Abstract
We determined the frequency of DNA recombination between Bombyx mori nucleopolyhedroviruses (BmNPVs) and between BmNPV and the closely related Autographa californica NPV (AcMNPV) in BmN cells, Sf-21 cells, and larvae of Heliothis virescens. The BmN cells were coinfected with two BmNPVs, one with a mutation at the polyhedrin gene (polh) locus and a second carrying a lacZ gene marker cassette. Eleven different BmNPV mutants carrying the lacZ gene marker at various distances (1.4 to 61.7 kb) from polh were used for the coinfections. The Sf-21 cells and larvae of H. virescens were coinfected with wild-type AcMNPV and 1 of the 11 lacZ-marked BmNPV mutants. In BmN cells, high-frequency recombination was detected as early as 15 h postcoinfection but not at 12 h postcoinfection. At 18 h postcoinfection, the mean frequency of recombination ranged between 20.0 and 35.4% when the polh and lacZ marker genes were separated by at least 9.7 kb. When these marker genes were separated by only 1.4 kb, the mean frequency of recombination was 2.7%. In BmN cells, the mean recombination frequency between two BmNPVs increased only marginally when the multiplicity of infection of each virus was increased 10-fold. In Sf-21 cells and the larvae of H. virescens, the recombination frequency between BmNPV and AcMNPV was ≤1.0%. AcMNPV DNA replication occurred normally after the coinfection of Sf-21 cells. BmNPV DNA replication, however, was not detected, indicating that normal DNA replication by both viruses is required for high-frequency recombination.
The Baculoviridae are characterized by large (to >178 kb) double-stranded, circular, DNA genomes and rod-shaped, enveloped virions. Several hundred baculovirus species have been identified and classified into two genera: nucleopolyhedrovirus (NPV) and granulovirus (2). The 133,894-bp genome of the baculovirus-type species Autographa californica NPV (AcMNPV) was the first to be completely sequenced (1). Subsequently, at least 12 baculoviruses have been sequenced (18), including the 128,413-bp genome of Bombyx mori NPV (BmNPV) (12). BmNPV shows >90% nucleotide sequence identity to AcMNPV over 78% of its genome (comparison of 115 of 136 homologous open reading frames [ORFs]) (12). Despite high homology at the nucleotide, amino acid, and genome organization levels (16-18), BmNPV and AcMNPV show unique host specificities. The host range of BmNPV is narrow, whereas that of AcMNPV is relatively wide (13). BmNPV replicates strongly in B. mori-derived cell lines and larvae, whereas AcMNPV replicates strongly in Spodoptera frugiperda-derived cell lines and larvae (22). Conversely, BmNPV (36) and AcMNPV (20) are only weakly permissive in S. frugiperda- and B. mori-derived cell lines, respectively.
During the baculovirus life cycle two forms of progeny are produced, a budded virus and an occluded virus. The occluded viruses of the NPV are referred to as polyhedra. The major protein component of the polyhedra is polyhedrin. Polyhedrin is not essential for virus replication in cell culture, and its gene (polh) is driven by a strong, very late promoter. The presence or absence of polyhedrin can be visualized under light microscopy. These characteristics were exploited by the Summers and Smith (52) and Miller (39) laboratories to establish a baculovirus-based eukaryotic gene expression system that is now in common use. This expression system depends upon homologous recombination between baculovirus genomic DNA and plasmid DNA carrying a foreign gene that is flanked by baculovirus target sequences. After the transfection of baculovirus and plasmid DNAs, recombinant viruses are observed at a frequency of ca. 1 to 2% (34).
Baculoviruses isolated from individual, field-collected insects are often composed of a mixture of genomic variants (5, 19, 29, 33, 49, 50). This suggests that recombination, mutation and/or transposition are common occurrences in baculoviruses. Transposition of AcMNPV by TED, a retrotransposon derived from insect Trichoplusia ni cells grown in culture, is the first example of the integration of a transposable element into a eukaryotic viral genome (38). The piggyBac (also known as IFP2) transposable element from T. ni is also involved in integration into the baculovirus genome (4, 10). Transposition may be responsible for the presence of 20 or more genes of putative eukaryotic and prokaryotic origin that are found in baculovirus genomes (3, 16, 28, 47). Recombination after the coinfection of baculoviruses with high to moderate homology has been demonstrated in cultured insect cells (7, 15, 25, 30, 44, 51, 53) and insect larvae (37, 44). In these studies, the recombination frequencies were in general described as “high” ranging from ca. 6.6% in larvae injected with AcMNPVs (37) to nearly 46.9% for genomic variants of Anticarsia gemmatalis MNPV (7). Recombination between homologous regions of AcMNPV and BmNPV after coinfection has been shown to result in AcMNPV-BmNPV chimeric viruses with expanded host ranges (25, 40, 56). The occurrence of defective interfering particles after serial passage of baculoviruses at high multiplicities of infection (MOIs) also suggests that recombination occurs frequently (26, 46, 55).
In the present study, the timing and frequency of homologous recombination were determined between two BmNPVs and between BmNPV and AcMNPV after coinfection. In BmN cells coinfected with two marked BmNPVs, high-frequency recombination was detected as early as 15 h postcoinfection but not at 12 h postcoinfection. High-frequency recombination occurred relatively uniformly throughout the BmNPV genome since long marker genes were separated by at least 9.7 kb. Increasing the MOI resulted in only marginal increases in the recombination frequency. High-frequency recombination was not found in Sf-21 cells or larvae of Heliothis virescens coinfected with BmNPV and AcMNPV. After the BmNPV-AcMNPV coinfections, AcMNPV showed normal levels of DNA replication, whereas BmNPV did not, indicating that normal DNA replication by both viruses is required for high-frequency recombination.
MATERIALS AND METHODS
Insect cell lines, viruses, and plaque assay.
The BmN-4 (BmN) (31) and IPLB-Sf-21-AE (Sf-21) (54) cell lines were cultured at 27°C in TC-100 medium supplemented with 10% fetal bovine serum and ExCell 401 medium (JRH Biosciences) supplemented with 2.5% fetal bovine serum, respectively. The BmNPV mutants (viruses A to L) (Fig. 1) were propagated in BmN cells. Virus A possesses two mutations: C to A at nucleotide 480 of polh and T to C at nucleotide 846 of orf1629. The C-to-A mutation within polh generates a premature stop codon (TAA) after amino acid residue 159 of polyhedrin. The T-to-C mutation within orf1629 is silent. Viruses B to L (11, 24) each carry a lacZ marker cassette (β-galactosidase gene driven by a Drosophila melanogaster heat shock promoter [hsp70]) within a single BmNPV ORF as indicated in Fig. 1. The 12 BmNPV mutants showed essentially indistinguishable growth curves on BmN cells, reaching plateau titers of at least 108 PFU/ml by 48 h postinfection (p.i.) (data not shown). The hsp70 promoter shows relatively strong activity in lepidopteran cells such as BmN and Sf-21 regardless of heat induction (23, 57). AcMNPV C6 (1) was propagated in Sf-21 cells. Viral titers and the phenotype of parental and recombinant viruses were determined by plaque assay on BmN or Sf-21 cells, as appropriate, as described previously (31). In order to visualize plaques and detect β-galactosidase expression, a second agarose overlay (3.0 ml) containing 0.1% (wt/vol) neutral red and 0.6 mg of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) was added at 4 or 5 days after the initial overlay. Under these conditions, virus A generated plaques that were polyhedrin negative and “white” and viruses B to L generated plaques that were polyhedrin positive and blue. These plaque phenotypes were easily visualized under light microscopy.
