Abstract
The title compound, [Mn(C7H4ClO2)2(C10H14N2O)2(H2O)2], is a monomeric complex with the MnII atom lying on an inversion center. It contains two 4-chlorobenzoate and two diethylnicotinamide ligands and two water molecules, all of which are monodentate. The four O atoms in the equatorial plane around the Mn atom form a slightly distorted square-planar arrangement, while the distorted octahedral geometry is completed by two N atoms in the axial positions. In the crystal structure, O—H⋯O hydrogen bonds link the molecules into an infinite chain.
Related literature
For general background, see: Adiwidjaja et al. (1978 ▶); Amiraslanov et al. (1979 ▶); Antolini et al. (1982 ▶); Antsyshkina et al. (1980 ▶); Nadzhafov et al. (1981 ▶); Shnulin et al. (1981 ▶). For related structures, see: Hökelek et al. (1995 ▶, 1997 ▶); Hökelek et al. (2007 ▶); Hökelek & Necefoğlu (1996 ▶, 1997 ▶, 2007 ▶).
Experimental
Crystal data
[Mn(C7H4ClO2)2(C10H14N2O)2(H2O)2]
M r = 758.54
Triclinic,
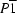
a = 7.3552 (1) Å
b = 8.6465 (2) Å
c = 15.9847 (3) Å
α = 84.500 (16)°
β = 78.616 (17)°
γ = 68.154 (17)°
V = 924.73 (12) Å3
Z = 1
Mo Kα radiation
μ = 0.56 mm−1
T = 294 (2) K
0.30 × 0.15 × 0.10 mm
Data collection
Enraf–Nonius TurboCAD-4 diffractometer
Absorption correction: ψ scan (North et al., 1968 ▶) T min = 0.902, T max = 0.950
4010 measured reflections
3752 independent reflections
2604 reflections with I > 2σ(I)
R int = 0.062
3 standard reflections frequency: 120 min intensity decay: 1%
Refinement
R[F 2 > 2σ(F 2)] = 0.080
wR(F 2) = 0.254
S = 1.04
3752 reflections
225 parameters
5 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 1.26 e Å−3
Δρmin = −1.31 e Å−3
Data collection: CAD-4 EXPRESS (Enraf–Nonius, 1989 ▶); cell refinement: CAD-4 EXPRESS; data reduction: XCAD4 (Harms & Wocadlo, 1995 ▶); program(s) used to solve structure: SIR92 (Altomare et al., 1994 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808005540/hy2120sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808005540/hy2120Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected geometric parameters (Å, °).
| Mn—O1 | 2.141 (3) |
| Mn—O4 | 2.205 (4) |
| Mn—N1 | 2.281 (4) |
| O1i—Mn—O4 | 90.38 (14) |
| O1—Mn—O4 | 89.62 (14) |
| O1—Mn—N1i | 92.23 (14) |
| O4—Mn—N1i | 92.72 (14) |
| O1—Mn—N1 | 87.77 (14) |
| O4—Mn—N1 | 87.28 (14) |
Symmetry code: (i)  .
.
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O4—H41⋯O2i | 0.99 (4) | 1.71 (5) | 2.670 (6) | 162 (7) |
| O4—H42⋯O3ii | 0.93 (5) | 1.85 (5) | 2.766 (6) | 168 (7) |
Symmetry codes: (i)  ; (ii)
; (ii) 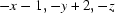 .
.
Acknowledgments
The authors acknowledge the purchase of a CAD-4 diffractometer under grant DPT/TBAG1 of the Scientific and Technical Research Council of Turkey.
supplementary crystallographic information
Comment
Transition metal complexes with biochemical molecules show interesting physical and/or chemical properties, through which they may find applications in biological systems (Antolini et al., 1982). The structural functions and coordination relationships of the arylcarboxylate ions in manganese(II) complexes of benzoic acid derivatives may be changed, depending on the nature and position of the substituted groups on the benzene ring, the nature of the additional ligand molecule or solvent, and the medium of the synthesis (Adiwidjaja et al., 1978; Amiraslanov et al., 1979; Antsyshkina et al., 1980; Nadzhafov et al., 1981; Shnulin et al., 1981).
