Abstract
West Nile virus (WNV) infects neurons and leads to encephalitis, paralysis, and death in humans, animals, and birds. We investigated the mechanism by which neuronal injury occurs after WNV infection. Neurons in the anterior horn of the spinal cords of paralyzed mice exhibited a high degree of WNV infection, leukocyte infiltration, and degeneration. Because it was difficult to distinguish whether neuronal injury was caused by viral infection or by the immune system response, a novel tissue culture model for WNV infection was established in neurons derived from embryonic stem (ES) cells. Undifferentiated ES cells were relatively resistant to WNV infection. After differentiation, ES cells expressed neural antigens, acquired a neuronal phenotype, and became permissive for WNV infection. Within 48 h of exposure to an exceedingly low multiplicity of infection (5 × 10−4), 50% of ES cell-derived neurons became infected, producing nearly 107 PFU of infectious virus per ml, and began to die by an apoptotic mechanism. The establishment of a tractable virus infection model in ES cell-derived neurons facilitates the study of the molecular basis of neurotropism and the mechanisms of viral and immune-mediated neuronal injury after infection by WNV or other neurotropic pathogens.
West Nile virus (WNV) is a neurotropic flavivirus that is transmitted by mosquitoes and causes West Nile encephalitis in humans, animals, and birds (35). Humans develop a febrile illness, with a subset of cases progressing to meningoencephalitis (56) or a paralytic or polio-like syndrome (38; T. J. John, Letter, N. Engl. J. Med. 348:564-566, 2003). WNV causes paralysis (14, 71), in part by destroying neurons in the anterior horn of the spinal cord, where motor neurons reside (19, 38). Although neuronal injury may be directly caused by viral infection (11, 71), destruction has also been attributed to infiltrating leukocytes, inflammatory cytokines, and activated microglial cells (19, 20, 24, 61).
In principle, tissue culture models of viral infection in primary neurons can distinguish injury that is caused by virus from injury that is caused by the immune response. For many neurotropic viruses (e.g., poliovirus, herpes simplex virus type 1, Japanese encephalitis virus, rabies virus, and Sindbis virus), cells from neuroblastomas and primary cultures from embryonic or neonatal mice and rats have been used as models of neuronal infection (11, 22, 28, 34, 40, 63). However, the existing primary culture systems have limitations, as they are difficult to establish and scale up for high-throughput applications. In addition, the cultures often contain cells of multiple neuronal cell types, and genetic manipulation is constrained in these postmitotic cells.
Embryonic stem (ES) cells are totipotent continuous cell lines that can be differentiated into neural, muscle, and hematopoietic cells (1, 27, 68, 70) and manipulated genetically (10, 50). We and others have efficiently differentiated ES cells into neurons (ESNC) after retinoic acid induction or by lineage selection (1, 21, 59, 74). Depending upon the induction method, several types of neurons can be generated, including motor neurons (53, 67), retinal neurons (74), dopaminergic neurons (57), interneurons (53), and GABAergic neurons (1, 66). The electrophysiology, morphology, and molecular properties of ESNC are similar to those of primary neuron cultures (1). For this study, using ESNC, we directly assessed the pathophysiology of WNV infection independent of the immune system response to address the mechanism of neuronal injury. Our studies demonstrate that ESNC offer a novel and flexible model system for infection with WNV and other neurotropic viruses.
MATERIALS AND METHODS
Nonneuronal cells, viruses, and antibodies.
BHK21 and C6/36 Aedes albopictus cells were cultured as described previously (13). All WNV infections used a viral isolate (strain 3000.0259, New York, 2000 [17]) that was passaged once in C6/36 cells. Viruses were injected into mice as described previously (13). The DEN strain was a prototype Thai hemorrhagic strain (16681) (55). Mouse monoclonal antibodies against WNV envelope (4G2) (7) or NS1 (1H4) (K. M. Chung, M. Engle, and M. Diamond, unpublished results) or dengue virus envelope (5D4-11, anti-DEN-type 3) (7) proteins were generated from hybridoma supernatants (12). A rabbit polyclonal antibody against the 145-kDa neuron-specific intermediate neurofilament protein was obtained from Chemicon International (Temecula, Calif.). A rat monoclonal antibody against mouse leukocyte common antigen (CD45) was obtained from BD Biosciences (San Diego, Calif.). A mouse monoclonal antibody (anti-NeuN) that recognizes a neuron-specific nuclear protein was also obtained from Chemicon International.
Mouse experiments and tissue preparation.
Strain C57BL/6J (H-2b) inbred wild-type mice were obtained (Jackson Laboratory, Bar Harbor, Maine) and were bred in the animal house of Washington University School of Medicine. Mouse experiments were approved and performed in a biosafety level 3 animal facility according to the guidelines of the Washington University School of Medicine Animal Safety Committee. Eight- to twelve-week-old mice were inoculated with 102 PFU of WNV by footpad injection. Mice were considered paralyzed after WNV infection if they were unable to move their limbs after stimulation or being lifted from the cage. Paralyzed and nonparalyzed mice on day 10 after infection were anesthetized and euthanized. Brains and spinal cords were removed and incubated in phosphate-buffered saline with 4% paraformaldehyde for 24 h at 4°C. After being embedded in paraffin, brain and spinal cord sections were stained with hematoxylin and eosin.
ES cell differentiation.
ES cells were derived from both 129/Sv and C57BL/6 mice and obtained from the ES cell core facility at Washington University. ES cells were grown and differentiated into neurons (ESNC) by use of retinoic acid according to published protocols (1). Briefly, ES cells were cultured in a gelatin-coated T25 flask in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 10% newborn calf serum, nucleotide solution (0.8 mg of adenosine per ml, 0.85 mg of guanosine per ml, 0.73 mg of cytidine per ml, 0.73 mg of uridine per ml, and 0.24 mg of thymidine per ml [Sigma Chemical]), 1,000 U of leukemia inhibitory factor (Chemicon International), and 10−4 M β-mercaptoethanol (Sigma Chemical). Nearly confluent ES cells were dissociated with 0.25% trypsin and cultured in a 100-mm-diameter suspension dish precoated with 0.1% agar (Fisher Scientific, Pittsburgh, Pa.) for 4 days in DMEM without leukemia inhibitory factor and β-mercaptoethanol, with a medium change every 2 days. During this period, dissociated cells aggregated to form embryoid bodies (EB). After 4 days, EB were induced for neuronal differentiation by the addition of all-trans retinoic acid (Sigma Chemical) (500 nM) and were cultured again for 4 days, with medium changes. After an induction period of 4 days, EB were dissociated with 0.25% trypsin and 1 mM EDTA, seeded in gelatin-coated 6-well plates (3 × 106 cells/well) in DMEM without retinoic acid, and incubated at 37°C for 24 h. To enrich for neurons, we switched the culture medium to Neurobasal medium supplemented with B27 (Invitrogen, Carlsbad, Calif.), and 2 days later, 10 μM cytosine arabinoside (AraC; Calbiochem) was added for 24 h to kill rapidly dividing cells. Six- to seven-day-old differentiated ES cell cultures were used for functional studies. ES cells were differentiated into hematopoietic cells as described previously (27, 68). Interleukin-3 and macrophage and granulocyte-macrophage colony-stimulating factors were added to enhance differentiation into macrophages. Recombinant human erythropoietin and kit ligand were added to promote differentiation of EB into primitive erythrocytes (27, 68). Phenotypes were confirmed by flow cytometry (CD11b+ for macrophages) or microscopy and gene expression (erythroid cell color and embryonic globin positive for primitive erythrocytes).