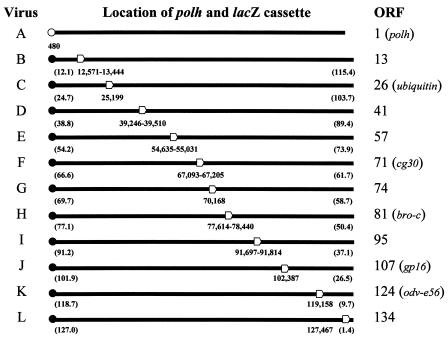
FIG. 1.
Linear maps of the circular genomes of viruses A to L. Each map initiates from the A (nucleotide 1) of the initiation codon (ATG) of the mutated (○) or wild-type (•) polyhedrin gene (polh). The open arrowhead indicates the relative location of the lacZ marker cassette of each virus with respect to polh. The numbers below the linear map indicate the location of the point mutation within polh or insertion coordinates of the lacZ marker cassette. The numbers in parentheses indicate the relative distances (in kilobases) between polh and the lacZ marker cassette of viruses B to L. The genotype of virus A is polh− lacZ−. The genotype of viruses B to L is polh+ lacZ+. The ORF column indicates the BmNPV ORF (see reference 12 for a complete list of BmNPV ORFs) that was inactivated by insertion of the lacZ marker cassette. If the ORF has been previously named, the name is given within the parentheses.
Coinfection of cell cultures.
BmN or Sf-21 cells (4 × 106 cells) in 60-mm-diameter culture dishes were coinfected with viruses at an MOI of 5 PFU per cell of each virus except as indicated. After the 1 -h viral adsorption period, the cells were washed twice with 2.0 ml of fresh medium and cultured at 27°C in 4.0 ml of fresh medium. In all experiments, time zero was defined as the point at which fresh medium was added after the 1 -h viral adsorption period. At the appropriate time postcoinfection, 200 μl of the culture supernatant was collected, centrifuged (10,000 × g for 3 min) to remove cell debris, and stored at 5°C or −80°C prior to plaque assay analysis as described above. Each coinfection experiment was repeated at least three times.
Dot blot hybridization of total cell DNA.
Viral DNA replication was measured by dot blot hybridization of total cell DNAs. BmN or Sf-21 cells (106 cells) in 35-mm-diameter culture dishes were coinfected with viruses at an MOI of 5 PFU per cell of each virus as described above. At 2, 12, 15, 18, and 24 h postcoinfection, the infected cells were detached from the culture surface with a rubber policeman, and 104 cells were collected by centrifugation (2,000 × g for 3 min), washed with phosphate-buffered saline (pH 6.2), and stored at −80°C. Total cell DNAs were released from the cell pellet as described previously (41) with supersaturated NaI, followed by boiling for 10 min. The released DNAs were dot blotted to a nylon membrane (Zeta-Probe; Bio-Rad Laboratories) and fixed by UV cross-linking. The fixed DNAs were hybridized with digoxigenin (DIG)-labeled AcMNPV genomic DNA or pUC-derived plasmid DNA (pTZ18R) containing the lacZ gene and detected with disodium 3-(4-methoxyspiro{1,2-dioxetane-3,2′-(5′-chloro)-tricyclo[3.3.1.13′7]decan}-4-yl)phenyl phosphate (CSPD) by using a DIG-High Prime DNA labeling and detection kit (Roche Molecular Biochemicals). The nylon membrane was rehybridized after the first probe (DIG-labeled AcMNPV genomic DNA) was stripped by boiling for 5 min in 10 mM Tris (pH 7.5)-0.1% sodium dodecyl sulfate, followed by incubation at 80°C for 1 h.
Insect larvae and insect inoculation.
Tobacco budworm (H. virescens) eggs were obtained from a colony maintained by the U.S. Department of Agriculture Agricultural Research Service in Stoneville, Mississippi. The larvae of H. virescens were reared on tobacco budworm artificial diet (Southland Products, Inc.). A colony of silkworms (B. mori) is continually maintained in the laboratory on Silkmate Series M artificial diet (Nosan, Yokohama, Japan). All larvae were reared at 27°C on a 12-h light—12-h dark cycle. Fifth-instar H. virescens or B. mori was injected with 10 μl of a viral suspension containing 105 PFU of AcMNPV or virus G, respectively, in order to generate polyhedra. Polyhedra were purified from infected larvae by centrifugation and resuspension in double-distilled H2O as described previously (45). Purified polyhedra were diluted in double-distilled H2O and quantified by using a hemacytometer with 1/400-mm2 grids (Kayagaki Irika Kogyo, Tokyo, Japan). In order to determine recombination frequencies, third-instar H. virescens was coinoculated with 3,000 polyhedra from AcMNPV and 3,000 polyhedra from virus G. The polyhedra were applied to a small plug of diet in individual containers, and only larvae that completely ingested the contaminated diet within 6 h were further reared. At 72 and 120 h p.i., 5 μl of the hemolymph was collected, the volume adjusted to 1 ml with ExCell 401 medium, filter sterilized, and stored at −80°C prior to plaque assay analysis as described above. Probit analysis (9) was performed with the aid of the POLO (48) computer program.
RESULTS
Frequency of recombination over time.
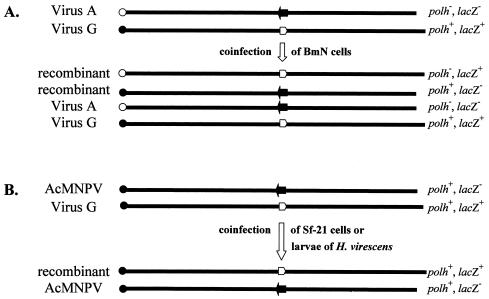
In order to determine when and to what extent homologous recombination occurs between two essentially identical baculoviruses, virus A and virus G were coinfected onto BmN cell monolayers at an MOI of 5 for each virus. The polh mutation of virus A and lacZ marker cassette of virus G are separated by 69.7 and 58.7 kb in the clockwise direction on the circular BmNPV map (32). At 2, 12, 15, 18, 24, and 48 h postcoinfection, progeny viruses in the supernatant were collected and analyzed by plaque assay on BmN cells. The parental viruses, viruses A and G, generated plaques that were polh− lacZ− and polh+ lacZ+, respectively. Should an even number of crossover events occur following the coinfection, the progeny viruses will generate only four types of plaques, the two original parental phenotypes and two recombinant phenotypes: polh− lacZ+ and polh+ lacZ− (Fig. 2A). The mean recombination frequencies after coinfection of viruses A and G are shown in Table 1. Recombinant viruses were not detected at 12 h or earlier times postcoinfection of viruses A and G. At 15 h postcoinfection, 11% of the plaques showed a recombinant phenotype. The mean recombination frequency was highest at 18 and 24 h postcoinfection at ca. 28% and decreased slightly to ca. 25% at 48 h postcoinfection. A typical growth curve (showing a logarithmic growth phase between 15 and 24 h p.i. and plateau titers in the 108 PFU/ml range) were generated after the coinfection of viruses A and G (data shown in the second column of Table 1). This indicated that neither virus interfered with nor enhanced the replication of the other.
