Abstract
Alpha-2a interferon (IFN-α2a) has beneficial clinical effects on human T-cell leukemia virus type 1 (HTLV-1) infection, but its antiviral mechanism of action is unknown. Antiviral effects of IFN-α2a were studied in 293T cells expressing HTLV-1 proviral DNA and in HTLV-1-infected cells (HOS/PL, MT2, and HUT102). In 293T cells, an 50% inhibitory concentration of 10 U of IFN-α2a/ml was determined by p19 antigen ELISA. Analysis of IFN-treated cells demonstrated no defect in viral protein synthesis but did show a decrease in the level of released virus, as determined by immunoblot assays. Electron microscopy studies of IFN-treated cells revealed neither a defect in the site of virus budding nor tethering of virus particles at the plasma membrane, thus arguing against an effect on virus release. Cell fractionation studies and confocal microscopy showed no effect of IFN on Gag association with membranes. However, the level of Gag association with lipid rafts was decreased, suggesting a role of IFN in inhibiting HTLV-1 assembly.
Human T-cell leukemia virus type 1 (HTLV-1) is the etiologic agent of adult T-cell lymphoma/leukemia (ATLL) (27) and a neurological disorder, HTLV-1-associated myelopathy/tropical spastic paraparesis (13). It is an oncogenic retrovirus that primarily infects and integrates into CD4+ T lymphocytes. HTLV-1 shares common features with other type C retroviruses, including morphology, genetics, and virus replication. Like the human immunodeficiency virus type 1 (HIV-1) Gag polyprotein, which is necessary and sufficient for assembly and budding of virus-like particles (9, 10, 28), the HTLV-1 Gag polyprotein is required for virus assembly and release from the plasma membrane (4, 16). Proper assembly, release, and infectivity of HTLV-1 particles are dependent on mammalian host cellular factors and myristoylation of the N-terminal glycine residue of the Gag protein (4), similar to HIV-1 Gag membrane targeting and assembly (5, 30).
Recent studies demonstrated HIV-1 Gag association with lipid rafts or raft-like microdomains at the plasma membrane. This interaction plays an important role in virus assembly and release. Lipid rafts are enriched in cholesterol and glycosphingolipid. Depletion of cellular cholesterol significantly reduces HIV-1 particle production and infectivity (19, 20, 23). Rafts provide the platforms for proteins involved in important cellular signaling processes, as well as for assembly and budding of viruses, such as measles and influenza viruses (15, 26, 29). Lipid rafts can be isolated as detergent-resistant microdomains (DRMs) by Triton X-100 extraction at low temperature and by equilibrium flotation centrifugation.
Alpha-2a interferon (IFN-α2a) is a cytokine with antiviral, antitumor, and immunomodulatory activities. Several clinical studies demonstrated the therapeutic effect of IFN-α2a in HTLV-1 infection (1, 32). Interestingly, combination therapy with zidovudine and IFN-α2a results in a high response rate in ATLL patients and significantly improves survival (2). The mechanism of action of this combination is not through regulation of cell proliferation, cell cycle distribution, or apoptosis in HTLV-1-transformed cells. No cytotoxic effect of zidovudine/IFN was observed on fresh ATLL cells in vitro (3). These studies suggest that IFN-α2a plays an important role in inhibiting HTLV-1 viral replication.
IFN blocks viral production at each stage of the replication cycle, i.e., viral entry and uncoating (simian virus 40 and retroviruses), viral RNA transcription (influenza virus, vesicular stomatitis virus, and picornaviruses), viral protein translation (adenovirus, reovirus, and vaccina virus), and particle maturation and release (vesicular stomatitis virus and retroviruses). In HIV-1 infection, IFN-α blocks proviral DNA and protein synthesis and late stages of assembly and budding. Few studies have addressed the activity of IFN in HTLV-1 infection. In the present study, we first determined that IFN inhibits HTLV-1 virion release into the extracellular medium but that it has no effect on virus protein synthesis in cell lysates, demonstrating that IFN induces a defect in virus assembly and/or release. We focused on several questions addressing the mechanism of inhibition induced by IFN: (i) Does IFN inhibit virus budding? (ii) Does IFN block Gag-membrane association? (iii) Is Gag-raft interaction blocked by IFN?
IFN-α2a reduces virion production.