ES cells and virus infection.
Both undifferentiated and differentiated ES cells were infected over a range of virus concentrations. The undifferentiated ES cells were seeded on gelatin-coated 6-well plates (4 × 105 cells/well), incubated for 24 h at 37°C, and infected. Six- to seven-day-old ESNC were grown in gelatin-coated 6-well plates and infected. After 1 h at 37°C, free virus was removed by serial washing with DMEM and cells were incubated for 48 to 72 h in Neurobasal medium. Supernatants were harvested for viral plaque assays and cells were collected for flow cytometric analysis.
Viral plaque assay.
After infection, culture supernatants were analyzed by plaque assays with BHK21 cells to evaluate the production of infectious virus as previously described (12, 14, 15).
Flow cytometry.
After infection, intracellular viral antigen was quantitated by flow cytometry of permeabilized cells as described previously (12, 13, 15). The neuronal phenotype was confirmed by use of antibody against the neuron-specific intermediate neurofilament protein. For one-color flow cytometry, cells were incubated with anti-WNV antibody (4G2) or negative control antibody (anti-DV3) (10 μg/ml) and then with Alexa 488-conjugated goat anti-mouse immunoglobulin G (IgG) (Molecular Probes, Eugene, Oreg.). For two-color flow cytometry, differentiated ES cells were incubated with both anti-neurofilament and anti-WNV antibodies for 1 h followed by Alexa 647-conjugated goat anti-rabbit IgG and Alexa 488-conjugated goat anti-mouse IgG secondary antibodies.
Immunohistochemistry.
WNV antigen in brain and spinal cord sections was detected by use of a polyclonal rat anti-WNV antiserum as described previously (14). Leukocyte infiltration in the spinal cord sections was detected by use of a rat monoclonal antibody against mouse leukocyte common antigen (CD45) according to the manufacturer's instructions (BD Biosciences).
Apoptosis assays.
Seven-day-old ESNC were infected at a multiplicity of infection (MOI) of 10. At 24-h intervals, the level of cell death was determined by flow cytometry using the annexin V apoptosis detection kit I (BD Biosciences). To confirm that apoptotic neurons were infected with WNV, cells were double stained with annexin V and anti-WNV NS1 monoclonal antibody. For the apoptosis experiments, which required intact nonpermeabilized cells, an anti-WNV NS1 protein monoclonal antibody was used because NS1 associates with the extracellular surface on most, but not all, infected neurons. To confirm that all neurons were infected with WNV, in parallel, we permeabilized all cells and then immunostained them for NS1 expression.
DNA fragmentation assay.
To detect DNA fragmentation, we lysed uninfected or infected ESNC with 0.2% Triton X-100. Lysates were centrifuged in a microcentrifuge (14,000 × g) for 20 min and the soluble supernatant fraction was collected. After undergoing treatment with RNase A (1 h, 37°C), samples were extracted with phenol-chloroform, precipitated with ethanol, and electrophoresed on 2% agarose gels.
Electron microscopy.
Seven-day-old ESNC were infected at an MOI of 10. At 48 h postinfection, cells were harvested, washed twice in phosphate-buffered saline, and fixed in electron microscopy grade 2% paraformaldehyde-2.5% glutaraldehyde in 100 mM phosphate buffer, pH 7.2, for 1 h at room temperature. Subsequently, cells were washed in phosphate buffer and treated with a 1% solution of osmium tetroxide (Polysciences Inc., Warrington, Pa.) for 1 h. Cells were then rinsed extensively in double-distilled water prior to en bloc staining with 1% aqueous uranyl acetate (Ted Pella Inc., Redding, Calif.) for 1 h. After several rinses in double-distilled water, samples were dehydrated in ethanol and embedded in Eponate 12 resin (Ted Pella Inc.). Sections (70 to 80 nm) were cut, stained with uranyl acetate and lead citrate, and viewed on a JEOL 1200 EX transmission electron microscope (JEOL USA, Inc., Peabody, Mass.).
In situ TUNEL assay.
Serial sections of brains and spinal cords were assayed for apoptosis by the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) technique. Sections were treated with proteinase K (Invitrogen, Carlsbad, Calif.) and incubated at 37°C for 1 h with a mixture of terminal deoxynucleotidyltransferase (Roche Pharmaceuticals, Nutley, N.J.) and biotin-dUTP (Enzo Biochem, Farmingdale, N.Y.) and then with streptavidin-conjugated horseradish peroxidase. Positive signals were visualized with aminoethyl carbazole as the chromogen (Zymed Laboratories, South San Francisco, Calif.). For double staining, spinal cord sections were first stained for neurons by use of a mouse monoclonal anti-NeuN antibody that recognizes neuron-specific nuclear protein (Chemicon International) and were then stained by the TUNEL technique. After dewaxing, sections were microwaved in citrate buffer for 6 min and immunohistochemistry was performed by a standard protocol. Positive signals were visualized with diaminobenzidine as a brown color. Sections were then stained by TUNEL as described above, and positive signals were visualized with aminoethyl carbazole as a red color.
RESULTS
Neuronal pathology after WNV infection in vivo.
In animals, WNV causes acute encephalitis that can progress to motor paralysis and death (14, 56, 71). In C57BL/6 mice, WNV infection disseminates to the central nervous system (CNS) within 4 to 6 days of infection, leading to a range of motor phenotypes, varying from no obvious motor weakness to individual limb paralysis to hemi- or paraplegia (14). For assessment of the mechanism by which WNV causes neuronal injury, mice were infected, assessed clinically, and analyzed histopathologically for changes in the spinal cord (Fig. 1). Spinal cords from uninfected mice demonstrated normal histology and neuronal morphology (Fig. 1A). Infected but nonparalyzed mice exhibited a low level of leukocyte infiltration, with near-normal neuron morphology (Fig. 1B and H). In contrast, an approximately 10-fold larger number of infiltrating CD45+ leukocytes was observed in the CNS of infected, paralyzed mice (Fig. 1C and I; Table 1). In spinal cords from paralyzed mice, many of the neurons appeared pyknotic, with evidence of altered morphology or frank degeneration; these were predominantly observed in the anterior horn region (Fig. 1C and Table 1). Similar results were observed in the brains of paralyzed mice compared to those of nonparalyzed mice (data not shown).
FIG. 1.