FIG. 2.
Linear maps of the progeny viruses that are released after coinfection of BmN cells with viruses A and G (A) or Sf-21 cells or larvae H. virescens with AcMNPV and virus G (B). Each map initiates from the mutated (○) or wild-type (•) polyhedrin gene. The open or filled arrows represent the lacZ marker cassette or wild-type ORF 74 locus, respectively.
TABLE 1.
Titer and mean recombination frequency after coinfection of virus A and virus G at an MOI of 5 for each virus
| Time (h) postcoinfection | Titer (PFU/ml) | Total no. of plaques and phenotype
|
Mean recombination frequencya (%) ± SD | |||
|---|---|---|---|---|---|---|
|
polh−
|
polh+
|
|||||
| lacZ− | lacZ+ | lacZ− | lacZ+ | |||
| 2 | 5.6 × 104 | 231 | 0 | 0 | 262 | 0 ± 0 |
| 12 | 1.2 × 105 | 182 | 0 | 0 | 268 | 0 ± 0 |
| 15 | 2.5 × 105 | 171 | 26 | 21 | 210 | 11.0 ± 0.5 |
| 18 | 2.7 × 106 | 250 | 88 | 110 | 258 | 28.3 ± 2.6 |
| 24 | 2.1 × 107 | 207 | 93 | 99 | 292 | 28.5 ± 3.3 |
| 48 | 1.6 × 108 | 190 | 45 | 71 | 163 | 24.7 ± 1.1 |
That is, [(polh− lacZ+ + polh+ lacZ−) ÷ (polh− lacZ− + polh− lacZ+ + polh+ lacZ− + polh+ lacZ+)] × 100%.
Frequency of recombination throughout the BmNPV genome.
In order to characterize the mean recombination frequency at other loci in the BmNPV genome, virus A was individually coinfected with 10 other BmNPV mutants each carrying a lacZ marker cassette (viruses B to F and H to L, Fig. 1). The polh mutation of virus A and lacZ marker cassette of viruses B, C, D, E, F, H, I, J, K, and L are separated by 12.1 and 115.4 kb, 24.7 and 103.7 kb, 38.8 and 89.4 kb, 54.2 and 73.9 kb, 66.6 and 61.7 kb, 77.1 and 50.4 kb, 91.2 and 37.1 kb, 101.9 and 26.5 kb, 118.7 and 9.7 kb, and 127.0 and 1.4 kb, respectively, in the clockwise direction on the circular map of BmNPV (32). The polh and lacZ markers are separated at most by 61.7 kb (viruses A and F) and at least by 1.4 kb (viruses A and L). Progeny viruses in the supernatant were collected at 2, 12, 15, 18, and 24 h postcoinfection of the BmN cells and analyzed by plaque assay on BmN cells. Recombinant viruses were not detected at 12 h postcoinfection or earlier times after the coinfection of most of the virus pairs (A-B, A-C, A-D, A-E, A-F, A-H, A-I, and A-L). One recombinant virus (polh− lacZ+) was found after the coinfection of viruses A and J, and another recombinant virus (polh+ lacZ−) was found after the coinfection of viruses A and K at 12 h postcoinfection. These recombinant viruses represented a mean recombination frequency of <0.5%. At 15 h postcoinfection the mean recombination frequencies ranged between 6.8 and 20.9% except after the coinfection of viruses A and L, which showed a mean recombination frequency of 1.5% (data not shown). The mean recombination frequencies at 18 h postcoinfection of virus A with viruses B to L are shown in Table 2. The mean recombination frequencies ranged between 20.0 and 35.4%, except after the coinfection of viruses A and L, which again showed a lower mean recombination frequency of 2.7%. At 24 h postcoinfection, the mean recombination frequencies for all of the coinfections were essentially the same as those observed at 18 h postcoinfection (data not shown). The viral titers at all of the time points tested were typical of wild-type BmNPV in BmN cells, indicating that there was no interference nor enhancement of virus replication following these coinfections (data not shown).
TABLE 2.
Titer and mean recombination frequency at 18 h p.i. after coinfection at an MOI of 5 for each virus
| Viruses coinfecteda | Titer (PFU/ml) | Total no. of plaques and phenotype
|
Mean recombination frequencyb (%) ± SD | |||
|---|---|---|---|---|---|---|
|
polh−
|
polh+
|
|||||
| lacZ− | lacZ+ | lacZ− | lacZ+ | |||
| A:B (12.1) | 2.7 × 106 | 260 | 57 | 84 | 304 | 20.0 ± 1.9 |
| A:C (24.7) | 3.1 × 106 | 227 | 76 | 108 | 262 | 27.3 ± 4.3 |
| A:D (38.8) | 1.7 × 106 | 250 | 89 | 108 | 180 | 31.0 ± 6.2 |
| A:E (54.2) | 2.5 × 106 | 261 | 102 | 139 | 235 | 32.9 ± 4.2 |
| A:F (61.7) | 2.2 × 106 | 336 | 71 | 99 | 116 | 27.3 ± 1.6 |
| A:G (58.7) | 2.7 × 106 | 250 | 88 | 110 | 258 | 28.3 ± 2.6 |
| A:H (50.4) | 2.4 × 106 | 401 | 68 | 187 | 74 | 35.0 ± 2.2 |
| A:I (37.1) | 2.2 × 106 | 305 | 83 | 94 | 182 | 26.6 ± 5.6 |
| A:J (26.5) | 2.0 × 106 | 337 | 98 | 122 | 201 | 28.9 ± 1.6 |
| A:K (9.7) | 2.9 × 106 | 239 | 119 | 117 | 192 | 35.4 ± 1.7 |
| A:L (1.4) | 2.6 × 106 | 445 | 14 | 5 | 239 | 2.7 ± 0.6 |
The numbers in parentheses indicate the closest distance between the markers in kilobases.
That is, [(polh− lacZ+ + polh+ lacZ−) ÷ (polh− lacZ− + polh− lacZ+ + polh+ lacZ− + polh+ lacZ+)] × 100%.
Effect of MOI on the frequency of recombination.
The effect of raising (10-fold) or lowering (5- and 50-fold) the MOI on the mean recombination frequency are shown in Tables 3 and 4. Recombinant viruses were not detected at 12 h or earlier times postcoinfection of viruses A and G or viruses A and L at an MOI of 50, 1, or 0.1 of each virus (data not shown). After the coinfection of viruses A and G (Table 3) at an MOI of 50 of each virus, the mean recombination frequency remained relatively constant at ca. 33% at 18 h and later times postcoinfection. After coinfection at an MOI of 1 of each virus, the mean recombination frequency decreased from ca. 24% at 18 and 24 h postcoinfection to roughly 18% at 36 and 48 h postcoinfection. In contrast, after coinfection at an MOI of 0.1 of each virus, the mean recombination frequency remained constant at ca. 7% until 36 h postcoinfection and then increased (16.3%) at 48 h postcoinfection. After the coinfection of viruses A and L (Table 4) at an MOI of 50 of each virus, the mean recombination frequency roughly doubled from 2.4% at 18 h postcoinfection to 4.8% at 24 h and later times postcoinfection. After the coinfection of viruses A and L at an MOI of 0.1 of each virus, the mean recombination frequency remained at <1% at 36 h and earlier times postcoinfection and then increased to 4% at 48 h postcoinfection.
