molecules form centrosymmetric dimers via O—H···O hydrogen bonds (Fig. 2).
Experimental
cis-4-(Methoxycarboxyl)cyclohexanemethanol (10 mmol), pyridine (11 mmol) and a small amount of 4-dimethylaminopyridine were dissolved in dichloromethane (20 ml), then p-toluenesulfonyl chloride (11 mmol) was added dropwise with vigorous stirring at room temperature. After 8 h the reaction was quenched by addition of water and the organic layer separated was evaporated under vacuum, the solid obtained was hydrolyzed in a mixed solution of methanol and aqueous NaOH (11 mmol) for 4 h at 323 K. The title compound was then obtained by acidification with hydrochloric acid followed by recrystallization from ethyl acetate. Colorless crystals suitable for X-ray analysis were obtained by slow evaporation in ethyl acetate at room temperature.
Refinement
The H atoms were placed in the calculated positions in the riding model approximation with C—H = 0.93 (aromatic-H) and 0.96 (methyl-H), O—H = 0.82 Å (hydroxyl) and with Uiso(H) = 1.2Ueq(aromatic-C) and 1.5Ueq(methyl-C, hydroxyl). Methyl and hydroxyl H atoms were allowed to rotate around the C—C and C—O axis but not to tilt to best fit the experimental electron density.
Figures
Fig. 1.
The molecular structure of the title compound with displacement ellipsoids drawn at the 30% probability level.
Fig. 2.
Packing diagram of the title compound.
Crystal data
| C15H20O5S | F000 = 664 |
| Mr = 312.37 | Dx = 1.309 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation λ = 0.71073 Å |
| a = 12.545 (4) Å | Cell parameters from 43 reflections |
| b = 10.085 (3) Å | θ = 4.4–7.3º |
| c = 12.654 (6) Å | µ = 0.22 mm−1 |
| β = 98.05 (3)º | T = 291 (2) K |
| V = 1585.1 (10) Å3 | Block, colourless |
| Z = 4 | 0.45 × 0.40 × 0.38 mm |
Data collection
| Enraf–Nonius CAD-4 diffractometer | Rint = 0.004 |
| Radiation source: fine-focus sealed tube | θmax = 25.5º |
| Monochromator: graphite | θmin = 1.6º |
| T = 291(2) K | h = −15→15 |
| ω/2θ scans | k = 0→12 |
| Absorption correction: none | l = −6→15 |
| 4142 measured reflections | 3 standard reflections |
| 2931 independent reflections | every 250 reflections |
| 1794 reflections with I > 2σ(I) | intensity decay: 0.8% |
Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.044 | w = 1/[σ2(Fo2) + (0.0689P)2 + 0.1341P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.130 | (Δ/σ)max < 0.001 |
| S = 1.03 | Δρmax = 0.24 e Å−3 |
| 2931 reflections | Δρmin = −0.26 e Å−3 |
| 197 parameters | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.0109 (15) |
| Secondary atom site location: difference Fourier map |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.88825 (5) | 1.11227 (6) | 0.13440 (6) | 0.0571 (2) | |