Histopathology, antigen expression, and leukocyte infiltration of WNV infection. (A to C) Histopathology of WNV infection in C57BL/6 mice. Spinal cords from mice that were uninfected (A), infected with WNV but not paralyzed (B), or infected and paralyzed (C) were harvested at 10 days, sectioned, and stained with hematoxylin and eosin (H&E). Typical sections are shown after reviewing >10 independent spinal cords from uninfected, infected and nonparalyzed, or infected and paralyzed wild-type mice. Thick black arrows identify infiltrating leukocytes and thin black arrows denote degenerating neurons. (D to F) WNV antigen expression in the spinal cords of uninfected (D), WNV infected and not paralyzed (E), or infected and paralyzed (F) mice. Spinal cords were harvested 10 days after infection, sectioned, and stained with a rat anti-WNV polyclonal serum. Typical sections are shown after reviewing between 5 and 10 independent spinal cords and brains from uninfected, infected and nonparalyzed, or infected and paralyzed wild-type mice. Green arrows indicate heavily infected neurons. (G to I) Leukocyte infiltration in the spinal cord. Sections from uninfected (G), WNV infected and not paralyzed (H), and infected and paralyzed (I) mice were stained for infiltrating leukocytes with a monoclonal antibody against CD45, a common leukocyte antigen. Red arrows indicate CD45+ leukocytes.
TABLE 1.
Leukocyte infiltration and neuronal injury in the spinal corda
| Condition of mice | No. of CD45+ cells per high-power field | % WNV-positive neurons per high-power field | % of neurons with altered morphology |
|---|---|---|---|
| Mock infected | <1 | 0 | 0 |
| Infected, nonparalyzed | 4 ± 2 | 4 ± 6 | 5 ± 12 |
| Infected, paralyzed | 37 ± 5 | 35 ± 14 | 44 ± 17 |
Results are based on scoring of at least 10 high-power-fields from several spinal cord sections. Altered neuronal morphology was defined by the presence of pyknotic nuclei and gross cell body shrinkage. Cells were confirmed to be of neuronal lineage by the presence of the NeuN antigen. The differences between nonparalyzed and paralyzed infected mice were significant in each of the columns as follows: for “no. of CD45+ cells per high-power field,” P < 0.0001; for “ % WNV-positive neurons per high-power field,” P = 0.0002; for “% of neurons with altered morphology,” P = 0.004. Results are means ± standard deviations.
WNV infection of neurons in vivo.
To confirm whether paralysis and neuronal injury correlated directly with the level of WNV infection, immunohistochemistry was performed.
(i) Brain.
High levels of WNV antigen were detected in neurons in a patchy distribution throughout the brain stem and cerebral cortex. Paralyzed mice consistently demonstrated a higher number of neurons that were infected with WNV (data not shown). A subset of paralyzed mice had high-grade neuronal infections in the cerebellum, specifically in the granular and Purkinjee neurons; these infections were associated with loss of neurons and neuronal architecture (data not shown).
(ii) Spinal cord.
In spinal cords from nonparalyzed mice, about 5% of neurons expressed moderate levels of WNV antigen yet appeared morphologically intact (Fig. 1E and Table 1). In paralyzed mice, a larger number (∼35%) of neurons were infected with WNV (Fig. 1F and Table 1); this was associated with increased neuron granularity, altered morphology, and an increase in the number of CD45+ inflammatory cells in the vicinity of infected neurons (Fig. 1F and I). Although increased levels of viral infection and leukocyte infiltrate in the CNS correlated with neuronal destruction, it was difficult to establish by virologic and histopathologic criteria whether neuronal injury and clinical motor dysfunction were caused by WNV infection or by the resultant host immune response.
WNV infection in ESNC.
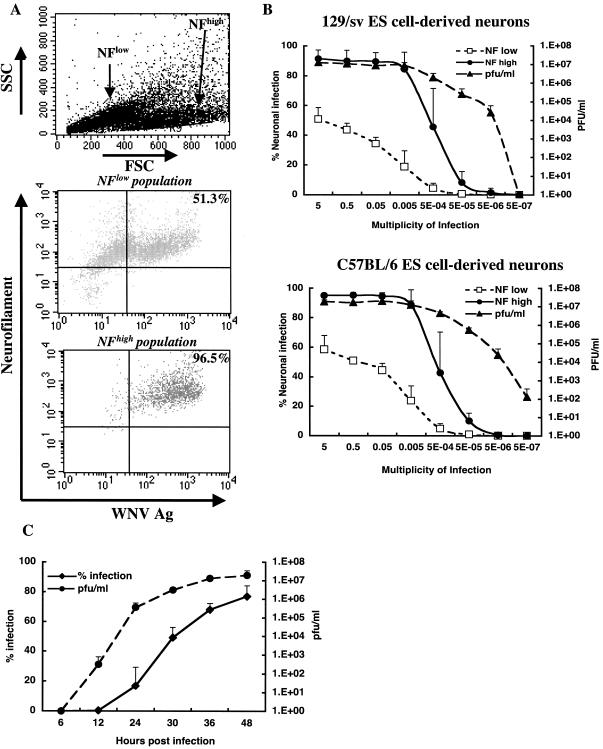
A tissue culture model of WNV infection was developed in ESNC to evaluate neuronal injury in the absence or presence of immune cells. Undifferentiated ES cells were relatively resistant to infection: at an MOI of 5, <2% of undifferentiated ES cells expressed WNV antigens 48 h after infection (Fig. 2). Highly enriched populations of almost pure neurons from C57BL/6 or 129/Sv mouse ES cells were generated after induction with retinoic acid; these neuronal cells are syngeneic with our in vivo infection studies. Treatment with AraC (10 μM) selectively depleted the rapidly dividing astrocytes and resulted in a population that was >95% neurons, as judged by morphology and expression of neuron-specific antigens (Fig. 3A). Based on electrophysiology experiments, differentiation of ES cells with retinoic acid generates a mixture of neurons that respond to the neurotransmitters glutamine, γ-aminobutyric acid, and glycine (reference 1 and data not shown). ESNC were highly permissive for WNV infection (Fig. 3A and B). Two neuronal cell populations were identified by flow cytometry (Fig. 3A). The smaller neurons expressed lower levels of neurofilament proteins (neurofilamentlow) and were somewhat less susceptible to infection. The larger neurons expressed higher levels of neurofilament proteins (neurofilamenthigh) and were highly susceptible to infection. Incubation with WNV at an MOI of 5 × 10−4 resulted in rapid propagation and spread of infection among neurons; even at this low initial MOI, 50% of cells became infected at 48 h. The ESNC secreted high levels of infectious virus into the culture medium, and within 48 h of infection at an MOI of 5 × 10−4, >107 PFU of virus per ml was detected (Fig. 3B).
FIG. 2.
WNV infection in undifferentiated ES cells. Undifferentiated ES 129 (A) or C57BL/6J (B) cells were infected with increasing amounts of WNV. At 48 h postinfection, cells and supernatants were harvested. Viral plaque assays were performed in BHK21 cells to determine the viral titers, which are expressed in PFU per milliliter. The percentage of cells infected was determined by a flow cytometric assay that reliably detects ≥1% of infected cells by intracellular staining of the viral envelope protein.