N,N-Diethylnicotinamide (DENA) is an important respiratory stimulant. The structures of several complexes obtained by reacting divalent transition metal ions with DENA have been determined in our laboratory, including those of Cu2(DENA)2(C6H5COO)4, (II), (Hökelek et al., 1995), [Zn2(DENA)2(C7H5O3)4].2H2O, (III), (Hökelek & Necefoğlu, 1996), [Co(DENA)2(C7H5O3)2(H2O)2], (IV), (Hökelek & Necefoğlu, 1997), [Cu(DENA)2(C7H4NO4)2(H2O)2], (V), (Hökelek et al., 1997) and [Zn(DENA)2(C7H4FO2)2(H2O)2], (VI), (Hökelek et al., 2007). The structure determination of the title compound, (I), a manganese(II) complex with two chlorobenzoate (CB), two DENA ligands and two water molecules, was undertaken in order to determine the properties of the CB and DENA ligands and also to compare the results obtained with those reported previously.
Compound (I) is a monomeric complex, with the Mn atom lying on a center of symmetry. It contains two CB, two DENA ligands and two water molecules (Fig. 1). All ligands are monodentate. The four O atoms (O1, O4, and their symmetry-related atoms) in the equatorial plane around the Mn atom form a slightly distorted square-planar arrangement, while the slightly distorted octahedral coordination geometry is completed by the two N atoms of the DENA ligands in the axial positions (Table 1 and Fig. 1).
The near equality of the C1—O1 [1.256 (6) Å] and C1—O2 [1.245 (6) Å] bonds in the carboxylate group indicates a delocalized bonding arrangement, rather than localized single and double bonds, and may be compared with the corresponding distances: 1.259 (9) and 1.273 (9) Å in (II), 1.279 (4) and 1.246 (4) Å in (III), 1.251 (6) and 1.254 (7) Å in (IV), 1.278 (3) and 1.246 (3) Å in (V) and 1.265 (6) and 1.275 (6) Å in [Mn(C9H10NO2)2(H2O)4].2H2O, (VII), (Hökelek & Necefoğlu, 2007). This may be due to the intramolecular O—H···O hydrogen bond involving the carboxylate O atom (Table 2). In (I), the average Mn—O bond length is 2.173 (3) Å. The Mn atom is displaced out of the least-squares plane of the carboxylate group (O1/C1/O2) by 0.890 (1) Å; this is reported as 2.185 (4) and 1.365 (3) Å, respectively, in (VII). The dihedral angle between the planar carboxylate group and the benzene ring A (C2 to C7) is 3.0 (4)°, while that between rings A and B (N1/C8 to C12) is 81.0 (4)°.
As can be seen from the packing diagram (Fig. 2), the Mn atoms are located at the corners of the unit cell and the molecules of (I) are linked into infinite chains along the a-axis by intermolecular O—H···O hydrogen bonds (Table 2).
Experimental
The title compound was prepared by the reaction of Mn(NO3)2 (1.79 g, 10 mmol) in H2O (25 ml) and DENA (3.56 g, 20 mmol) in H2O (25 ml) with sodium p-chlorobenzoate (3.57 g, 20 mmol) in H2O (100 ml). The mixture was filtered and set aside to crystallize at ambient temperature for several days, giving colorless single crystals.
Refinement
H atoms of the water molecule were located in a difference Fourier map and refined isotropically. The remaining H atoms were positioned geometrically and refined as riding atoms, with C—H = 0.93 (aromatic), 0.97 (methylene) and 0.96 Å (methyl) and with Uiso(H) = xUeq(C), where x = 1.0 for H atoms of C15 methyl, 1.5 for H atoms of C17 methyl, and 1.2 for other H atoms. The restrains on the C14—C15 bond length and O—H bond lengths and H—O—H bond angle of water molecule were applied. The highest residual electron density was found 0.92 Å from H15B and the deepest hole 0.14 Å from C15.