TABLE 3.
Titer and mean recombination frequency following coinfection of virus A and virus G at an MOI of 50, 1, or 0.1 for each virus
| Time (h) postcoinfection | MOI | Titer (PFU/ml) | Total no. of plaques and phenotype
|
Mean recombination frequencya (%) ± SD | |||
|---|---|---|---|---|---|---|---|
|
polh−
|
polh+
|
||||||
| lacZ− | lacZ+ | lacZ− | lacZ+ | ||||
| 18 | 50:50 | 4.1 × 106 | 164 | 68 | 85 | 167 | 31.7 ± 0.9 |
| 1:1 | 2.0 × 105 | 129 | 27 | 41 | 91 | 25.4 ± 5.0 | |
| 0.1:0.1 | 4.8 × 103 | 154 | 9 | 13 | 126 | 7.3 ± 0.8 | |
| 24 | 50:50 | 3.3 × 107 | 66 | 36 | 50 | 98 | 33.7 ± 4.4 |
| 1:1 | 7.1 × 106 | 95 | 24 | 29 | 80 | 23.3 ± 2.7 | |
| 0.1:0.1 | 1.9 × 105 | 147 | 12 | 11 | 99 | 8.6 ± 0.6 | |
| 36 | 50:50 | 1.6 × 108 | 73 | 33 | 39 | 82 | 32.1 ± 3.2 |
| 1:1 | 1.0 × 108 | 151 | 29 | 25 | 107 | 17.6 ± 4.6 | |
| 0.1:0.1 | 7.8 × 106 | 149 | 6 | 9 | 103 | 5.6 ± 0.3 | |
| 48 | 50:50 | 2.0 × 108 | 80 | 50 | 34 | 81 | 34.5 ± 1.1 |
| 1:1 | 2.0 × 108 | 123 | 22 | 29 | 104 | 18.3 ± 0.5 | |
| 0.1:0.1 | 6.2 × 107 | 115 | 19 | 24 | 120 | 16.3 ± 2.8 | |
That is, [(polh− lacZ+ + polh+ lacZ−) ÷ (polh− lacZ− + polh− lacZ+ + polh+ lacZ− + polh+ lacZ+)] × 100%.
TABLE 4.
Titer and mean recombination frequency after coinfection of virus A and virus L at an MOI of 50 or 0.1 for each virus
| Time (h) postcoinfection | MOI | Titer (PFU/ml) | Total no. of plaques and phenotype
|
Mean recombination frequencya (%) ± SD | |||
|---|---|---|---|---|---|---|---|
|
polh−
|
polh+
|
||||||
| lacZ− | lacZ+ | lacZ− | lacZ+ | ||||
| 18 | 50:50 | 7.9 × 106 | 238 | 4 | 7 | 213 | 2.4 ± 0.2 |
| 0.1:0.1 | 4.6 × 103 | 170 | 0 | 1 | 116 | 0.3 ± 0.4 | |
| 24 | 50:50 | 3.3 × 107 | 111 | 8 | 5 | 123 | 4.6 ± 3.3 |
| 0.1:0.1 | 2.8 × 105 | 140 | 0 | 0 | 96 | 0.0 ± 0.0 | |
| 36 | 50:50 | 1.6 × 108 | 120 | 6 | 8 | 101 | 5.3 ± 1.7 |
| 0.1:0.1 | 1.0 × 107 | 147 | 1 | 1 | 95 | 0.9 ± 0.3 | |
| 48 | 50:50 | 2.5 × 108 | 133 | 6 | 5 | 111 | 4.4 ± 1.2 |
| 0.1:0.1 | 6.2 × 107 | 138 | 4 | 4 | 85 | 4.0 ± 2.8 | |
That is, [(polh− lacZ+ + polh+ lacZ−) ÷ (polh− lacZ− + polh− lacZ+ + polh+ lacZ− + polh+ lacZ+)] × 100%.
Frequency of recombination between BmNPV and AcMNPV.
As mentioned in the introduction, BmNPV and AcMNPV share very high homology at the nucleotide sequence and genome organization levels but have essentially nonoverlapping host ranges. In order to analyze the effect of relatively small nucleotide sequence differences between BmNPV and AcMNPV on the recombination frequency, viruses B to L were individually coinfected with AcMNPV onto Sf-21 cells at an MOI of 5 of each virus. At 12, 18, and 24 h postcoinfection, viruses in the supernatant were collected and analyzed by plaque assay on Sf-21 cells. By using Sf-21 cells for plaque assay, the BmNPV parent viruses are selected against and only two plaque phenotypes are generated (Fig. 2B). Thus, blue plaques most likely result from a recombination event that transfers the lacZ marker cassette from the BmNPV mutant to AcMNPV, whereas white plaques are most likely generated by AcMNPV. Both the blue and white plaques produce polyhedra (polh+) assuming that random mutation of polh does not occur. After these coinfections, recombinant viruses were not detected at 12 h postcoinfection. The recombination frequencies were in general 1.0% or lower at 18 h postcoinfection (Table 5) and 1.9% or lower at 24 h postcoinfection (data not shown). The virus titers in the supernatant at 12 h (low-105 PFU/ml range), 18 h (mid-106 PFU/ml range), and 24 h (low- 107 PFU/ml range) postcoinfection were similar to those observed after the single infection of Sf-21 cells with AcMNPV, indicating that BmNPV did not inhibit nor enhance the replication of AcMNPV.
TABLE 5.
Titer and recombination frequency at 18 h p.i. after the coinfection of Sf-21 cells at an MOI of 5 of AcMNPV and BmNPV mutant
| Viruses coinfected | Titer (PFU/ml) | Total no. of plaques (phenotype)
|
Recombination frequencya (%) ± SD | |
|---|---|---|---|---|
| Wild-type AcMNPV (polh+lacZ−) | Recombinant (polh+lacZ+) | |||
| AcMNPV:B | 6.3 × 106 | 7,000 | 71 | 1.0 ± 0.5 |
| AcMNPV:C | 6.1 × 106 | 6,680 | 64 | 1.0 ± 0.3 |
| AcMNPV:D | 6.4 × 106 | 6,880 | 48 | 0.7 ± 0.1 |
| AcMNPV:E | 5.9 × 106 | 6,720 | 31 | 0.5 ± 0.6 |
| AcMNPV:F | 5.8 × 106 | 6,900 | 18 | 0.3 ± 0.4 |
| AcMNPV:G | 3.6 × 106 | 8,980 | 54 | 0.6 ± 0.2 |
| AcMNPV:H | 9.2 × 106 | 9,550 | 19 | 0.2 ± 0.2 |
| AcMNPV:I | 9.8 × 106 | 7,080 | 4 | 0.1 ± 0.1 |
| AcMNPV:J | 6.0 × 106 | 7,210 | 14 | 0.2 ± 0.1 |
| AcMNPV:K | 6.4 × 106 | 6,730 | 22 | 0.3 ± 0.1 |
| AcMNPV:L | 5.6 × 106 | 6,730 | 70 | 1.0 ± 0.7 |
That is, [(polh+ lacZ+) ÷ (polh+ lacZ− + polh+ lacZ+)] × 100%.