FIG. 3.
WNV infection of differentiated ES cells. (A) Flow cytometric scatter and fluorescence dot plots of WNV-infected ESNC. Differentiation with retinoic acid yielded two populations of neurons by forward scatter (FSC) and side scatter (SSC) analysis (top). The larger population expressed higher levels of neurofilament (NF) and WNV antigens (compare middle and bottom panels) after infection. (B) Dose-dependent infection of ESNC. ESNC from 129/Sv (top) or C57BL/6 (bottom) mice were infected with increasing amounts of WNV and harvested 2 days later for flow cytometric and viral plaque assays. For flow cytometric analysis, neuronal populations were separated by their relative expression (low or high) of neurofilament protein. Data are the averages of three independent experiments and error bars reflect the standard deviations. (C) Kinetics of WNV infection in ESNC. Neurons were infected at an MOI of 0.05 with WNV. At the indicated times after infection, cells were harvested for flow cytometric analysis of viral antigen and supernatants were assessed for infectious virus as described for Fig. 2. Data are the averages of three independent experiments and error bars reflect the standard deviations.
The kinetics of WNV infection in ESNC were characterized (Fig. 3C). During the first 6 h of infection, viral antigen and infectious virus were not detected. However, by 12 h, a small percentage of cells (0.3%) expressed viral antigen and 5 × 102 PFU/ml was detected in the culture medium. Subsequently, infection spread rapidly through the culture. By 30 h, ∼50% of cells were infected and >106 PFU of virus per ml was present in the culture supernatant.
To confirm that differentiation of ES cells, per se, did not confer permissiveness to WNV infection, we examined the susceptibility of ES cells that were differentiated into primitive erythrocytes and macrophages. After infection at an MOI of 5, only 0.6% of primitive erythrocytes and 29% of macrophages became infected (data not shown). Thus, only specific differentiation of ES cells into neurons conferred the highest level of permissiveness to WNV infection. As an additional specificity control, we examined the susceptibility of ESNC to a pathogenic strain of dengue virus (DEN), a related flavivirus. At an MOI of 5, only 2% of neurofilamentlow and 22% of neurofilamenthigh cells expressed DEN viral antigens and significantly less (∼104 PFU/ml) infectious virus was recovered from the culture medium (data not shown).
WNV infection and neuronal cell death.
Our studies with ESNC demonstrated that the acquisition of a neural phenotype conferred susceptibility to WNV infection. Subsequently, we evaluated whether WNV directly caused cell death in primary neurons. Time course studies were performed after an exposure to a high inoculating dose (MOI of 10) of WNV. As judged by the failure to exclude trypan blue or the binding of DNA-avid dye (7-amino-actinomycin D [7-AAD]), increased cell death was detected within 24 h, and by 72 h most neurons had detached and were not viable (data not shown). Although a prior study suggested that WNV induced cell death in the N2a neuroblastoma cell line (48) through an apoptotic mechanism, it was unclear whether this occurred in primary neurons. To evaluate this, ESNC were infected and analyzed kinetically for the earliest stages of apoptosis by annexin V staining (36, 37). Because annexin V also binds to phosphatidylserine on the inner leaflet of the membrane of necrotic cells that have lost membrane integrity, cells were counterstained with 7-AAD to distinguish viable apoptotic cells from dead cells by two-color flow cytometry. Within 48 h of infection, a marked increase in the number of apoptotic neurons was detected (Fig. 4A and B). Because the annexin V studies required the use of intact, nonpermeabilized cells, neurons were evaluated for WNV infection by the presence of cell surface-associated WNV NS1 antigen; many cells that are infected with flaviviruses have detectable NS1 on their extracellular surfaces through a noncovalent interaction (58, 69). Although many infected neurons expressed WNV NS1 antigen on their cell surfaces, almost all expressed WNV NS1 and E proteins intracellularly (Fig. 4C and D; data not shown); thus, most, if not all, neurons undergoing apoptosis were infected with WNV. DNA fragmentation assays confirmed that WNV caused neuronal cell death by an apoptotic mechanism. Infection of ESNC resulted in the formation of 180- to 200-bp DNA ladders, which is characteristic of the endonucleolytic DNA cleavage associated with apoptosis (Fig. 4E). For final confirmation, electron microscopy was performed on WNV-infected ESNC (Fig. 5). Forty-eight hours after infection, ESNC demonstrated features of neuronal apoptosis, including chromatin clumping and marginalization (Fig. 5B and C) and the formation of large aggregates of dense granular material (Fig. 5D) in the nucleus (16, 23, 73).
FIG. 4.
Apoptosis assays with WNV-infected neurons. (A) Flow cytometry profiles with annexin V and 7-AAD staining of uninfected (left) and WNV-infected neurons at 24 and 48 h (middle and right, respectively). (B) Summary of apoptosis data in neurons derived from ES cells. Data are the averages of three independent experiments and error bars reflect the standard deviations. (C) Intracellular staining of NS1 antigen confirms that ∼90% of neurons are infected at 48 h after infection. (D) WNV infection and neuronal phenotype in annexin V-positive apoptotic cells. Left panel, negative control (anti-DV3 antibody) staining; middle, the majority of annexin V-positive cells also express WNV NS1 protein on their surfaces; right, neurofilament protein staining on the surfaces of annexin V-positive cells. (E) DNA fragmentation in WNV-infected ESNC. ESNC were not infected or infected with WNV at an MOI of 10 and incubated for 48 h. DNA was extracted from cells and resolved by agarose gel electrophoresis. The gel mobility standards (S) are included to the right of the figure. Three independent samples are shown for each condition.
FIG. 5.
Electron micrographs of WNV-infected ESNC. Uninfected (A) or infected (B to D) (48-h time point; MOI of 10) cells were harvested, fixed, sectioned, and processed by electron microscopy. Typical sections are shown after a review of >15 independent images. WNV-infected ESNC show evidence of apoptosis, including chromatin condensation and marginalization (CC) along the nuclear membrane. Scale bar, 1 μm.
To confirm that cell death occurred in vivo after WNV infection, brain and spinal cord sections from infected mice were analyzed by TUNEL assay (Fig. 6). Uninfected and nonparalyzed mice showed virtually no evidence of apoptotic neurons in the CNS (Fig. 6A and B; data not shown). In contrast, significant numbers of dying cells were detected in multiple regions of the brains and spinal cords of infected, paralyzed mice (Fig. 6C and data not shown). In the spinal cords of paralyzed mice, the majority of TUNEL-positive cells were neurons and the most intense staining was observed in the anterior horn (Fig. 6D and data not shown).
FIG. 6.
In situ TUNEL staining of spinal cord neurons after WNV infection. Sections from WNV-infected and nonparalyzed (A and B) and WNV-infected and paralyzed (C and D) mice were analyzed for cell death by TUNEL staining (A to D) and expression of the NeuN neuronal antigen (B and D). Cells that are undergoing cell death (black arrows) appear red after incubation with the chromogenic substrate. In the paralyzed mice, many of the TUNEL-positive cells stained positively for the NeuN neuronal nuclear antigen (green arrows) (D).