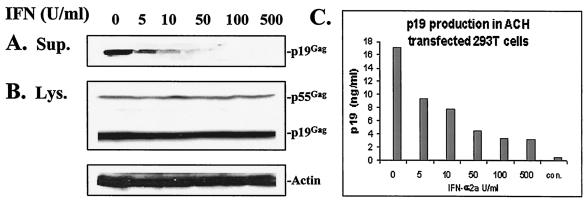
Comparison of viral protein levels in cell lysates and supernatants can be used to examine the process of viral replication, assembly, and release. For this purpose, an HTLV-1 proviral DNA plasmid, pACH (14), was transfected into 293T cells with or without IFN treatment. HTLV-1 proteins in supernatants and lysates were assessed by immunoblot and p19Gag antigen enzyme-linked immunosorbent assay (ELISA). Briefly, 293T cells (n = 3 × 105) were seeded onto six-well plates and were pretreated with various amounts of IFN-α2a (0, 5, 10, 50, 100, or 500 U/ml) overnight. Two micrograms of pACH was then transfected into these cells by using TransIT (Mirus, Madison, Wis.) and was incubated in the presence of IFN for an additional 72 h. Pelleted viral particles from supernatants, as well as viral proteins from cell lysates, were subjected to sodium dodecyl sulfate (SDS)-10% polyacrylamide gels and were then blotted onto polyvinylidene difluoride membranes. Immunoblot analyses were performed with monoclonal antibody (MAb) against viral p19Gag (ZeptoMetrix Corporation, Buffalo, N.Y.), and polyclonal antibody against cellular actin (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.). Horseradish peroxidase-conjugated secondary antibody and an ECL Western blotting detection system (Amersham, Little Chalfont, United Kingdom) were used to detect viral Gag protein. Supernatant virion production under various IFN treatments was assayed by p19Gag antigen ELISA according to the manufacturer's protocol (ZeptoMetrix).
Figure 1A and B demonstrate viral protein expression during IFN-α2a treatment in both cell lysates and supernatants. With increasing doses of IFN-α2a treatment, there was a dose-dependent inhibition of extracellular viral structural protein p19Gag (Fig. 1A). In contrast, viral structural protein p19Gag and p55Gag levels in cell lysates were not affected by increasing doses of IFN-α2a treatment (Fig. 1B). These findings suggest that IFN-α2a did not affect transfection efficiency, transcription, or translation. Levels of actin in cellular lysates were also unaffected by these doses of IFN-α2a (Fig. 1B). To study the effect of IFN-α2a on extracellular viral production in ACH-transfected 293T cells, p19Gag antigen was quantitated by ELISA. Viral p19 protein synthesis was significantly reduced with increasing amounts of IFN-α2a (50% inhibitory concentration = 10 U/ml) (Fig. 1C). This indicates that the process of viral assembly or budding is impaired, but earlier steps in virus replication are not affected by IFN-α2a treatment.
FIG. 1.
Viral protein synthesis during IFN treatment. 293T cells were pretreated with various amounts of IFN-α2a (0, 5, 10 50, 100, and 500 U/ml) for 16 h and were then transfected with proviral DNA, pACH, in the presence of IFN-α2a for 72 h. The same amount of protein from cell lysates and supernatants was loaded in SDS-10% polyacrylamide gel electrophoresis. Anti-HTLV-1 human MAb against p19Gag and polyclonal antibody against cellular actin were used to react with blots. IFN reduced virion p19Gag in cell supernatants in a dose-dependent manner (A) and had no effect on the viral structural protein Gag, and cellular actin in cell lysates (B). IFN significantly reduced p19Gag production in supernatants, based on quantitation of virion production with p19 ELISA. Representative data from four independent experiments are indicated in panel C. The bar labeled “con” indicated mock-transfected cells.
In addition, a similar inhibitory effect of IFN-α2a on HTLV-1 production was also identified by immunoblot analysis and p19Gag ELISA in other HTLV-1-infected cells, including HOS/PL, MT2, and HUT102. This inhibitory effect was reversible after IFN removal in these cells (data not shown). Because high doses of IFN-α2a inhibit cell growth (more than 1,000 U/ml), the effect of 5 to 100 U of IFN-α2a/ml on cell survival was examined in these cell lines by trypan blue uptake. Cell growth was not affected by IFN at 5 to 100 U/ml (data not shown).
IFN-α2a has no inhibitory effect on virus budding.