Dot blot hybridization.
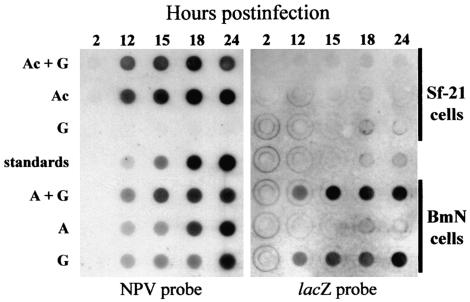
Compared to the high-frequency recombination that was found after the coinfection of two BmNPVs, the recombination frequencies after the coinfection of BmNPV and AcMNPV were dramatically lower. In order to determine whether this dramatic reduction occurred in conjunction with a deficiency in viral DNA replication, dot blot hybridizations of total cellular DNAs isolated from coinfected or singly infected Sf-21 or BmN cells were performed (Fig. 3). In Sf-21 cells, virus-specific DNA replication of ca. 50 ng of DNA per 104 cells was initially detected at 12 h postcoinfection of virus G and AcMNPV. This amount gradually increased to ca. 400 ng/104 cells by 24 h postcoinfection. Hybridization of the same membrane with a second, virus G-specific probe indicated that this DNA was solely the result of AcMNPV DNA replication. The timing and level of AcMNPV DNA replication in BmNPV-AcMNPV-coinfected Sf-21 cells was the same as that found in Sf-21 cells singly infected with AcMNPV, indicating that BmNPV did not interfere with AcMNPV DNA replication. Furthermore, virus G-specific DNA replication was not detected in virus G-inoculated Sf-21 cells.
FIG. 3.
Dot blot hybridization of total cell DNAs isolated from 104 Sf-21 or BmN cells at 2, 12, 15, 18, or 24 h p.i. with AcMNPV and virus G (Ac+G), AcMNPV (Ac), virus G (G), viruses A and G (A+G), or virus A (A). The DNAs were fixed to a nylon membrane and hybridized with labeled AcMNPV DNA (NPV probe) or virus G-specific probe (lacZ probe). The standards consist of 0, 5, 20, 100, and 500 ng of AcMNPV DNA.
In BmN cells that were coinfected with viruses A and G, ca. 25 ng of virus-specific DNA per 104 cells was detected at 12 h p.i. This amount gradually increased to ca. 300 ng per 104 cells by 24 h p.i. In BmN cells singly infected with virus A or virus G, ca. 15 to 25 ng of virus-specific DNA/104 cells was detected at 12 and 15 h p.i. This amount gradually increased to ca. 200 and 300 ng/104 cells at 18 and 24 h p.i., respectively. Virus-specific DNA was not detected in mock-infected Sf-21 or BmN cells (data not shown).
Frequency of recombination in insect larvae.
In order to analyze the recombination frequency through the natural route of infection in insect larvae, third-instar H. virescens was fed a mixture of 3,000 AcMNPV polyhedra and 3,000 virus G polyhedra. At 72 and 120 h postcoinfection, progeny viruses in the hemolymph were collected and analyzed by plaque assay on Sf-21 cells. As is the case after the coinfection of Sf-21 cells with AcMNPV and virus G, only two plaque phenotypes (Fig. 2B) were observed after plaque assay on the Sf-21 cell monolayers. The recombination frequencies in larvae (Table 6) were in general ∼50-fold lower than that found after the coinfection of Sf-21 cells. At 72 and 120 h postcoinfection, the virus titer in the hemolymph was 2.8 × 108 and 8.2 × 108 PFU/ml, respectively, these titers were similar to those found in third instar H. virescens that were singly infected with AcMNPV. Time-mortality analysis of larvae orally infected with AcMNPV and virus G indicated that the lethal time needed to produce 50% mortality was 115.8 h (102.4 to 126.5 h, 95% fiducial limits). Oral inoculation with 3,000 AcMNPV polyhedra showed similar mortality, whereas mortality did not occur after oral inoculation with 3,000 virus G polyhedra.
TABLE 6.
Titer and recombination frequency after oral coinfection of third-instar H. virescens with 3,000 polyhedra from AcMNPV and virus G
| Time (h) postcoinfection | Titer (PFU/ml of hemolymph) | Total no. of plaques (phenotype)
|
Recombination frequencya (%) ± SD | |
|---|---|---|---|---|
| Wild-type AcMNPV (polh+lacZ−) | Recombinant (polh+lacZ+) | |||
| 72 | 2.8 × 108 | 170,000 | 51 | 0.02 ± 0.02 |
| 120 | 8.2 × 108 | 139,000 | 55 | 0.03 ± 0.02 |
That is, [(polh+ lacZ+) ÷ (polh+ lacZ− + polh+ lacZ+)] × 100%.
DISCUSSION
We established here a model system to analyze the onset and frequency of DNA recombination between essentially identical or highly homologous baculoviruses, each carrying marker genes that generated unique phenotypes that were easily visualized by light microscopy. After the coinfection of two BmNPVs (viruses A and G) in which the marker genes were separated by at least 58.7 kb, the mean recombination frequency reached a maximum of ca. 28% at 18 and 24 h postcoinfection and decreased slightly to ca. 25% at 48 h postcoinfection. Recombination was not detected at 12 h or earlier times postcoinfection but could be detected at a frequency of 11% at 15 h postcoinfection. Our findings are the first to show that high-frequency recombination occurs as early as 18 h postcoinfection and possibly as early as 15 h postcoinfection. Here, we arbitrarily define high-frequency recombination as a mean recombination frequency of ≥20%. Hajos et al. (15) observed a mean frequency of recombination of 23% at 72 h in Sf-21 cells coinfected with two AcMNPV mutants at an MOI of 5 of each virus. In similar experiments, Croizier and Ribeiro (7) observed recombination frequencies of between 26.5 and 46.9% at 14 days in Sf-9 cells coinfected with two Anticarsia gemmatalis NPV variants at an MOI of 8 of each virus. The recombination rates that were determined in our study and by other researchers are based upon recombination events that could be detected due to a change in phenotype. Recombination events that (i) occur between the marker genes or (ii) result in the reversion to the parental phenotype (i.e., the occurrence of an even number of recombination events) should increase these “observed” recombination frequencies.
In order to assess the effects of the physical distance between the marker genes and/or regional differences in the sequences flanking the marker genes on the recombination frequency, virus A was individually coinfected with viruses B to L (Fig. 1) at an MOI of 5 of each virus. At 15 h postcoinfection, the mean recombination frequencies after these coinfections ranged between 6.8 and 20.9% when the marker genes were separated by at least 9.7 kb. At 18 h postcoinfection, the mean recombination frequencies in general increased by twofold in comparison to those found at 15 h p.i. The mean recombination frequencies at 18 h postcoinfection ranged between 20.0 and 35.4%. When two marker genes were relatively close together (i.e., separated by only 1.4 kb) as was the case for viruses A and L, the mean recombination frequency was significantly lower (i.e., 1.5 and 2.7% at 15 and 18 h postcoinfection, respectively). It is possible that this low frequency of recombination was an inherent characteristic of the ORF 134 locus. However, the fact that low-frequency recombination was not detected between any of the other marked loci suggests that there is a minimum separation requirement for high-frequency recombination. Kumar and Miller (27) identified two regions within the PstI-G and PstI-I fragments (8.6 to 10.2 and 14.3 to 17.9 map units) of AcMNPV in which deletions frequently occur after serial passage of AcMNPV in cultured cells of Trichoplusia ni. In our experiments, these regions corresponded most closely to the location of the lacZ marker genes in virus B (ORF 13) and virus C (ORF 26). In our hands, the lacZ marker genes of viruses B and C did not show any increased propensity to undergo recombination after coinfection with either virus A (Table 2) or AcMNPV (Table 5).