| O1 | 0.86629 (16) | 1.13334 (18) | 0.24047 (15) | 0.0714 (6) | |
| O2 | 0.92693 (13) | 0.96607 (16) | 0.12094 (14) | 0.0587 (5) | |
| O3 | 0.96662 (14) | 1.19132 (18) | 0.09296 (16) | 0.0734 (6) | |
| O4 | 0.61080 (15) | 0.4878 (2) | 0.08885 (17) | 0.0756 (6) | |
| O5 | 0.60020 (17) | 0.5097 (3) | −0.08567 (17) | 0.0921 (7) | |
| H5 | 0.5356 | 0.5095 | −0.0812 | 0.138* | |
| C1 | 0.6708 (2) | 1.1419 (3) | 0.0859 (2) | 0.0655 (7) | |
| H1 | 0.6689 | 1.1457 | 0.1591 | 0.079* | |
| C2 | 0.5771 (2) | 1.1563 (3) | 0.0140 (3) | 0.0768 (9) | |
| H2 | 0.5122 | 1.1706 | 0.0399 | 0.092* | |
| C3 | 0.5778 (2) | 1.1499 (3) | −0.0942 (3) | 0.0718 (8) | |
| C4 | 0.6745 (3) | 1.1276 (3) | −0.1305 (2) | 0.0744 (8) | |
| H4 | 0.6763 | 1.1214 | −0.2035 | 0.089* | |
| C5 | 0.7684 (2) | 1.1143 (3) | −0.0613 (2) | 0.0673 (7) | |
| H5A | 0.8331 | 1.1002 | −0.0876 | 0.081* | |
| C6 | 0.7667 (2) | 1.1218 (2) | 0.0466 (2) | 0.0517 (6) | |
| C7 | 0.4746 (3) | 1.1693 (4) | −0.1705 (3) | 0.1080 (12) | |
| H7A | 0.4173 | 1.1920 | −0.1309 | 0.162* | |
| H7B | 0.4569 | 1.0887 | −0.2094 | 0.162* | |
| H7C | 0.4843 | 1.2394 | −0.2196 | 0.162* | |
| C8 | 0.8744 (2) | 0.8621 (2) | 0.1757 (2) | 0.0574 (7) | |
| H8A | 0.8005 | 0.8870 | 0.1802 | 0.069* | |
| H8B | 0.9116 | 0.8509 | 0.2477 | 0.069* | |
| C9 | 0.87667 (18) | 0.7337 (2) | 0.11476 (18) | 0.0475 (6) | |
| H9 | 0.9516 | 0.7140 | 0.1065 | 0.057* | |
| C10 | 0.8351 (2) | 0.6228 (2) | 0.1803 (2) | 0.0537 (6) | |
| H10A | 0.8800 | 0.6170 | 0.2490 | 0.064* | |
| H10B | 0.7623 | 0.6434 | 0.1926 | 0.064* | |
| C11 | 0.8356 (2) | 0.4903 (2) | 0.1233 (2) | 0.0628 (7) | |
| H11A | 0.9095 | 0.4643 | 0.1201 | 0.075* | |
| H11B | 0.8036 | 0.4237 | 0.1644 | 0.075* | |
| C12 | 0.7748 (2) | 0.4936 (3) | 0.0110 (2) | 0.0618 (7) | |
| H12 | 0.7914 | 0.4111 | −0.0242 | 0.074* | |
| C13 | 0.8138 (2) | 0.6083 (3) | −0.0531 (2) | 0.0620 (7) | |
| H13A | 0.8869 | 0.5905 | −0.0659 | 0.074* | |
| H13B | 0.7687 | 0.6138 | −0.1218 | 0.074* | |
| C14 | 0.81088 (19) | 0.7401 (2) | 0.00422 (18) | 0.0509 (6) | |
| H14A | 0.7369 | 0.7627 | 0.0108 | 0.061* | |
| H14B | 0.8396 | 0.8090 | −0.0373 | 0.061* | |
| C15 | 0.6550 (2) | 0.4981 (2) | 0.0095 (2) | 0.0608 (7) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0529 (4) | 0.0520 (4) | 0.0648 (5) | −0.0071 (3) | 0.0025 (3) | −0.0085 (3) |
| O1 | 0.0762 (13) | 0.0768 (13) | 0.0589 (12) | −0.0026 (10) | 0.0017 (10) | −0.0190 (9) |
| O2 | 0.0526 (10) | 0.0551 (10) | 0.0697 (12) | −0.0066 (8) | 0.0133 (9) | −0.0022 (8) |
| O3 | 0.0561 (12) | 0.0641 (11) | 0.0990 (15) | −0.0186 (9) | 0.0074 (10) | 0.0001 (10) |