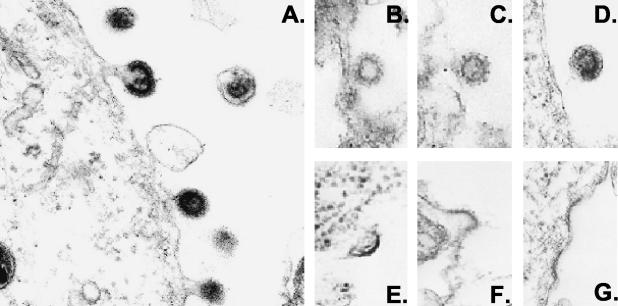
To examine whether IFN blocks virus release, we first examined virus particle formation and budding. ACH-transfected 293T cells were treated with 100 U of IFN-α2a/ml for 3 days and analyzed by transmission electron microscopy. As a control, a separate culture of ACH-transfected 293T cells was treated with 50 μM lactacystin, a proteasome inhibitor, for 2.5 h prior to harvest, to block virus release. Cells were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, washed in the same buffer overnight, and then postfixed with 1% osmium tetroxide at 4°C for 1 h. Dehydration with graded ethanol was performed. The samples were embedded, sectioned, and examined with a Zeiss EM 900 electron microscope.
Virus particles were observed in the extracellular medium of cells transfected with pACH in the absence of IFN (Fig. 2A) and also in the presence of IFN, although the levels were decreased (Fig. 2B to D). There was no defect observed in the site of virus budding or increased tethering of virus particles at the plasma membrane after IFN treatment. As a comparison, no virus particles were detected in cells treated with the proteasome inhibitor lactacystin. There was an accumulation of slightly curved, electron-dense thickenings underneath the cell surface, or there was tethering of virus particles at the plasma membrane (Fig. 2E to G). This result suggests that IFN does not inhibit virus budding.
FIG. 2.
Electron microscopic analysis of viral budding. 293T cells were transfected with ACH in the absence or presence of 100 U of IFN/ml for 3 days. Cells were fixed, and viral particles were visualized by electron microscopy. Virus particles are seen budding through the plasma membrane of ACH-expressing cells in the absence of IFN (A) or the presence of IFN treatment (B to D). No virus particles were detected when cells treated with 50 μM proteasome inhibitor lactacystin for 2.5 h prior to harvest. Slightly curved and electron-dense thickenings are seen in panels E to G. Magnification, ×50,000.
IFN-α2a does not affect Gag membrane localization.
We next tested whether IFN has an effect on membrane association and localization of HTLV-1 Gag. Forty thousand HeLa cells were seeded onto a glass chamber slide (Lab-Tec; Nunc, Inc.) overnight in the presence of IFN-α2a. pACH was transfected into HeLa cells, followed by 2 days of IFN treatment. Cells were then fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min at room temperature. After three washes with PBS, cells were permeabilized with PBS containing 0.01% Triton X-100 for 20 min at room temperature, washed with PBS, and then blocked in PBS containing 10% normal goat serum. Immunostaining was performed with a primary antibody against p19Gag, and with goat anti-mouse immunoglobulin G (IgG) conjugated with fluorescein isothiocyanate. The stained cells were observed with a confocal laser scanning microscope (MRC-1000; Bio-Rad Laboratories, with a 60× objective; Nikon). As depicted in Fig. 3A, membrane localization of p19Gag was detected in ACH-expressing cells both in the absence and in the presence of 100 U of IFN/ml. The punctate fluorescence pattern of p19Gag was unchanged after treatment with IFN, demonstrating that IFN has no inhibitory effect on Gag membrane localization. Similar results were abtained in pACH-transfected 293T cells (not shown).
FIG. 3.
Confocal and immunoblot analysis of Gag membrane localization after IFN treatment. ACH-transfected HeLa cells were treated with IFN for 2 days and then were fixed, permeabilized, and stained with anti-p19Gag. There was a discrete, punctate pattern of Gag protein at the plasma membrane in untreated cells (A), as well as in IFN-treated cells (B). Postnuclear supernatants derived from 293T cells expressing ACH with or without IFN treatment were subjected to equilibrium flotation centrifugation. p19Gag was detected by immunoblot in membrane fractions 1 to 4 and nonmembrane fractions 8 to 10 (C).