DISCUSSION
Neurons are targets for WNV infection (14, 32, 62). Infection in the brain and spinal cord causes destruction and leads to encephalitis and paralysis (14, 38, 39, 71; T. Kelley, R. Prayson, and C. Isada, Letter, N. Engl. J. Med. 348:564-566, 2003). It remains unclear how WNV infection and the host immune system response contribute to neuronal injury. In this study, we first compared the histopathology and immunohistochemistry of spinal cords from infected, paralyzed or nonparalyzed, mice. Although the degree of WNV infection and leukocyte infiltration in the spinal cord correlated with the destruction of anterior horn neurons and clinical paralysis, it was difficult to distinguish viral and immune-mediated neuronal injury. To directly address the pathological effect of WNV in neurons, we developed a tissue culture model of neuron infection by using ES cells. Within 72 h of exposure to WNV, virtually all ESNC became infected and many underwent cell death by an apoptotic mechanism.
WNV infection in the CNS.
In C57BL/6 wild-type mice, WNV disseminates to the CNS within 4 to 6 days of infection (4, 14). However, variable phenotypes are observed: some mice recover, whereas others progress to paralysis, encephalitis, or death. Pathological analysis of CNS tissues from paralyzed mice demonstrated high levels of WNV antigen in neurons and infiltrating leukocytes in the proximity of dying neurons, results that were consistent with previously published studies (14, 18, 46, 47, 71). In contrast, in time-matched nonparalyzed mice, lower levels of viral antigen, leukocyte infiltration, and neuronal injury were detected. Nonetheless, by histopathologic criteria alone, it is difficult to conclude whether paralysis is caused by immune-mediated or viral injury of spinal cord motor neurons. The development of flaccid paralysis or a polio-like syndrome is a more common feature of WNV-induced CNS disease than was previously believed. Our studies demonstrate that infection and destruction of motor neurons in the anterior horn of the spinal cord correlate with clinical paralysis. These results are consistent with autopsy studies on human patients infected with WNV (38, 39).
ESNC as a model for WNV infection.
To determine mechanisms of neuronal injury, we established a novel model for WNV infection in ESNC. Although pathological studies clearly indicate that WNV is a neurotropic virus, no prior infection studies have been performed with primary neurons. In cell culture, WNV infects neuroblastoma cells, astrocytes, and oligodendrocytes (26, 42, 48), yet in vivo in the CNS, only infected neurons are reliably detected (14, 56, 60, 71). Undifferentiated ES cells and ES cells differentiated to nonneuronal fates were less susceptible to WNV infection. Significant infection, however, was observed in ES cell-derived macrophages, which agrees with earlier reports that suggest that macrophages can be infected productively with WNV (8, 30, 49). Our data contrast with those for the only other published stem cell model of viral infection; stem cells derived from 14-day-old embryo brains were susceptible to cytomegalovirus infection without further neuronal differentiation (33). In contrast, acquisition of neuron-specific markers corresponded with permissiveness for WNV infection. The level of susceptibility for a primary cell was striking, as infection of neurons at an MOI of 5 × 10−4 resulted in 50% infection within 48 h. Even the addition of as few as 5 PFU of WNV to 106 ESNC resulted in significant infection within 48 h. Such a degree of susceptibility and spread in tissue culture was observed for very few other cell types; indeed, ESNC were almost as sensitive as the BHK21 cells that were used for determining the viral titer.
Neuronal injury and WNV.
Identification of the mechanism of neuronal injury after WNV infection has remained controversial. At least three hypotheses have been proposed as follows. (i) Neurons undergo cell death directly as a result of viral infection. Studies in neuroblastoma cells suggest that expression of WNV or other flaviviral proteins induces apoptosis (51, 52, 72). (ii) Infected cells in the CNS are targeted by cytotoxic T lymphocytes (CTL). Infection by WNV up-regulates class I major histocompatibility complex (MHC) expression (29, 43, 44), which may lead to antigen-restricted killing of infected neurons (41). Consistent with this, mice that lack granzyme and perforin, the effector proteins in cytotoxic granules of CTL, have decreased mortality after infection with the related Murray Valley encephalitis flavivirus (40a). Similarly, mice that lack CD8+ T cells or β2-microglobulin have improved outcomes relative to congenic wild-type mice after infection with the encephalitic Sindbis alphavirus (40a). However, the relationship between infected neurons, CTL, and disease outcome after WNV infection is complex; our recent studies indicate that genetic deficiencies of CD8+ T cells or classical class I MHC molecules result in enhanced CNS viral burden and mortality (B. Shrestha and M. Diamond, unpublished data). (iii) Neurons may undergo cell death as a result of bystander injury (9). Neurons that are dying secondary to viral infection or immune system targeting may release toxic cytokines that induce injury in uninfected cells. Alternatively, activated lymphocytes or microglial cells may secrete inflammatory mediators that compromise neuron survival (2, 3, 5, 6, 20, 64, 65). Based on our pathological data, the percentage of degenerating neurons correlated directly with WNV infection. We did not observe significant numbers of injured neurons that stained negatively for WNV antigen. Thus, in the spinal cord, bystander injury did not appear to be a primary mechanism for neuronal death.
The studies with ESNC demonstrated that infection by WNV causes apoptosis and cell death in the absence of activated lymphocytes or microglial cells. Apoptosis of ESNC occurred within 48 h of infection, kinetics that are similar to those observed with neuroblastoma cells or neurons infected with the Langat flavivirus (51) or Sindbis alphavirus (25, 45). The induction of apoptosis in ESNC by WNV was confirmed by three independent assays, namely, annexin V staining, DNA fragmentation, and electron microscopy. These data are consistent with previous studies that demonstrated TUNEL-positive neurons in WNV-infected hamsters (31, 54) and DNA fragmentation in WNV-infected tumor cell lines (31, 54). In situ TUNEL assays confirmed neuronal death in paralyzed mice after WNV infection. Studies with immunodeficient mice are under way to determine the mechanisms of cell death after WNV infection in vivo in the absence of an adaptive immune response and whether activated lymphocytes protect against or contribute to neuronal injury.
The development of a WNV infection model in ESNC may help to elucidate the pathogenesis of WNV infection in the CNS. Beyond defining the mechanism of neuronal injury by WNV, important questions such as the molecular basis of neurotropism and the neurobiology of infection may now be readily studied. Experiments are under way to determine why undifferentiated ES cells but not ESNC lack permissiveness for WNV infection and the basis for rapid dissemination of WNV infection in neuronal cultures. ES cells are immortal, amenable to gene targeting (10, 50), and easy to culture and differentiate into neurons (1, 67). Gain-of-function genetic screens with cDNA libraries generated from ESNC may identify the neuronal proteins that confer permissiveness for WNV infection. Our description of a viral infection model for WNV also has significant implications for other neurotropic pathogens. In addition to its use for studying infections in neurons that are syngeneic to many mouse models, this technology may be applicable to human ESNC (59). This is particularly important, as there are few accessible tissue culture models for infection of human neurons. Lastly, an available source of neurons in tissue culture provides a platform for the development and testing of novel small-molecule inhibitors that are directed at aborting infection caused by neurotropic pathogens.