| O4 | 0.0562 (12) | 0.1058 (16) | 0.0645 (13) | −0.0209 (10) | 0.0070 (10) | −0.0001 (11) |
| O5 | 0.0640 (13) | 0.139 (2) | 0.0713 (14) | −0.0219 (14) | 0.0036 (11) | 0.0193 (13) |
| C1 | 0.0573 (17) | 0.0739 (18) | 0.0659 (18) | −0.0063 (14) | 0.0111 (15) | −0.0126 (14) |
| C2 | 0.0484 (17) | 0.091 (2) | 0.091 (2) | −0.0003 (15) | 0.0110 (16) | −0.0210 (18) |
| C3 | 0.0640 (19) | 0.0699 (18) | 0.077 (2) | −0.0004 (14) | −0.0058 (17) | −0.0145 (15) |
| C4 | 0.076 (2) | 0.089 (2) | 0.0558 (18) | 0.0038 (17) | 0.0018 (16) | −0.0006 (15) |
| C5 | 0.0593 (17) | 0.0799 (19) | 0.0640 (19) | 0.0029 (14) | 0.0128 (15) | −0.0006 (14) |
| C6 | 0.0525 (15) | 0.0462 (13) | 0.0558 (15) | −0.0047 (11) | 0.0055 (12) | −0.0056 (11) |
| C7 | 0.077 (2) | 0.130 (3) | 0.106 (3) | 0.012 (2) | −0.025 (2) | −0.018 (2) |
| C8 | 0.0589 (16) | 0.0625 (16) | 0.0506 (15) | −0.0092 (12) | 0.0071 (13) | 0.0009 (12) |
| C9 | 0.0410 (13) | 0.0525 (13) | 0.0482 (14) | −0.0033 (11) | 0.0035 (11) | 0.0020 (11) |
| C10 | 0.0464 (14) | 0.0613 (15) | 0.0517 (14) | −0.0021 (12) | 0.0010 (11) | 0.0106 (12) |
| C11 | 0.0499 (15) | 0.0558 (15) | 0.082 (2) | 0.0018 (12) | 0.0072 (14) | 0.0111 (13) |
| C12 | 0.0616 (17) | 0.0529 (14) | 0.0721 (19) | −0.0055 (12) | 0.0138 (14) | −0.0088 (12) |
| C13 | 0.0559 (15) | 0.0810 (18) | 0.0511 (15) | −0.0119 (14) | 0.0144 (13) | −0.0095 (14) |
| C14 | 0.0487 (14) | 0.0576 (14) | 0.0466 (14) | −0.0070 (11) | 0.0070 (11) | 0.0062 (11) |
| C15 | 0.0596 (17) | 0.0564 (15) | 0.0647 (19) | −0.0165 (13) | 0.0023 (15) | 0.0002 (13) |
Geometric parameters (Å, °)
| S1—O3 | 1.4218 (18) | C7—H7C | 0.9600 |
| S1—O1 | 1.423 (2) | C8—C9 | 1.510 (3) |
| S1—O2 | 1.5688 (18) | C8—H8A | 0.9700 |
| S1—C6 | 1.759 (3) | C8—H8B | 0.9700 |
| O2—C8 | 1.464 (3) | C9—C14 | 1.523 (3) |
| O4—C15 | 1.217 (3) | C9—C10 | 1.526 (3) |
| O5—C15 | 1.306 (3) | C9—H9 | 0.9800 |
| O5—H5 | 0.8200 | C10—C11 | 1.519 (3) |
| C1—C6 | 1.380 (4) | C10—H10A | 0.9700 |
| C1—C2 | 1.390 (4) | C10—H10B | 0.9700 |
| C1—H1 | 0.9300 | C11—C12 | 1.516 (4) |
| C2—C3 | 1.372 (4) | C11—H11A | 0.9700 |
| C2—H2 | 0.9300 | C11—H11B | 0.9700 |
| C3—C4 | 1.374 (4) | C12—C15 | 1.501 (4) |
| C3—C7 | 1.515 (4) | C12—C13 | 1.532 (4) |
| C4—C5 | 1.372 (4) | C12—H12 | 0.9800 |
| C4—H4 | 0.9300 | C13—C14 | 1.517 (3) |
| C5—C6 | 1.371 (4) | C13—H13A | 0.9700 |
| C5—H5A | 0.9300 | C13—H13B | 0.9700 |
| C7—H7A | 0.9600 | C14—H14A | 0.9700 |
| C7—H7B | 0.9600 | C14—H14B | 0.9700 |
| O3—S1—O1 | 119.95 (12) | C8—C9—C10 | 108.55 (19) |
| O3—S1—O2 | 104.28 (11) | C14—C9—C10 | 110.34 (18) |
| O1—S1—O2 | 110.26 (11) | C8—C9—H9 | 108.4 |