To measure the level of membrane-bound p19Gag under IFN treatment, equilibrium flotation centrifugation was performed as previously described (5, 30, 31). IFN-pretreated 293T cells were transfected with pACH, followed by exposure to 100 U of IFN-α2a/ml for three days. Cells were rinsed with PBS, scraped, pelleted by centrifugation at 1,000 × g for 10 min, and resuspended in ice-cold hypotonic buffer (1 mM MgCl2, 10 mM Tris-HCl, pH 7.4, 1 mM EDTA) containing protease inhibitors. Cells were then broken with 45 strokes of a Dounce homogenizer with a Teflon pestle to release cytoplasmic proteins. Postnuclear supernatants were obtained by removal of the nuclei and unbroken cells with centrifugation for 10 min at 1,000 × g after adjusting the final salt concentration to 0.15 M NaCl. The soluble cytosol fractions were separated from the membrane fractions by adjusting the postnuclear supernatant to 80% (wt/vol) sucrose in Tris-EDTA (TE) on the bottom of a centrifuge tube. On the top of this mixture was layered TE containing 55% (wt/vol) sucrose and 5% (wt/vol) sucrose. The gradients were centrifuged at 45,000 rpm for 18 h at 4°C in a Beckman SW55 rotor. Ten fractions were collected from the top of the centrifuge tube. Fractionated samples were analyzed by Western blotting (21, 24) with anti-p19Gag MAb. Figure 3B shows the levels of p19Gag in membrane fractions (fractions 1 to 3) and cytosol fractions (fractions 8 to 10). IFN-α2a (100 U/ml) did not alter the p19 levels in membrane or cytosol fractions, indicating that IFN-α2a does not inhibit Gag membrane binding.
IFN-α2a blocks Gag-raft association.
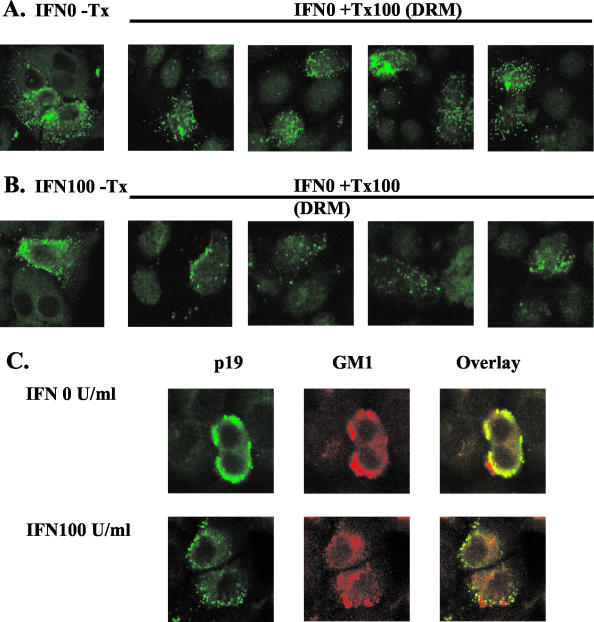
Rafts or raft-like microdomains that are enriched in cholesterol and glycosphingolipids have been shown to be required for HIV-1 Gag assembly (19, 23). Raft-associated proteins are resistant to extraction with nonionic detergents, such as Triton X-100. In order to determine whether IFN increases the sensitivity to Triton X-100 extraction through effects on Gag-raft interaction, confocal microscopy analysis was used to determine p19Gag expression, which was compared to that of a lipid raft-specific ganglioside, GM1. HeLa cells, grown on chamber slides, were pretreated with 100 U of IFN/ml overnight, and transfected with pACH for an additional 2 days. Cells were washed with PBS and were extracted with PBS containing 0.5% Triton X-100 for 20 min on ice and then were fixed with 4% paraformaldehyde in PBS for 20 min at room temperature. For detecting Gag-raft association, ACH-expressing cells were immunostained with either anti-p19 MAb, followed by goat anti-mouse IgG-fluorescein isothiocyanate, or with rabbit anti-GM1 antibody (CalbioChem, San Diego, Calif.), followed by goat anti-rabbit IgG-phycoerythrin.
In the absence of IFN, the punctate fluorescent staining pattern for p19Gag (green) was largely preserved after Triton X-100 extraction (Fig. 4A), suggesting Gag-raft association. However, with IFN treatment, the majority of the signal was lost after Triton X-100 extraction, indicating that IFN reduced Gag-raft association by increasing the solubility of Gag to detergent (Fig. 4B). GM-1 and Gag colocalized, confirming Gag-raft interaction (Fig. 4C, upper panel). Interestingly, the majority of Gag expression after IFN treatment was not colocalized with GM1 (Fig. 4C lower panel), consistent with IFN-α2a inhibition of Gag-raft association.
FIG. 4.