Acknowledgments
We thank T. Ley for the C57BL/6 ES cells; K. H. Choi for help with hematopoietic differentiation of ES cells; and T. Chambers, A. Pekosz, D. Leib, L. Morrison, P. Olivo, P. Stuart, E. Johnson, and members of their laboratories for experimental advice. We thank D. Leib for critical reading of the manuscript, J. Olney for help with the evaluation of the electron micrographs, and the Ophthalmology and Electron Microscopy Core Facilities at Washington University for technical assistance with the pathological sections.
This work was supported by grants from the Centers for Disease Control and Prevention (U50/CCU720545-02), the Pharmacia Biomedical Program, and the Edward Mallinckrodt, Jr., Foundation.
REFERENCES
- 1.Bain, G., D. Kitchens, M. Yao, J. E. Huettner, and D. I. Gottlieb. 1995. Embryonic stem cells express neuronal properties in vitro. Dev. Biol. 168:342-357. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, J., C. G. Bien, and H. Lassmann. 2002. Rasmussen's encephalitis: a role for autoimmune cytotoxic T lymphocytes. Curr. Opin. Neurol. 15:197-200. [DOI] [PubMed] [Google Scholar]
- 3.Bauer, J., T. Sminia, F. G. Wouterlood, and C. D. Dijkstra. 1994. Phagocytic activity of macrophages and microglial cells during the course of acute and chronic relapsing experimental autoimmune encephalomyelitis. J. Neurosci. Res. 38:365-375. [DOI] [PubMed] [Google Scholar]
- 4.Bernard, K. A., and L. D. Kramer. 2001. West Nile virus activity in the United States, 2001. Viral Immunol. 14:319-338. [DOI] [PubMed] [Google Scholar]
- 5.Bien, C. G., J. Bauer, T. L. Deckwerth, H. Wiendl, M. Deckert, O. D. Wiestler, J. Schramm, C. E. Elger, and H. Lassmann. 2002. Destruction of neurons by cytotoxic T cells: a new pathogenic mechanism in Rasmussen's encephalitis. Ann. Neurol. 51:311-318. [DOI] [PubMed] [Google Scholar]
- 6.Boje, K. M., and P. K. Arora. 1992. Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 587:250-256. [DOI] [PubMed] [Google Scholar]
- 7.Brandt, W. E., J. M. McCown, M. K. Gentry, and P. K. Russell. 1982. Infection enhancement of dengue type 2 virus in the U-937 human monocyte cell line by antibodies to flavivirus cross-reactive determinants. Infect. Immun. 36:1036-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardosa, M. J., S. Gordon, S. Hirsch, T. A. Springer, and J. S. Porterfield. 1986. Interaction of West Nile virus with primary murine macrophages: role of cell activation and receptors for antibody and complement. J. Virol. 57:952-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan, W. L., T. Javanovic, and M. L. Lukic. 1989. Infiltration of immune T cells in the brain of mice with herpes simplex virus-induced encephalitis. J. Neuroimmunol. 23:195-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung, S., K. C. Sonntag, T. Andersson, L. M. Bjorklund, J. J. Park, D. W. Kim, U. J. Kang, O. Isacson, and K. S. Kim. 2002. Genetic engineering of mouse embryonic stem cells by Nurr1 enhances differentiation and maturation into dopaminergic neurons. Eur. J. Neurosci. 16:1829-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Couderc, T., F. Guivel-Benhassine, V. Calaora, A. S. Gosselin, and B. Blondel. 2002. An ex vivo murine model to study poliovirus-induced apoptosis in nerve cells. J. Gen. Virol. 83:1925-1930. [DOI] [PubMed] [Google Scholar]
- 12.Diamond, M. S., D. Edgil, T. G. Roberts, B. Lu, and E. Harris. 2000. Infection of human cells by dengue virus is modulated by different cell types and viral strains. J. Virol. 74:7814-7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamond, M. S., T. G. Roberts, D. Edgil, B. Lu, J. Ernst, and E. Harris. 2000. Modulation of dengue virus infection in human cells by alpha, beta, and gamma interferons. J. Virol. 74:4957-4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diamond, M. S., B. Shrestha, A. Marri, D. Mahan, and M. Engle. 2003. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J. Virol. 77:2578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diamond, M. S., M. Zachariah, and E. Harris. 2002. Mycophenolic acid inhibits dengue virus infection by preventing replication of viral RNA. Virology 304:211-221. [DOI] [PubMed] [Google Scholar]
- 16.Dikranian, K., M. J. Ishimaru, T. Tenkova, J. Labruyere, Y. Q. Qin, C. Ikonomidou, and J. W. Olney. 2001. Apoptosis in the in vivo mammalian forebrain. Neurobiol. Dis. 8:359-379. [DOI] [PubMed] [Google Scholar]
- 17.Ebel, G. D., A. P. Dupuis, 2nd, K. Ngo, D. Nicholas, E. Kauffman, S. A. Jones, D. Young, J. Maffei, P. Y. Shi, K. Bernard, and L. D. Kramer. 2001. Partial genetic characterization of West Nile virus strains, New York state, 2000. Emerg. Infect. Dis. 7:650-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eldadah, A. H., and N. Nathanson. 1967. Pathogenesis of West Nile virus encephalitis in mice and rats. II. Virus multiplication, evolution of immunofluorescence, and development of histological lesions in the brain. Am. J. Epidemiol. 86:776-790. [DOI] [PubMed] [Google Scholar]
- 19.Girard, S., T. Couderc, J. Destombes, D. Thiesson, F. Delpeyroux, and B. Blondel. 1999. Poliovirus induces apoptosis in the mouse central nervous system. J. Virol. 73:6066-6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez-Scarano, F., and G. Baltuch. 1999. Microglia as mediators of inflammatory and degenerative diseases. Annu. Rev. Neurosci. 22:219-240. [DOI] [PubMed] [Google Scholar]
- 21.Guan, K., H. Chang, A. Rolletschek, and A. M. Wobus. 2001. Embryonic stem cell-derived neurogenesis. Retinoic acid induction and lineage selection of neuronal cells. Cell Tissue Res. 305:171-176. [DOI] [PubMed] [Google Scholar]
- 22.Heneka, M. T., and D. L. Feinstein. 2001. Expression and function of inducible nitric oxide synthase in neurons. J. Neuroimmunol. 114:8-18. [DOI] [PubMed] [Google Scholar]
- 23.Ishimaru, M. J., C. Ikonomidou, T. I. Tenkova, T. C. Der, K. Dikranian, M. A. Sesma, and J. W. Olney. 1999. Distinguishing excitotoxic from apoptotic neurodegeneration in the developing rat brain. J. Comp. Neurol. 408:461-476. [PubMed] [Google Scholar]