Figures
Fig. 1.
The molecular structure of the title compound. Displacement ellipsoids are drawn at the 20% probability level. Hydrogen bonds are shown as dashed lines. [Symmetry code: (i) -x, 2 - y, -z.]
Fig. 2.
A packing diagram of the title compound, viewed down the a-axis, showing hydrogen bonds (dashed lines) linking the molecules into chains. H atoms not involved in hydrogen bonds have been omitted for clarity.
Crystal data
| [Mn(C7H4ClO2)2(C10H14N2O)2(H2O)2] | Z = 1 |
| Mr = 758.54 | F000 = 395 |
| Triclinic, P1 | Dx = 1.362 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation λ = 0.71073 Å |
| a = 7.3552 (1) Å | Cell parameters from 25 reflections |
| b = 8.6465 (2) Å | θ = 5.2–11.6º |
| c = 15.9847 (3) Å | µ = 0.56 mm−1 |
| α = 84.500 (16)º | T = 294 (2) K |
| β = 78.616 (17)º | Block, colorless |
| γ = 68.154 (17)º | 0.30 × 0.15 × 0.10 mm |
| V = 924.73 (12) Å3 |
Data collection
| Enraf–Nonius TurboCAD-4 diffractometer | Rint = 0.062 |
| Radiation source: fine-focus sealed tube | θmax = 26.3º |
| Monochromator: graphite | θmin = 3.0º |
| T = 294(2) K | h = −8→9 |
| ω scans | k = 0→10 |
| Absorption correction: ψ scan(North et al., 1968) | l = −19→19 |
| Tmin = 0.902, Tmax = 0.950 | 3 standard reflections |
| 4010 measured reflections | every 120 min |
| 3752 independent reflections | intensity decay: 1% |
| 2604 reflections with I > 2σ(I) |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.080 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.254 | w = 1/[σ2(Fo2) + (0.1471P)2 + 1.5562P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.04 | (Δ/σ)max < 0.001 |
| 3752 reflections | Δρmax = 1.26 e Å−3 |
| 225 parameters | Δρmin = −1.31 e Å−3 |
| 5 restraints | Extinction correction: none |
| Primary atom site location: structure-invariant direct methods |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Mn | 0.0000 | 1.0000 | 0.0000 | 0.0323 (3) | |
| Cl | −0.7397 (3) | 0.8445 (3) | 0.46709 (13) | 0.0925 (7) | |
| O1 | −0.1119 (5) | 0.8758 (4) | 0.1086 (2) | 0.0408 (8) | |
| O2 | 0.0744 (5) | 0.8690 (5) | 0.2036 (3) | 0.0508 (10) | |
| O3 | −0.8388 (6) | 1.3270 (5) | 0.1267 (3) | 0.0576 (11) | |