A single gene was deleted in each of the 12 BmNPV mutants (viruses A to L) that were used in these studies. Each of these mutants showed identical replication rates (i.e., generated growth curves that were statistically identical [data not shown]) to the each other and to the wild-type parent BmNPV. Furthermore, the viral titers found in the supernatant of coinfected BmN cells were statistically identical to each other. These findings suggest that deletion of the individual genes did not affect the fitness of the mutant BmNPVs or their ability to undergo homologous recombination. We did, however, find that the calculated recombination frequency with respect to a single locus (i.e., the frequency of the generation of only one of the recombinant phenotypes in relation to the only one of the parental phenotypes) was not uniform after several of the coinfections. This was most pronounced after the coinfection of viruses A and H (ORF 81) in which virus H appeared to lose the lacZ marker cassette approximately five times (i.e., 71.8% ÷ 14.5% = {[187/(187 + 74) ÷ [68/(68 + 401]}) more easily than it was gained by virus A. If we assume that there is sufficient sequence homology flanking the lacZ marker cassette at the ORF 81 locus, it is possible that an intramolecular recombination event (after the first single crossover, but prior to the second single crossover) resulted in the deletion of the lacZ cassette. Coinfection of virus A and another BmNPV mutant in which ORF 80 (immediately upstream of ORF 81) was deleted, however, did not show this tendency (data not shown). Thus, a more likely explanation for this discrepancy is that the fitness of the recombinant (i.e., virus lacking the lacZ cassette at the ORF 81 locus) was slightly improved over the parental virus, resulting in an increase or faster release of progeny virions.
The mean recombination frequency increased marginally from 28.3 to 31.7%, a 12.0% increase, at 18 h postcoinfection after a 10-fold increase in the MOIs of viruses A and G from 5 to 50 of each virus. In similar experiments, Hajos et al. (15) reported that the mean recombination frequency of two AcMNPVs increased from 23 to 41% by increasing the MOIs from 5 to 20. Summers et al. (53) inoculated T. ni cells with AcMNPV and the closely related Rachiplusia ou NPV at an MOI of 500 of each virus and reported a minimum recombination frequency of 7%. These findings suggest that increasing or decreasing the MOI beyond what is minimally necessary (i.e., for each cell to be infected with at least one of each virus) for coinfection has relatively little effect on increasing the recombination frequency. On the other hand, even following the inoculations at an MOI of 0.1 of viruses A and G, the mean recombination frequency appeared to significantly decrease. Poisson distribution predicts that after the inoculation of a population of cells at an MOI of 0.1 of each virus, roughly 82% of the cells will be uninfected, 16% will be infected with one virus, and 2% will be infected with two or more viruses. Thus, after the inoculations at an MOI of 0.1 of viruses A and G, the detected recombination frequency was most likely skewed lower by the release of nonrecombinant progeny from cells (16% of population) infected with only one of the parental viruses. Thus, the results after either high or low MOI coinfections suggest that the viral replication process itself is critical for high-frequency homologous recombination.
BmNPV and AcMNPV share high genome homology, and BmNPV is considered to be weakly permissive in Sf-21 cells. Thus, after the coinfection of Sf-21 cells with BmNPV and AcMNPV, we initially predicted that the recombination frequency between BmNPV and AcMNPV would only be marginally lower than that found after the BmNPV-BmNPV coinfections. To our surprise, however, the recombination frequencies between BmNPV and AcMNPV (Table 5) were significantly lower than those found after the BmNPV-BmNPV coinfections (Table 2). The recombination frequencies between BmNPV and AcMNPV were similar to that found after the transfection of genomic viral DNA and recombinant transfer vector plasmid DNA for the generation of recombinant baculoviruses. Early events in the BmNPV infection cycle such as attachment, nucleocapsid translocation, and uncoating occur normally occur normally in most Sf-21 cells (41, 42). Thus, our findings suggest that a late event such as DNA replication is critical for high-frequency recombination to occur. After the coinfection of viruses A and G (BmNPV-BmNPV coinfection) onto BmN cells, ca. 25 and 150 ng of BmNPV DNA (per 104 cells) were detected at 12 and 15 h postcoinfection, respectively, indicating that BmNPV DNA replication is occurring actively at these times. In contrast, BmNPV-specific DNA replication was not detected in the BmNPV-AcMNPV coinfected Sf-21 cells at 24 h or early times postcoinfection. These findings indicate that the lack of BmNPV DNA replication was responsible for the dramatic drop in the recombination frequency after BmNPV-AcMNPV coinfection. On the basis of in vitro transposition studies by using AcMNPV sequences, Martin and Weber (35) also found that high-frequency recombination is strictly dependent upon viral DNA replication. More recently, Crouch and Passarelli (8) have shown by cotransfection studies that a subset of AcMNPV genes that are involved in DNA replication is required for high-frequency homologous recombination of AcMNPV.
Wild-type baculoviruses are used as safe and effective biopesticides in the Americas, Europe, and Asia, with particular success in Brazil for the protection of soybean (43). The general use of baculoviruses in developed countries, however, has been limited primarily due to their slow speed of kill compared to synthetic chemical pesticides. The speed of kill of the wild-type baculovirus has been significantly improved by genetic engineering (reviewed in reference 21). Public concern, however, has been raised over the release of genetically modified baculoviruses into the field (6, 14). One focus of this concern has been the potential risk of the drift through either homologous or heterologous recombination of, for example, an insect-selective scorpion toxin gene carried by the recombinant baculovirus. The findings of the present study provide insight into the recombination frequencies that are obtained under the most optimal laboratory conditions (i.e., recombination between identical viruses after coinfection of cultured cells at a known MOI). Under these optimal conditions, we found that the recombination frequency was high. One would expect, however, that under suboptimal conditions (e.g., recombination between heterologous viruses or within the larval host) such as those found in the field, the recombination frequency or potential of recombination should be significantly lower. In the present study, the detected recombination frequency in larva was roughly 50-fold lower than that observed in vitro. We also found an ∼30-fold drop in the recombination frequency even between two highly homologous baculoviruses when both viruses were not able to replicate their DNAs. We believe that future risk assessment studies will be greatly enhanced by the recent availability of the complete genome sequences of numerous other baculoviruses (18). By using baculoviruses that show lower and lower homology for coinfection studies, one may be able to extrapolate the relationship between baculovirus heterogeneity and recombination frequency, thus getting a better understanding of the relative risks associated with the use baculovirus insecticides.
Acknowledgments
We thank Josie Wei Chua, Sumiko Gomi, and Taro Ohkawa for help with the construction of the mutant BmNPVs; Ahmet Bora Inceoglu for help with the in vivo experiments; and Just M. Vlak for access to data prior to publication and helpful discussions.