Inhibitory effect of IFN on Gag-raft association. IFN increases the detergent solubility of Gag (A). ACH-transfected 293T cells in the absence (upper panel) or presence (lower panel) of IFN were treated with or without 0.5% Triton X-100 (Tx100) on ice for 20 min before fixation. Gag membrane localization was detected by confocal analysis by using anti-p19Gag. In the presence of Triton X-100, the punctate fluorescence pattern was largely preserved in DRMs without IFN. However, it was largely lost in DRMs from cells treated with 100 U of IFN/ml. IFN blocks Gag-raft colocalization (B). ACH-expressing cells were stained with anti-p19Gag MAb (green) and anti-GM1 (red). p19Gag immunofluorescence colocalized with raft-associated marker GM1 in the absence of IFN (the upper panel), whereas only a small portion of p19Gag was colocalized with GM1 following IFN treatment (the lower panel).
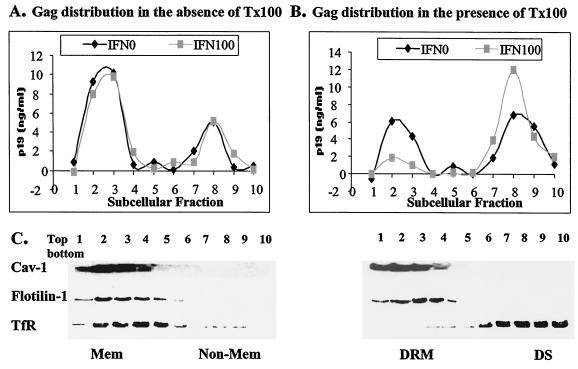
To measure the level of p19Gag in detergent-resistant domains during IFN treatment, flotation assays were employed with various raft-associated markers. Briefly, postnuclear supernatants from pACH-transfected 293T cell homogenates were treated with or without 0.25% Triton X-100 for 20 min on ice and were then subjected to a discontinuous sucrose gradient (5 to 80%) (22, 23). Aliquots of each clarified fraction were precipitated with 20% trichloroacetic acid; proteins were analyzed by p19Gag antigen ELISA and by SDS-polyacrylamide gel electrophoresis followed by immunoblotting with MAbs against human transferrin receptor (TfR) (Zymed Laboratories Inc., South San Francisco, Calif.) and flotilin-1 (BD Biotech) and with polyclonal antibody against caveolin-1 (Santa Cruz).
Without detergent treatment, both the raft-associated proteins caveolin-1 and flotilin-1 and non-raft-associated protein TfR were recovered in membrane fractions 1 to 4. After detergent treatment, TfR was detected predominantly in detergent-sensitive cytosol (fractions 7 to 10), whereas caveolin-1 and flotilin-1 distribution remained in DRM fractions (Fig. 5C). A similar result was observed in human influenza virus hemagglutinin-expressing cells when human influenza virus hemagglutinin was used as a raft marker (data not shown). In the absence of detergent treatment, p19Gag expression and distribution, detected by ELISA, were not affected by IFN treatment (Fig. 5A). About 50% of p19Gag was recovered in DRMs in the absence of IFN-α2a, but with IFN-α2a treatment, only 15% of p19Gag was detected in DRM fractions (Fig. 5B).
FIG. 5.
IFN blocks Gag-raft association. Postnuclear supernatants derived from ACH-transfected 293T cells were treated with or without 0.25% Triton X-100 (Tx100) on ice and were subjected to equilibrium flotation centrifugation. In the absence of IFN, no changes in level of Gag-raft association were detected (A), whereas IFN significantly reduced the level of Gag-raft association (B). Raft-associated proteins caveolin-1 and flotilin-1 were used to determine membrane and DRMs, and TfR was used as a detergent-sensitive (DS), nonraft marker (C).
Discussion.
In this study, we have assessed the inhibitory effect of IFN-α2a on HTLV-1 virion production, focusing our studies on the late stages of virus replication, including viral release, Gag-membrane binding, and Gag-raft interaction. The results show that IFN reduces HTLV-1 virion production in ACH-transfected 293T cells and in other HTLV-1 cell lines (Fig. 1). There was no defect in the process of virus budding after IFN treatment, suggesting that IFN impairs viral assembly (Fig. 2). We also determined that IFN has no effects on virus entry and reverse transcription (M. Wielgosz, personal communication). Further studies revealed that IFN treatment has no effect on Gag-membrane localization (Fig. 3) but rather that IFN affects a late stage of virus assembly by blocking Gag-raft association (Fig. 4 and 5).