| O4 | −0.2247 (6) | 0.9829 (5) | −0.0681 (2) | 0.0463 (9) | |
| H41 | −0.195 (10) | 1.042 (7) | −0.122 (2) | 0.07 (2)* | |
| H42 | −0.221 (12) | 0.880 (5) | −0.083 (4) | 0.08 (2)* | |
| N1 | −0.2326 (6) | 1.2447 (5) | 0.0532 (3) | 0.0360 (9) | |
| N2 | −0.8349 (8) | 1.4183 (8) | 0.2506 (4) | 0.0649 (15) | |
| C1 | −0.0808 (7) | 0.8688 (6) | 0.1836 (3) | 0.0366 (11) | |
| C2 | −0.2462 (7) | 0.8636 (5) | 0.2547 (3) | 0.0350 (10) | |
| C3 | −0.4206 (7) | 0.8580 (6) | 0.2372 (3) | 0.0381 (11) | |
| H3 | −0.4358 | 0.8589 | 0.1807 | 0.046* | |
| C4 | −0.5733 (8) | 0.8509 (7) | 0.3024 (4) | 0.0487 (13) | |
| H4 | −0.6898 | 0.8463 | 0.2904 | 0.058* | |
| C5 | −0.5477 (9) | 0.8509 (8) | 0.3850 (4) | 0.0525 (14) | |
| C6 | −0.3789 (9) | 0.8586 (8) | 0.4047 (4) | 0.0533 (14) | |
| H6 | −0.3655 | 0.8585 | 0.4613 | 0.064* | |
| C7 | −0.2282 (8) | 0.8665 (7) | 0.3392 (3) | 0.0434 (12) | |
| H7 | −0.1138 | 0.8739 | 0.3520 | 0.052* | |
| C8 | −0.2050 (7) | 1.3893 (6) | 0.0424 (3) | 0.0390 (11) | |
| H8 | −0.0855 | 1.3917 | 0.0107 | 0.047* | |
| C9 | −0.3452 (8) | 1.5356 (6) | 0.0760 (4) | 0.0461 (13) | |
| H9 | −0.3203 | 1.6343 | 0.0676 | 0.055* | |
| C10 | −0.5227 (8) | 1.5332 (6) | 0.1223 (4) | 0.0442 (13) | |
| H10 | −0.6197 | 1.6304 | 0.1458 | 0.053* | |
| C11 | −0.5554 (7) | 1.3848 (6) | 0.1334 (3) | 0.0359 (11) | |
| C12 | −0.4080 (7) | 1.2446 (6) | 0.0965 (3) | 0.0380 (11) | |
| H12 | −0.4315 | 1.1453 | 0.1020 | 0.046* | |
| C13 | −0.7519 (7) | 1.3715 (6) | 0.1710 (3) | 0.0413 (12) | |
| C14 | −0.7427 (13) | 1.4762 (10) | 0.3087 (5) | 0.080 (2) | |
| H14A | −0.6247 | 1.4935 | 0.2770 | 0.096* | |
| H14B | −0.8350 | 1.5823 | 0.3318 | 0.096* | |
| C15 | −0.6883 (17) | 1.3572 (14) | 0.3785 (7) | 0.132 | |
| H15A | −0.6290 | 1.3989 | 0.4151 | 0.132* | |
| H15B | −0.5949 | 1.2527 | 0.3558 | 0.132* | |
| H15C | −0.8052 | 1.3412 | 0.4106 | 0.132* | |
| C16 | −1.0402 (11) | 1.4197 (10) | 0.2815 (6) | 0.080 (2) | |
| H16A | −1.1031 | 1.4942 | 0.3291 | 0.096* | |
| H16B | −1.1177 | 1.4616 | 0.2362 | 0.096* | |
| C17 | −1.0391 (16) | 1.2541 (11) | 0.3085 (7) | 0.107 (3) | |
| H17A | −1.1734 | 1.2589 | 0.3272 | 0.160* | |