| O3—S1—C6 | 108.65 (12) | C14—C9—H9 | 108.4 |
| O1—S1—C6 | 108.78 (13) | C10—C9—H9 | 108.4 |
| O2—S1—C6 | 103.69 (10) | C11—C10—C9 | 111.2 (2) |
| C8—O2—S1 | 117.09 (15) | C11—C10—H10A | 109.4 |
| C15—O5—H5 | 109.5 | C9—C10—H10A | 109.4 |
| C6—C1—C2 | 118.7 (3) | C11—C10—H10B | 109.4 |
| C6—C1—H1 | 120.7 | C9—C10—H10B | 109.4 |
| C2—C1—H1 | 120.7 | H10A—C10—H10B | 108.0 |
| C3—C2—C1 | 121.7 (3) | C12—C11—C10 | 113.0 (2) |
| C3—C2—H2 | 119.2 | C12—C11—H11A | 109.0 |
| C1—C2—H2 | 119.2 | C10—C11—H11A | 109.0 |
| C2—C3—C4 | 118.1 (3) | C12—C11—H11B | 109.0 |
| C2—C3—C7 | 120.3 (3) | C10—C11—H11B | 109.0 |
| C4—C3—C7 | 121.6 (3) | H11A—C11—H11B | 107.8 |
| C5—C4—C3 | 121.5 (3) | C15—C12—C11 | 112.6 (2) |
| C5—C4—H4 | 119.3 | C15—C12—C13 | 111.4 (2) |
| C3—C4—H4 | 119.3 | C11—C12—C13 | 110.9 (2) |
| C6—C5—C4 | 119.9 (3) | C15—C12—H12 | 107.2 |
| C6—C5—H5A | 120.1 | C11—C12—H12 | 107.2 |
| C4—C5—H5A | 120.1 | C13—C12—H12 | 107.2 |
| C5—C6—C1 | 120.2 (3) | C14—C13—C12 | 112.2 (2) |
| C5—C6—S1 | 119.5 (2) | C14—C13—H13A | 109.2 |
| C1—C6—S1 | 120.2 (2) | C12—C13—H13A | 109.2 |
| C3—C7—H7A | 109.5 | C14—C13—H13B | 109.2 |
| C3—C7—H7B | 109.5 | C12—C13—H13B | 109.2 |
| H7A—C7—H7B | 109.5 | H13A—C13—H13B | 107.9 |
| C3—C7—H7C | 109.5 | C13—C14—C9 | 110.81 (19) |
| H7A—C7—H7C | 109.5 | C13—C14—H14A | 109.5 |
| H7B—C7—H7C | 109.5 | C9—C14—H14A | 109.5 |
| O2—C8—C9 | 109.29 (19) | C13—C14—H14B | 109.5 |
| O2—C8—H8A | 109.8 | C9—C14—H14B | 109.5 |
| C9—C8—H8A | 109.8 | H14A—C14—H14B | 108.1 |
| O2—C8—H8B | 109.8 | O4—C15—O5 | 121.8 (3) |
| C9—C8—H8B | 109.8 | O4—C15—C12 | 123.9 (3) |
| H8A—C8—H8B | 108.3 | O5—C15—C12 | 114.3 (3) |
| C8—C9—C14 | 112.73 (19) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O5—H5···O4i | 0.82 | 1.83 | 2.642 (3) | 173 |
Symmetry codes: (i) −x+1, −y+1, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: ZL2098).
References
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Gabe, E. J., Le Page, Y., Charland, J.-P., Lee, F. L. & White, P. S. (1989). J. Appl. Cryst.22, 384–387.
- Gabe, E. J., White, P. S. & Enright, G. D. (1993). DIFRAC. American Crystallographic Association, Pittsburgh Meeting, Abstract PA 104.
- Hoshina, K., Yamazaki, Y., Takeshita, T. & Naruchi, T. (1984). IUPHAP 9th International Congress of Pharmacology, p. 697. London.
- Koningsveld, H. van (1972). Acta Cryst. B28, 1189–1195.
- Qi, Q.-R., Huang, W.-C. & Zheng, H. (2008). Acta Cryst. E64, o405. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808003176/zl2098sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808003176/zl2098Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report