Several lines of evidence demonstrate that HIV-1 assembly takes place at the plasma membrane and that virus particles selectively bud from areas rich in raft-associated proteins. First, HIV-1 matrix protein (MA) and transmembrane protein (TM) interact with DRMs in infected Jurkat cells (20). Second, the Gag protein mediates HIV-1 virus assembly, and Gag-raft association is critical for assembly. Gag-raft association, mediated by the N terminus of Gag, occurs after Gag binds to the membrane (23). Gag multimerization enhances its association with raft-like membrane microdomains, which are denser complexes designated “barges” that are resistant to Triton X-100 extraction and are segregated from lipid raft markers (19). Ding et al. (7) recently confirmed earlier work of Ono and Freed, as well as Lindwasser and Resh, who showed cosedimentation of Gag on gradients with raft markers. In addition, they further demonstrated that the I and M domains of Gag combine to mediate Gag-raft interaction under similar gradient conditions. However, by altering the gradient conditions, using graded step iodixanol gradients, they found that Gag protein complexes are denser than typical lipid rafts, which interact with DRMs at the plasma membrane.
This is the first report of Gag-membrane association and Gag-raft interaction in HTLV-infected cells. Our observations are consistent with the studies by Ono and Freed (21-24), as well as those of Resh and colleagues (12, 19). The punctate fluorescence pattern of Gag proteins was localized at the plasma membrane, and Gag also was colocalized with a raft marker, GM1. IFN treatment disrupted Gag interaction with lipid rafts, as determined by Triton X-100 extraction and confocal image analyses, and flotation assays (Fig. 4 and 5). The punctate staining pattern was retained in IFN-treated cells, suggesting that IFN did not interfere with Gag multimerization. Our data suggest that IFN inhibition of Gag-raft interaction is the underlying mechanism in blocking HTLV-1 assembly. Gag multimerization and association with lipid rafts may occur sequentially or independently. Although cholesterol extraction by methyl-β-cyclodextrin mβCD) in HIV-1 infection results in significant reduction in virion production and Gag-raft interaction, it is difficult to repeat this experiment in HTLV-1 infected. HTLV-1 virion production is 1,000-fold lower than that of in HIV-1, and mβCD treatment is toxic to cells; thus, we were unable to assess the effects of cholesterol extraction on HTLV-1 assembly (not shown).
Virion production from ACH-transfected cells is significantly reduced by IFN, whereas viral protein synthesis from cell lysates remains unchanged (Fig. 1). It is possible that IFN alters co- or posttranslational modifications of viral proteins by affecting Gag protein myristoylation, RNA association, or Gag protein conformational changes. However, Gag-membrane targeting appears to be unaffected by IFN, based on the results from both immunostaining and subcellular fractionation experiments (Fig. 3). This indicates that IFN has no effect on Gag modifications, including N-terminal myristoylation of the Gag protein.
It is also possible that IFN blocks virus release by altering the function of the L domain, which is required for Gag budding (17), or by inhibiting ubiquitin ligase-like cellular proteins involved in retrovirus release, such as vacuolar protein sorting proteins (Vps) and tumor susceptibility gene 101 (Tsg 101) (11, 18, 25). However, no inhibitory effect by IFN on virus budding was detected in pACH-transfected 293 T cells compared to the cells treated with proteasome inhibitor. Although the amounts of virus particles were significantly reduced, virus particles were detected in IFN-treated cells (Fig. 2). This indicates that IFN has no effect on virus budding.
The composition of plasma membrane lipid rafts may be altered by IFN treatment. Therefore, there could be altered affinity of Gag with other raft-associated proteins. To examine this possibility, various raft or non-raft-associated markers were used during flotation assays and immunostaining. The raft-associated markers, caveolin-1, flotilin-1, and influenza virus hemagglutinin, remained in the DRMs in the absence or the presence of IFN treatment. As expected, a nonraft marker, TfR, redistributed into detergent-sensitive fractions with or without IFN treatment, following Triton X-100 extraction. However, the amount of Gag protein in the DRMs was significantly reduced (Fig. 5C). These data indicate that IFN does not affect the distribution of most raft-associated markers but does modify Gag-raft association.