| H17B | −0.9659 | 1.2137 | 0.3546 | 0.160* | |
| H17C | −0.9773 | 1.1802 | 0.2614 | 0.160* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Mn | 0.0261 (5) | 0.0275 (5) | 0.0373 (6) | −0.0030 (4) | −0.0004 (4) | −0.0107 (4) |
| Cl | 0.0696 (12) | 0.144 (2) | 0.0638 (12) | −0.0517 (13) | 0.0190 (9) | −0.0096 (12) |
| O1 | 0.043 (2) | 0.0384 (19) | 0.0390 (19) | −0.0133 (16) | −0.0033 (15) | −0.0072 (14) |
| O2 | 0.034 (2) | 0.061 (3) | 0.057 (2) | −0.0162 (18) | −0.0097 (17) | −0.0010 (19) |
| O3 | 0.043 (2) | 0.066 (3) | 0.070 (3) | −0.022 (2) | −0.0078 (19) | −0.022 (2) |
| O4 | 0.043 (2) | 0.046 (2) | 0.054 (2) | −0.0195 (17) | −0.0090 (17) | −0.0081 (18) |
| N1 | 0.030 (2) | 0.026 (2) | 0.046 (2) | −0.0054 (16) | −0.0002 (17) | −0.0086 (16) |
| N2 | 0.052 (3) | 0.079 (4) | 0.061 (3) | −0.026 (3) | 0.011 (2) | −0.024 (3) |
| C1 | 0.031 (2) | 0.022 (2) | 0.049 (3) | −0.0010 (18) | −0.007 (2) | −0.0055 (19) |
| C2 | 0.034 (2) | 0.022 (2) | 0.044 (3) | −0.0032 (18) | −0.006 (2) | −0.0050 (18) |
| C3 | 0.037 (3) | 0.033 (3) | 0.043 (3) | −0.012 (2) | −0.007 (2) | −0.005 (2) |
| C4 | 0.036 (3) | 0.058 (3) | 0.055 (3) | −0.021 (3) | −0.005 (2) | −0.005 (3) |
| C5 | 0.044 (3) | 0.055 (3) | 0.051 (3) | −0.015 (3) | 0.005 (3) | −0.005 (3) |
| C6 | 0.055 (3) | 0.064 (4) | 0.038 (3) | −0.018 (3) | −0.006 (2) | −0.001 (3) |
| C7 | 0.036 (3) | 0.045 (3) | 0.047 (3) | −0.010 (2) | −0.008 (2) | −0.008 (2) |
| C8 | 0.032 (2) | 0.032 (2) | 0.051 (3) | −0.009 (2) | −0.005 (2) | −0.009 (2) |
| C9 | 0.042 (3) | 0.030 (3) | 0.066 (4) | −0.012 (2) | −0.007 (3) | −0.009 (2) |
| C10 | 0.040 (3) | 0.027 (2) | 0.059 (3) | −0.003 (2) | −0.005 (2) | −0.018 (2) |
| C11 | 0.031 (2) | 0.032 (2) | 0.040 (3) | −0.0040 (19) | −0.0066 (19) | −0.0103 (19) |
| C12 | 0.030 (2) | 0.028 (2) | 0.050 (3) | −0.0054 (19) | 0.000 (2) | −0.010 (2) |
| C13 | 0.031 (2) | 0.038 (3) | 0.051 (3) | −0.007 (2) | −0.003 (2) | −0.013 (2) |
| C14 | 0.085 (5) | 0.078 (5) | 0.072 (5) | −0.029 (4) | −0.002 (4) | −0.009 (4) |
| C15 | 0.180 | 0.156 | 0.138 | −0.130 | −0.127 | 0.126 |
| C16 | 0.060 (4) | 0.067 (5) | 0.098 (6) | −0.015 (4) | 0.018 (4) | −0.026 (4) |