This research was supported by grants 96-33120-3726, 98-35302-6955, and 2001-35302-09919 from the U.S. Department of Agriculture.
Footnotes
This paper is dedicated to the memory of Susumu Maeda.
REFERENCES
- 1.Ayres, M. D., S. C. Howard, J. Kuzio, M. Lopez-Ferber, and R. D. Possee. 1994. The complete DNA sequence of Autographa californica nuclear polyhedrosis virus. Virology 202:586-605. [DOI] [PubMed] [Google Scholar]
- 2.Blissard, G., B. Black, N. Crook, B. A. Keddie, R. Possee, G. Rohrmann, D. Theilmann, and L. Volkman. 2000. Family Baculoviridae, p. 195-202. In M. H. V. van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, D. J. McGeoch, J. Maniloff, M. A. Mayo, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy: classification and nomenclature of viruses. Academic Press, Inc., San Diego, Calif.
- 3.Blissard, G. W., and G. F. Rohrmann. 1990. Baculovirus diversity and molecular biology. Annu. Rev. Entomol. 35:127-135. [DOI] [PubMed] [Google Scholar]
- 4.Cary, L. C., M. Goebel, B. G. Corsaro, H. G. Wang, E. Rosen, and M. J. Fraser. 1989. Transposon mutagenesis of baculoviruses: analysis of Trichoplusia ni transposon IFP2 insertions with the FP-locus of nuclear polyhedrosis viruses. Virology 172:156-169. [DOI] [PubMed] [Google Scholar]
- 5.Cherry, C. L., and M. D. Summers. 1985. Genotypic variation among wild isolates of two nuclear polyhedrosis viruses isolated from Spodoptera littoralis. J. Invertebr. Pathol. 46:289-295. [Google Scholar]
- 6.Cory, J. S. 2000. Assessing the risks of releasing genetically modified virus insecticides: progress to date. Crop Prot. 19:779-785. [Google Scholar]
- 7.Croizier, G., and H. C. T. Ribeiro. 1992. Recombination as a possible major cause of genetic heterogeneity in Anticarsia gemmatalis nuclear polyhedrosis virus wild populations. Virus Res. 26:183-196. [Google Scholar]
- 8.Crouch, E. A., and A. L. Passarelli. 2002. Genetic requirements for homologous recombination in Autographa californica nucleopolyhedrovirus. J. Virol. 76:9323-9334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finney, D. J. 1952. Probit analysis, a statistical treatment of the sigmoid response curve, 2nd ed. Cambridge University Press, Cambridge United Kingdom.
- 10.Fraser, M. J., G. E. Smith, and M. D. Summers. 1983. Acquisition of host cell DNA sequences by baculoviruses: relationship between host DNA insertions and FP mutants of Autographa californica and Galleria mellonella nuclear polyhedrosis viruses. J. Virol. 47:287-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomi, S., S. G. Kamita, and S. Maeda. 1999. Deletion analysis of all genes of Bombyx mori nucleopolyhedrovirus (BmNPV). RIKEN Rev. 22:39-41. [Google Scholar]
- 12.Gomi, S., K. Majima, and S. Maeda. 1999. Sequence analysis of the genome of Bombyx mori nucleopolyhedrovirus. J. Gen. Virol. 80:1323-1337. [DOI] [PubMed] [Google Scholar]
- 13.Groner, A. 1986. Specificity and safety of baculoviruses, p. 178-196. In R. R. Granados and B. A. Federici (ed.), The biology of baculoviruses, vol. 1. CRC Press, Inc., Boca Raton, Fla.
- 14.Hails, R. 2001. Natural and genetically modified baculoviruses: environmentally friendly pest control or an ecological threat? Outlook Agric. 30:171-178. [Google Scholar]
- 15.Hajos, J. P., J. Pijnenburg, M. Usmany, D. Zuidema, P. Zavodszky, and J. M. Vlak. 2000. High frequency recombination between homologous baculoviruses in cell culture. Arch. Virol. 145:159-164. [DOI] [PubMed] [Google Scholar]
- 16.Hayakawa, T., G. F. Rohrmann, and Y. Hashimoto. 2000. Patterns of genome organization and content in lepidopteran baculoviruses. Virology 278:1-12. [DOI] [PubMed] [Google Scholar]
- 17.Herniou, E. A., T. Luque, X. Chen, J. M. Vlak, D. Winstanley, J. S. Cory, and D. R. O'Reilly. 2001. Use of whole genome sequence data to infer baculovirus phylogeny. J. Virol. 75:8117-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herniou, E. A., J. A. Olszewski, J. S. Cory, and D. R. O'Reilly. 2003. The genome sequence and evolution of baculoviruses. Annu. Rev. Entomol. 48:211-234. [DOI] [PubMed] [Google Scholar]
- 19.Hodgson, D. J., A. J. Vanbergen, A. D. Watt, R. S. Hails, and J. S. Cory. 2001. Phenotypic variation between naturally co-existing genotypes of a lepidopteran baculovirus. Evol. Ecol. Res. 3:687-701. [Google Scholar]
- 20.Ikeda, M., Y. Katou, Y. Yamada, S. Chaeychomsri, and M. Kobayashi. 2001. Characterization of Autographa californica nucleopolyhedrovirus infection in cell lines from Bombyx mori. J. Insect Bio/Technol. Sericol. 70:49-58. [Google Scholar]
- 21.Inceoglu, A. B., S. G. Kamita, A. C. Hinton, Q. Huang, T. F. Severson, K.-D. Kang, and B. D. Hammock. 2001. Recombinant baculoviruses for insect control. Pest Management Sci. 57:981-987. [DOI] [PubMed] [Google Scholar]
- 22.Kamita, S. G., and S. Maeda. 1993. Inhibition of Bombyx mori nuclear polyhedrosis virus (NPV) replication by the putative DNA helicase gene of Autographa californica NPV. J. Virol. 67:6239-6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamita, S. G., K. Majima, and S. Maeda. 1993. Identification and characterization of the p35 gene of Bombyx mori nuclear polyhedrosis virus that prevents virus-induced apoptosis. J. Virol. 67:455-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang, W., M. Suzuki, E. Zemskov, K. Okano, and S. Maeda. 1999. Characterization of baculovirus repeated open reading frames (bro) in Bombyx mori nucleopolyhedrovirus. J. Virol. 73:10339-10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kondo, A., and S. Maeda. 1991. Host range expansion by recombination of the baculoviruses Bombyx mori nuclear polyhedrosis virus and Autographa californica nuclear polyhedrosis virus. J. Virol. 65:3625-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kool, M., J. W. Voncken, F. L. J. Van Lier, J. Tramper, and J. M. Vlak. 1991. Detection and analysis of Autographa californica nuclear polyhedrosis virus mutants with defective interfering properties. Virology 183:739-746. [DOI] [PubMed] [Google Scholar]
- 27.Kumar, S., and L. K. Miller. 1987. Effects of serial passage of Autographa californica nuclear polyhedrosis virus in cell culture. Virus Res. 7:335-350. [DOI] [PubMed] [Google Scholar]
- 28.Kuzio, J., M. N. Pearson, S. H. Harwood, C. J. Funk, J. T. Evans, J. M. Slavicek, and G. F. Rohrmann. 1999. Sequence and analysis of the genome of a baculovirus pathogenic for Lymantria dispar. Virology 253:17-34. [DOI] [PubMed] [Google Scholar]
- 29.Lee, H. H., and L. K. Miller. 1978. Isolation of genotypic variants of Autographa californica polyhedrosis virus. J. Virol. 27:754-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, H. H., and L. K. Miller. 1979. Isolation, complementation, and initial characterization of temperature-sensitive mutants of the baculovirus Autographa californica nuclear polyhedrosis virus. J. Virol. 31:240-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maeda, S. 1989. Gene transfer vectors of a baculovirus, Bombyx mori, and their use for expression of foreign genes in insect cells, p. 167-181. In J. Mitsuhashi (ed.), Invertebrate cell system applications, vol. I. CRC Press, Inc., Boca Raton, Fla.