- 24.Iwasaki, Y., J. X. Zhao, T. Yamamoto, and H. Konno. 1986. Immunohistochemical demonstration of viral antigens in Japanese encephalitis. Acta Neuropathol. (Berlin) 70:79-81. [DOI] [PubMed] [Google Scholar]
- 25.Jan, J. T., S. Chatterjee, and D. E. Griffin. 2000. Sindbis virus entry into cells triggers apoptosis by activating sphingomyelinase, leading to the release of ceramide. J. Virol. 74:6425-6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jordan, I., T. Briese, N. Fischer, J. Y. Lau, and W. I. Lipkin. 2000. Ribavirin inhibits West Nile virus replication and cytopathic effect in neural cells. J. Infect. Dis. 182:1214-1217. [DOI] [PubMed] [Google Scholar]
- 27.Keller, G., M. Kennedy, T. Papayannopoulou, and M. V. Wiles. 1993. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol. Cell. Biol. 13:473-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy, P. G., J. Gairns, and A. R. MacLean. 2000. Replication of the herpes simplex virus type 1 RL1 mutant 1716 in primary neuronal cell cultures—possible relevance to use as a viral vector. J. Neurol. Sci. 179:108-114. [DOI] [PubMed] [Google Scholar]
- 29.Kesson, A. M., and N. J. King. 2001. Transcriptional regulation of major histocompatibility complex class I by flavivirus West Nile is dependent on NF-kappaB activation. J. Infect. Dis. 184:947-954. [DOI] [PubMed] [Google Scholar]
- 30.Kimura, T., S. W. Gollins, and J. S. Porterfield. 1986. The effect of pH on the early interaction of West Nile virus with P388D1 cells. J. Gen. Virol. 67:2423-2433. [DOI] [PubMed] [Google Scholar]
- 31.Kimura, T., and D. E. Griffin. 2000. The role of CD8+ T cells and major histocompatibility complex class I expression in the central nervous system of mice infected with neurovirulent Sindbis virus. J. Virol. 74:6117-6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komar, N., S. Langevin, S. Hinten, N. Nemeth, E. Edwards, D. Hettler, B. Davis, R. Bowen, and M. Bunning. 2003. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg. Infect. Dis. 9:311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kosugi, I., Y. Shinmura, H. Kawasaki, Y. Arai, R. Y. Li, S. Baba, and Y. Tsutsui. 2000. Cytomegalovirus infection of the central nervous system stem cells from mouse embryo: a model for developmental brain disorders induced by cytomegalovirus. Lab. Investig. 80:1373-1383. [DOI] [PubMed] [Google Scholar]
- 34.Kosugi, I., Y. Shinmura, R. Y. Li, S. Aiba-Masago, S. Baba, K. Miura, and Y. Tsutsui. 1998. Murine cytomegalovirus induces apoptosis in noninfected cells of the developing mouse brain and blocks apoptosis in primary neuronal culture. Acta Neuropathol. (Berlin) 96:239-247. [DOI] [PubMed] [Google Scholar]
- 35.Kramer, L. D., and K. A. Bernard. 2001. West Nile virus infection in birds and mammals. Ann. N. Y. Acad. Sci. 951:84-93. [DOI] [PubMed] [Google Scholar]
- 36.Kuypers, F. A., R. A. Lewis, M. Hua, M. A. Schott, D. Discher, J. D. Ernst, and B. H. Lubin. 1996. Detection of altered membrane phospholipid asymmetry in subpopulations of human red blood cells using fluorescently labeled annexin V. Blood 87:1179-1187. [PubMed] [Google Scholar]
- 37.Lee, G., and H. B. Pollard. 1997. Highly sensitive and stable phosphatidylserine liposome aggregation assay for annexins. Anal. Biochem. 252:160-164. [DOI] [PubMed] [Google Scholar]
- 38.Leis, A. A., J. Fratkin, D. S. Stokic, T. Harrington, R. M. Webb, and S. A. Slavinski. 2003. West Nile poliomyelitis. Lancet Infect. Dis. 3:9-10. [DOI] [PubMed] [Google Scholar]
- 39.Leis, A. A., D. S. Stokic, J. L. Polk, V. Dostrow, and M. Winkelmann. 2002. A poliomyelitis-like syndrome from West Nile virus infection. N. Engl. J. Med. 347:1279-1280. [DOI] [PubMed] [Google Scholar]
- 40.Liao, C. L., Y. L. Lin, J. J. Wang, Y. L. Huang, C. T. Yeh, S. H. Ma, and L. K. Chen. 1997. Effect of enforced expression of human Bcl-2 on Japanese encephalitis virus-induced apoptosis in cultured cells. J. Virol. 71:5963-5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40a.Licon Luna, R. M., E. Lee, A. Müllbacher, R. V. Blanden, R. Langman, and M. Lobigs. 2002. Lack of both Fas ligand and perforin protects from flavivirus-mediated encephalitis in mice. J. Virol. 76:3202-3211 [DOI] [PMC free article] [PubMed]
- 41.Liu, Y., N. King, A. Kesson, R. V. Blanden, and A. Mullbacher. 1989. Flavivirus infection up-regulates the expression of class I and class II major histocompatibility antigens on and enhances T cell recognition of astrocytes in vitro. J. Neuroimmunol. 21:157-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu, Y., N. King, A. Kesson, R. V. Blanden, and A. Mullbacher. 1988. West Nile virus infection modulates the expression of class I and class II MHC antigens on astrocytes in vitro. Ann. N. Y. Acad. Sci. 540:483-485. [DOI] [PubMed] [Google Scholar]
- 43.Lobigs, M., R. V. Blanden, and A. Mullbacher. 1996. Flavivirus-induced up-regulation of MHC class I antigens; implications for the induction of CD8+ T-cell-mediated autoimmunity. Immunol. Rev. 152:5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Momburg, F., A. Mullbacher, and M. Lobigs. 2001. Modulation of transporter associated with antigen processing (TAP)-mediated peptide import into the endoplasmic reticulum by flavivirus infection. J. Virol. 75:5663-5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nargi-Aizenman, J. L., and D. E. Griffin. 2001. Sindbis virus-induced neuronal death is both necrotic and apoptotic and is ameliorated by N-methyl-d-aspartate receptor antagonists. J. Virol. 75:7114-7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nathanson, N., and G. A. Cole. 1970. Immunosuppression and experimental virus infection of the nervous system. Adv. Virus Res. 16:397-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nathanson, N., and G. A. Cole. 1971. Immunosuppression: a means to assess the role of the immune response in acute virus infections. Fed. Proc. 30:1822-1830. [PubMed] [Google Scholar]
- 48.Parquet, M. C., A. Kumatori, F. Hasebe, K. Morita, and A. Igarashi. 2001. West Nile virus-induced bax-dependent apoptosis. FEBS Lett. 500:17-24. [DOI] [PubMed] [Google Scholar]