| C17 | 0.116 (8) | 0.071 (6) | 0.112 (7) | −0.031 (5) | 0.030 (6) | −0.014 (5) |
Geometric parameters (Å, °)
| Mn—O1i | 2.141 (3) | C7—C6 | 1.383 (8) |
| Mn—O1 | 2.141 (3) | C7—H7 | 0.9300 |
| Mn—O4 | 2.205 (4) | C8—H8 | 0.9300 |
| Mn—O4i | 2.205 (4) | C9—C8 | 1.376 (7) |
| Mn—N1i | 2.281 (4) | C9—H9 | 0.9300 |
| Mn—N1 | 2.281 (4) | C10—C9 | 1.373 (8) |
| Cl—C5 | 1.741 (6) | C10—H10 | 0.9300 |
| O1—C1 | 1.256 (6) | C11—C12 | 1.378 (6) |
| O2—C1 | 1.245 (6) | C11—C10 | 1.380 (7) |
| O3—C13 | 1.214 (6) | C12—H12 | 0.9300 |
| O4—H41 | 0.99 (4) | C13—N2 | 1.328 (7) |
| O4—H42 | 0.93 (5) | C13—C11 | 1.494 (7) |
| N1—C8 | 1.330 (6) | C14—C15 | 1.453 (9) |
| N1—C12 | 1.339 (6) | C14—H14A | 0.9700 |
| N2—C14 | 1.471 (10) | C14—H14B | 0.9700 |
| N2—C16 | 1.489 (9) | C15—H15A | 0.9600 |
| C2—C3 | 1.384 (7) | C15—H15B | 0.9600 |
| C2—C7 | 1.387 (7) | C15—H15C | 0.9600 |
| C2—C1 | 1.502 (7) | C16—C17 | 1.453 (11) |
| C3—H3 | 0.9300 | C16—H16A | 0.9700 |
| C4—C5 | 1.369 (8) | C16—H16B | 0.9700 |
| C4—C3 | 1.388 (7) | C17—H17A | 0.9600 |
| C4—H4 | 0.9300 | C17—H17B | 0.9600 |
| C5—C6 | 1.366 (9) | C17—H17C | 0.9600 |
| C6—H6 | 0.9300 | ||
| O1i—Mn—O1 | 180.000 (1) | C6—C7—H7 | 119.7 |
| O1i—Mn—O4 | 90.38 (14) | C2—C7—H7 | 119.7 |
| O1—Mn—O4 | 89.62 (14) | N1—C8—C9 | 122.9 (5) |
| O1i—Mn—O4i | 89.62 (14) | N1—C8—H8 | 118.5 |
| O1—Mn—O4i | 90.38 (14) | C9—C8—H8 | 118.5 |
| O4—Mn—O4i | 180.00 (16) | C10—C9—C8 | 118.8 (5) |
| O1i—Mn—N1i | 87.77 (14) | C10—C9—H9 | 120.6 |
| O1—Mn—N1i | 92.23 (14) | C8—C9—H9 | 120.6 |
| O4—Mn—N1i | 92.72 (14) | C9—C10—C11 | 119.2 (4) |
| O4i—Mn—N1i | 87.28 (14) | C9—C10—H10 | 120.4 |
| O1i—Mn—N1 | 92.23 (14) | C11—C10—H10 | 120.4 |
| O1—Mn—N1 | 87.77 (14) | C12—C11—C10 | 118.2 (5) |
| O4—Mn—N1 | 87.28 (14) | C12—C11—C13 | 117.2 (4) |
| O4i—Mn—N1 | 92.72 (14) | C10—C11—C13 | 123.8 (4) |
| N1i—Mn—N1 | 180.0 | N1—C12—C11 | 123.0 (5) |
| C1—O1—Mn | 127.5 (3) | N1—C12—H12 | 118.5 |
| Mn—O4—H41 | 100 (4) | C11—C12—H12 | 118.5 |
| Mn—O4—H42 | 121 (5) | O3—C13—N2 | 120.8 (5) |
| H41—O4—H42 | 107 (4) | O3—C13—C11 | 119.4 (5) |
| C8—N1—C12 | 117.7 (4) | N2—C13—C11 | 119.7 (5) |
| C8—N1—Mn | 123.5 (3) | C15—C14—N2 | 111.7 (7) |