There are several possible explanations for the inhibitory effect of IFN on Gag-raft association and virus assembly. IFN may inhibit Gag oligomerization, preventing formation of the Gag complexes that promote Gag-raft association. Secondly, following Gag-membrane targeting, Gag protein binds to nonraft domains and moves laterally to rafts. IFN may block the Gag distributions to rafts. It is possible that IFN-α2a inhibits Gag-raft interaction by decreasing membrane fluidity (6, 8). Finally, IFN may alter specific cellular proteins that are responsible for Gag-raft association. Future studies will focus on determining whether IFN affects Gag multimerization and Gag redistribution at the plasma membrane and will further define the IFN-dependent cell mediator for this effect.
Acknowledgments
We thank M. Levy for assistance with electron microscopy and M. Wielgosz for information on virus entry and reverse transcription.
This study was supported by PHS grants and the AIDS Malignancy Consortium.
REFERENCES
- 1.Bazarbachi, A., M. E. El-Sabban, R. Nasr, F. Quignon, C. Awaraji, J. Kersual, L. Dianoux, Y. Zermati, J. H. Haidar, O. Hermine, and H. de The. 1999. Arsenic trioxide and interferon-alpha synergize to induce cell cycle arrest and apoptosis in human T-cell lymphotropic virus type I-transformed cells. Blood 93:278-283. [PubMed] [Google Scholar]
- 2.Bazarbachi, A., and O. Hermine. 1996. Treatment with a combination of zidovudine and alpha-interferon in naive and pretreated adult T-cell leukemia/lymphoma patients. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 13:S186-S190. [DOI] [PubMed] [Google Scholar]
- 3.Bazarbachi, A., R. Nasr, M. E. El-Sabban, A. Mahe, R. Mahieux, A. Gessain, N. Darwiche, G. Dbaibo, J. Kersual, Y. Zermati, L. Dianoux, M. K. Chelbi-Alix, H. de The, and O. Hermine. 2000. Evidence against a direct cytotoxic effect of alpha interferon and zidovudine in HTLV-I associated adult T cell leukemia/lymphoma. Leukemia 14:716-721. [DOI] [PubMed] [Google Scholar]
- 4.Bouamr, F., L. Garnier, F. Rayne, A. Verna, N. Rebeyrotte, M. Cerutti, and R. Z. Mamoun. 2000. Differential budding efficiencies of human T-cell leukemia virus type I (HTLV-I) Gag and Gag-Pro polyproteins from insect and mammalian cells. Virology 278:597-609. [DOI] [PubMed] [Google Scholar]
- 5.Bryant, M., and L. Ratner. 1990. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc. Natl. Acad. Sci. USA 87:523-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darmani, H., J. L. Harwood, and S. K. Jackson. 1996. Differential effects of interferon-gamma and -beta on fatty acid turnover, lipid bilayer fluidity and TNF-alpha release in murine macrophage J774.2 cells. Life Sci. 59:1109-1119. [PMC free article] [PubMed] [Google Scholar]
- 7.Ding, L., A. Derdowski, J. J. Wang, and P. Spearman. 2003. Independent segregation of human immunodeficiency virus type 1 Gag protein complexes and lipid rafts. J. Virol. 77:1916-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feld, L. J., E. A. Jaffe, L. M. Pfeffer, H. M. Han, and D. B. Donner. 1991. The down-regulation of alpha-interferon receptors in human lymphoblastoid cells: relation to cellular responsiveness to the antiproliferative action of alpha-interferon. J. Biol. Chem. 266:2685-2688. [PubMed] [Google Scholar]
- 9.Freed, E. O. 1998. HIV-1 gag proteins: diverse functions in the virus life cycle. Virology 251:1-15. [DOI] [PubMed] [Google Scholar]
- 10.Garnier, L., J. B. Bowzard, and J. W. Wills. 1998. Recent advances and remaining problems in HIV assembly. AIDS 12:S5-S16. [PubMed] [Google Scholar]
- 11.Garrus, J. E., U. K. von Schwedler, O. W. Pornillos, S. G. Morham, K. H. Zavitz, H. E. Wang, D. A. Wettstein, K. M. Stray, M. Cote, R. L. Rich, D. G. Myszka, and W. I. Sundquist. 2001. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107:55-65. [DOI] [PubMed] [Google Scholar]
- 12.Hermida-Matsumoto, L., and M. D. Resh. 2000. Localization of human immunodeficiency virus type 1 Gag and Env at the plasma membrane by confocal imaging. J. Virol. 74:8670-8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson, S., C. S. Raine, E. S. Mingioli, and D. E. McFarlin. 1988. Isolation of an HTLV-1-like retrovirus from patients with tropical spastic paraparesis. Nature 331:540-543. [DOI] [PubMed] [Google Scholar]