- 49.Peiris, J. S., and J. S. Porterfield. 1979. Antibody-mediated enhancement of flavivirus replication in macrophage-like cell lines. Nature 282:509-511. [DOI] [PubMed] [Google Scholar]
- 50.Prelle, K., N. Zink, and E. Wolf. 2002. Pluripotent stem cells—model of embryonic development, tool for gene targeting, and basis of cell therapy. Anat. Histol. Embryol. 31:169-186. [DOI] [PubMed] [Google Scholar]
- 51.Prikhod'ko, G. G., E. A. Prikhod'ko, J. I. Cohen, and A. G. Pletnev. 2001. Infection with Langat flavivirus or expression of the envelope protein induces apoptotic cell death. Virology 286:328-335. [DOI] [PubMed] [Google Scholar]
- 52.Prikhod'ko, G. G., E. A. Prikhod'ko, A. G. Pletnev, and J. I. Cohen. 2002. Langat flavivirus protease NS3 binds caspase-8 and induces apoptosis. J. Virol. 76:5701-5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Renoncourt, Y., P. Carroll, P. Filippi, V. Arce, and S. Alonso. 1998. Neurons derived in vitro from ES cells express homeoproteins characteristic of motoneurons and interneurons. Mech. Dev. 79:185-197. [DOI] [PubMed] [Google Scholar]
- 54.Rowell, J. F., and D. E. Griffin. 2002. Contribution of T cells to mortality in neurovirulent Sindbis virus encephalomyelitis. J. Neuroimmunol. 127:106-114. [DOI] [PubMed] [Google Scholar]
- 55.Russell, P. K., and A. Nisalak. 1967. Dengue virus identification by the plaque reduction neutralization test. J. Immunol. 99:291-296. [PubMed] [Google Scholar]
- 56.Sampson, B. A., C. Ambrosi, A. Charlot, K. Reiber, J. F. Veress, and V. Armbrustmacher. 2000. The pathology of human West Nile virus infection. Hum. Pathol. 31:527-531. [DOI] [PubMed] [Google Scholar]
- 57.Sasai, Y. 2002. Generation of dopaminergic neurons from embryonic stem cells. J. Neurol. 249(Suppl. 2):II41-II44. [DOI] [PubMed] [Google Scholar]
- 58.Schlesinger, J. J., M. W. Brandriss, J. R. Putnak, and E. E. Walsh. 1990. Cell surface expression of yellow fever virus non-structural glycoprotein NS1: consequences of interaction with antibody. J. Gen. Virol. 71:593-599. [DOI] [PubMed] [Google Scholar]
- 59.Schuldiner, M., R. Eiges, A. Eden, O. Yanuka, J. Itskovitz-Eldor, R. S. Goldstein, and N. Benvenisty. 2001. Induced neuronal differentiation of human embryonic stem cells. Brain Res. 913:201-205. [DOI] [PubMed] [Google Scholar]
- 60.Shieh, W. J., J. Guarner, M. Layton, A. Fine, J. Miller, D. Nash, G. L. Campbell, J. T. Roehrig, D. J. Gubler, and S. R. Zaki. 2000. The role of pathology in an investigation of an outbreak of West Nile encephalitis in New York, 1999. Emerg. Infect. Dis. 6:370-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sugamata, M., M. Miyazawa, S. Mori, G. J. Spangrude, L. C. Ewalt, and D. L. Lodmell. 1992. Paralysis of street rabies virus-infected mice is dependent on T lymphocytes. J. Virol. 66:1252-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tesh, R. B., J. Arroyo, A. P. Travassos da Rosa, H. Guzman, S. Y. Xiao, and T. P. Monath. 2002. Efficacy of killed virus vaccine, live attenuated chimeric virus vaccine, and passive immunization for prevention of West Nile virus encephalitis in hamster model. Emerg. Infect. Dis. 8:1392-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tucker, P. C., D. E. Griffin, S. Choi, N. Bui, and S. Wesselingh. 1996. Inhibition of nitric oxide synthesis increases mortality in Sindbis virus encephalitis. J. Virol. 70:3972-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weiland, F., J. H. Cox, S. Meyer, E. Dahme, and M. J. Reddehase. 1992. Rabies virus neuritic paralysis: immunopathogenesis of nonfatal paralytic rabies. J. Virol. 66:5096-5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weissenbock, H., M. Hornig, W. F. Hickey, and W. I. Lipkin. 2000. Microglial activation and neuronal apoptosis in Borna virus infected neonatal Lewis rats. Brain Pathol. 10:260-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Westmoreland, J. J., C. R. Hancock, and B. G. Condie. 2001. Neuronal development of embryonic stem cells: a model of GABAergic neuron differentiation. Biochem. Biophys. Res. Commun. 284:674-680. [DOI] [PubMed] [Google Scholar]
- 67.Wichterle, H., I. Lieberam, J. A. Porter, and T. M. Jessell. 2002. Directed differentiation of embryonic stem cells into motor neurons. Cell 110:385-397. [DOI] [PubMed] [Google Scholar]
- 68.Wiles, M. V., and G. Keller. 1991. Multiple hematopoietic lineages develop from embryonic stem (ES) cells in culture. Development 111:259-267. [DOI] [PubMed] [Google Scholar]
- 69.Winkler, G., S. E. Maxwell, C. Ruemmler, and V. Stollar. 1989. Newly synthesized dengue-2 virus nonstructural protein NS1 is a soluble protein but becomes partially hydrophobic and membrane-associated after dimerization. Virology 171:302-305. [DOI] [PubMed] [Google Scholar]
- 70.Wobus, A. M., K. Guan, H. T. Yang, and K. R. Boheler. 2002. Embryonic stem cells as a model to study cardiac, skeletal muscle, and vascular smooth muscle cell differentiation. Methods Mol. Biol. 185:127-156. [DOI] [PubMed] [Google Scholar]
- 71.Xiao, S. Y., H. Guzman, H. Zhang, A. P. Travassos da Rosa, and R. B. Tesh. 2001. West Nile virus infection in the golden hamster (Mesocricetus auratus): a model for West Nile encephalitis. Emerg. Infect. Dis. 7:714-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang, J. S., M. P. Ramanathan, K. Muthumani, A. Y. Choo, S. H. Jin, Q. C. Yu, D. S. Hwang, D. K. Choo, M. D. Lee, K. Dang, W. Tang, and J. J. Kim. 2002. Induction of inflammation by West Nile virus capsid through the caspase-9 apoptotic pathway. Emerg. Infect. Dis. 8:1379-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yasuhara, S., Y. Zhu, T. Matsui, N. Tipirneni, Y. Yasuhara, M. Kaneki, A. Rosenzweig, and J. A. Martyn. 2003. Comparison of comet assay, electron microscopy, and flow cytometry for detection of apoptosis. J. Histochem. Cytochem. 51:873-885. [DOI] [PubMed] [Google Scholar]
- 74.Zhao, X., J. Liu, and I. Ahmad. 2002. Differentiation of embryonic stem cells into retinal neurons. Biochem. Biophys. Res. Commun. 297:177. [DOI] [PubMed] [Google Scholar]