- 32.Maeda, S., and K. Majima. 1990. Molecular cloning and physical mapping of the genome of Bombyx mori nuclear polyhedrosis virus. J. Gen. Virol. 71:1851-1856. [DOI] [PubMed] [Google Scholar]
- 33.Maeda, S., Y. Mukohara, and A. Kondo. 1990. Characteristically distinct isolates of the nuclear polyhedrosis virus from Spodoptera litura. J. Gen. Virol. 71:2631-2640. [DOI] [PubMed] [Google Scholar]
- 34.Martens, J. W. M., M. M. Van Oers, B. D. Van De Bilt, P. Oudshoorn, and J. M. Vlak. 1995. Development of a baculovirus vector that facilitates the generation of p10-based recombinants. J. Virol. Methods 52:15-19. [DOI] [PubMed] [Google Scholar]
- 35.Martin, D. W., and P. C. Weber. 1997. DNA replication promotes high-frequency homologous recombination during Autographa californica multiple nuclear polyhedrosis virus infection. Virology 232:300-309. [DOI] [PubMed] [Google Scholar]
- 36.Martin, O., and G. Croizier. 1997. Infection of a Spodoptera frugiperda cell line with Bombyx mori nucleopolyhedrovirus. Virus Res. 47:179-185. [DOI] [PubMed] [Google Scholar]
- 37.Merryweather-Clarke, A. T., M. Hirst, and R. D. Possee. 1994. Recombination between genetically modified and unmodified Autographa californica nuclear polyhedrosis virus in Trichoplusia ni larvae. Acta Virol. 38:311-315. [PubMed] [Google Scholar]
- 38.Miller, D. W., and L. K. Miller. 1982. A virus mutant with an insertion of a copia-like transposable element. Nature 299:562-564. [DOI] [PubMed] [Google Scholar]
- 39.Miller, L. K. 1988. Baculoviruses as gene expression vectors. Annu. Rev. Entomol. 42:177-199. [DOI] [PubMed] [Google Scholar]
- 40.Mori, H., H. Nakazawa, N. Shirai, N. Shibata, M. Sumida, and F. Matsubara. 1992. Foreign gene expression by a baculovirus vector with an expanded host range. J. Gen. Virol. 73:1877-1880. [DOI] [PubMed] [Google Scholar]
- 41.Morris, T. D., and L. K. Miller. 1993. Characterization of productive and non-productive AcMNPV infection in selected insect cell lines. Virology 197:339-348. [DOI] [PubMed] [Google Scholar]
- 42.Morris, T. D., and L. K. Miller. 1992. Promoter Influence on baculovirus-mediated gene expression in permissive and nonpermissive insect cell lines. J. Virol. 66:7397-7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moscardi, F. 1999. Assessment of the application of baculoviruses for control of Lepidoptera. Annu. Rev. Entomol. 44:257-289. [DOI] [PubMed] [Google Scholar]
- 44.Munoz, D., J. M. Vlak, and P. Caballero. 1997. In vivo recombination between two strains of the genus nucleopolyhedrovirus in its natural host, Spodoptera exigua. Appl. Environ. Microbiol. 63:3025-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Reilly, D. R., L. K. Miller, and V. A. Luckow. 1992. Baculovirus expression vectors: a laboratory manual. W. H. Freeman and Co., New York, N.Y.
- 46.Pijlman, G. P., E. van den Born, D. E. Martens, and J. M. Vlak. 2001. Autographa californica baculoviruses with large genomic deletions are rapidly generated in infected insect cells. Virology 283:132-138. [DOI] [PubMed] [Google Scholar]
- 47.Possee, R. D., and G. F. Rohrmann. 1997. Baculovirus genome organization and evolution, p. 109-140. In L. K. Miller (ed.), The baculoviruses. Plenum Press, Inc., New York, N.Y.
- 48.Russell, R. M., J. L. Robertson, and N. E. Savin. 1977. POLO: a new computer program for probit analysis. Entomol. Soc. Am. Bull. 23:209-213. [Google Scholar]
- 49.Shapiro, D. I., J. R. Fuxa, H. D. Braymer, and D. P. Pashley. 1991. DNA restriction polymorphism in wild isolates of Spodoptera frugiperda nuclear polyhedrosis virus. J. Invertebr. Pathol. 58:96-105. [DOI] [PubMed] [Google Scholar]
- 50.Smith, G. E., and M. D. Summers. 1978. Analysis of baculovirus genomes with restriction endonucleases. Virology 89:517-527. [DOI] [PubMed] [Google Scholar]
- 51.Smith, G. E., and M. D. Summers. 1980. Restriction maps of Rachiplusia ou and Rachiplusia ou-Autographa californica baculovirus recombinants. J. Virol. 33:311-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Summers, M. D., and G. E. Smith. 1987. A manual of methods for baculovirus vectors and insect cell culture procedures. Tex. Agric. Exp. Stn. Bull. 1555:1-55. [Google Scholar]
- 53.Summers, M. D., G. E. Smith, J. D. Knell, and J. P. Burand. 1980. Physical maps of Autographa californica and Rachiplusia ou nuclear polyhedrosis virus recombinants. J. Virol. 34:693-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vaughn, J. L., R. H. Goodwin, G. J. Tompkins, and P. McCawley. 1977. The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera: Noctuidae). In Vitro 13:213-217. [DOI] [PubMed] [Google Scholar]
- 55.Wickham, T. J., T. Davis, R. R. Granados, D. A. Hammer, M. L. Shuler, and H. A. Wood. 1991. Baculovirus defective interfering particles are responsible for variations in recombinant protein production as a function of multiplicity of infection. Biotechnol. Lett. 13:483-488. [Google Scholar]
- 56.Woo, S. D., W. J. Kim, H. S. Kim, B. R. Jin, Y. H. Lee, and S. K. Kang. 1998. The morphology of the polyhedra of a host range-expanded recombinant baculovirus and its parents. Arch. Virol. 143:1209-1214. [DOI] [PubMed] [Google Scholar]
- 57.Zuidema, D., A. Schouten, M. Usmany, A. J. Maule, G. J. Belsham, J. Roosien, E. C. Klinge-Roode, J. W. M. Van Lent, and J. M. Vlak. 1990. Expression of cauliflower mosaic virus gene I in insect cells using a novel polyhedrin-based baculovirus expression vector. J. Gen. Virol. 71:2201-2210. [DOI] [PubMed] [Google Scholar]