- 14.Kimata, J. T., F. H. Wong, J. J. Wang, and L. Ratner. 1994. Construction and characterization of infectious human T-cell leukemia virus type 1 molecular clones. Virology 204:656-664. [DOI] [PubMed] [Google Scholar]
- 15.Lafont, V., J. Liautard, J. P. Liautard, and J. Favero. 2001. Production of TNF-alpha by human V gamma 9V delta 2 T cells via engagement of Fc gamma RIIIA, the low affinity type 3 receptor for the Fc portion of IgG, expressed upon TCR activation by nonpeptidic antigen. J. Immunol. 166:7190-7199. [DOI] [PubMed] [Google Scholar]
- 16.Le Blanc, I., V. Blot, I. Bouchaert, J. Salamero, B. Goud, A. R. Rosenberg, and M. C. Dokhelar. 2002. Intracellular distribution of human T-cell leukemia virus type 1 Gag proteins is independent of interaction with intracellular membranes. J. Virol. 76:905-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Blanc, I., M. C. Prevost, M. C. Dokhelar, and A. R. Rosenberg. 2002. The PPPY motif of human T-cell leukemia virus type 1 Gag protein is required early in the budding process. J. Virol. 76:10024-10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, L., and S. N. Cohen. 1996. Tsg101: a novel tumor susceptibility gene isolated by controlled homozygous functional knockout of allelic loci in mammalian cells. Cell 85:319-329. [DOI] [PubMed] [Google Scholar]
- 19.Lindwasser, O. W., and M. D. Resh. 2001. Multimerization of human immunodeficiency virus type 1 Gag promotes its localization to barges, raft-like membrane microdomains. J. Virol. 75:7913-7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen, D. H., and J. E. Hildreth. 2000. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 74:3264-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ono, A., D. Demirov, and E. O. Freed. 2000. Relationship between human immunodeficiency virus type 1 Gag multimerization and membrane binding. J. Virol. 74:5142-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ono, A., and E. O. Freed. 1999. Binding of human immunodeficiency virus type 1 Gag to membrane: role of the matrix amino terminus. J. Virol. 73:4136-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ono, A., and E. O. Freed. 2001. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc. Natl. Acad. Sci. USA 98:13925-13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ono, A., J. M. Orenstein, and E. O. Freed. 2000. Role of the Gag matrix domain in targeting human immunodeficiency virus type 1 assembly. J. Virol. 74:2855-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez, O. D., and G. P. Nolan. 2001. Resistance is futile: assimilation of cellular machinery by HIV-1. Immunity 15:687-690. [DOI] [PubMed] [Google Scholar]
- 26.Puertollano, R., J. A. Martinez-Menarguez, A. Batista, J. Ballesta, and M. A. Alonso. 2001. An intact dilysine-like motif in the carboxyl terminus of MAL is required for normal apical transport of the influenza virus hemagglutinin cargo protein in epithelial Madin-Darby canine kidney cells. Mol. Biol. Cell 12:1869-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ratner, L., and B. J. Poiesz. 1988. Leukemias associated with human T-cell lymphotropic virus type I in a non-endemic region. Medicine (Baltimore) 67:401-422. [DOI] [PubMed] [Google Scholar]
- 28.Sakalian, M., and E. Hunter. 1998. Molecular events in the assembly of retrovirus particles. Adv. Exp. Med. Biol. 440:329-339. [DOI] [PubMed] [Google Scholar]
- 29.Scheiffele, P., A. Rietveld, T. Wilk, and K. Simons. 1999. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J. Biol. Chem. 274:2038-2044. [DOI] [PubMed] [Google Scholar]
- 30.Spearman, P., J. J. Wang, N. Vander Heyden, and L. Ratner. 1994. Identification of human immunodeficiency virus type 1 Gag protein domains essential to membrane binding and particle assembly. J. Virol. 68:3232-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang, L., and L. Ratner. 2002. Interaction of HIV-1 gag and membranes in a cell-free system. Virology 302:164-173. [DOI] [PubMed] [Google Scholar]
- 32.Yatsuhashi, H., K. Yamasaki, T. Aritomi, P. Maria, D. Carmen, O. Inoue, M. Koga, and M. Yano. 1997. Quantitative analysis of interferon alpha/beta receptor mRNA in the liver of patients with chronic hepatitis C: correlation with serum hepatitis C virus-RNA levels and response to treatment with interferon. J. Gastroenterol. Hepatol. 12:460-467. [DOI] [PubMed] [Google Scholar]