| C12—N1—Mn | 118.8 (3) | C15—C14—H14A | 109.3 |
| C13—N2—C14 | 124.8 (6) | N2—C14—H14A | 109.3 |
| C13—N2—C16 | 117.3 (6) | C15—C14—H14B | 109.3 |
| C14—N2—C16 | 117.8 (6) | N2—C14—H14B | 109.3 |
| O2—C1—O1 | 125.2 (5) | H14A—C14—H14B | 107.9 |
| O2—C1—C2 | 117.6 (5) | C14—C15—H15A | 109.5 |
| O1—C1—C2 | 117.1 (4) | C14—C15—H15B | 109.5 |
| C3—C2—C7 | 118.6 (5) | H15A—C15—H15B | 109.5 |
| C3—C2—C1 | 120.8 (5) | C14—C15—H15C | 109.5 |
| C7—C2—C1 | 120.6 (5) | H15A—C15—H15C | 109.5 |
| C2—C3—C4 | 121.2 (5) | H15B—C15—H15C | 109.5 |
| C2—C3—H3 | 119.4 | C17—C16—N2 | 111.5 (7) |
| C4—C3—H3 | 119.4 | C17—C16—H16A | 109.3 |
| C5—C4—C3 | 118.2 (5) | N2—C16—H16A | 109.3 |
| C5—C4—H4 | 120.9 | C17—C16—H16B | 109.3 |
| C3—C4—H4 | 120.9 | N2—C16—H16B | 109.3 |
| C6—C5—C4 | 122.2 (5) | H16A—C16—H16B | 108.0 |
| C6—C5—Cl | 119.3 (5) | C16—C17—H17A | 109.5 |
| C4—C5—Cl | 118.5 (5) | C16—C17—H17B | 109.5 |
| C5—C6—C7 | 119.1 (5) | H17A—C17—H17B | 109.5 |
| C5—C6—H6 | 120.5 | C16—C17—H17C | 109.5 |
| C7—C6—H6 | 120.5 | H17A—C17—H17C | 109.5 |
| C6—C7—C2 | 120.6 (5) | H17B—C17—H17C | 109.5 |
| O4—Mn—O1—C1 | −164.0 (4) | C7—C2—C1—O2 | 2.8 (7) |
| O4i—Mn—O1—C1 | 16.0 (4) | C3—C2—C1—O1 | 3.3 (6) |
| N1i—Mn—O1—C1 | 103.3 (4) | C7—C2—C1—O1 | −175.9 (4) |
| N1—Mn—O1—C1 | −76.7 (4) | C7—C2—C3—C4 | −1.7 (7) |
| O1i—Mn—N1—C8 | −32.0 (4) | C1—C2—C3—C4 | 179.1 (5) |
| O1—Mn—N1—C8 | 148.0 (4) | C5—C4—C3—C2 | 0.5 (8) |
| O4—Mn—N1—C8 | −122.3 (4) | C3—C4—C5—C6 | 0.4 (9) |
| O4i—Mn—N1—C8 | 57.7 (4) | C3—C4—C5—Cl | 179.2 (4) |
| O1i—Mn—N1—C12 | 146.8 (4) | C4—C5—C6—C7 | −0.1 (10) |
| O1—Mn—N1—C12 | −33.2 (4) | Cl—C5—C6—C7 | −178.9 (5) |
| O4—Mn—N1—C12 | 56.6 (4) | C2—C7—C6—C5 | −1.2 (9) |
| O4i—Mn—N1—C12 | −123.4 (4) | C10—C9—C8—N1 | −0.6 (9) |
| Mn—O1—C1—O2 | −31.6 (7) | C11—C10—C9—C8 | −0.2 (8) |
| Mn—O1—C1—C2 | 146.9 (3) | C12—C11—C10—C9 | −0.7 (8) |
| Mn—N1—C8—C9 | −178.9 (4) | C13—C11—C10—C9 | −170.7 (5) |
| C12—N1—C8—C9 | 2.2 (8) | C10—C11—C12—N1 | 2.4 (8) |
| C8—N1—C12—C11 | −3.2 (8) | C13—C11—C12—N1 | 173.1 (5) |
| Mn—N1—C12—C11 | 177.9 (4) | O3—C13—N2—C14 | −179.1 (6) |
| C13—N2—C14—C15 | −111.0 (9) | C11—C13—N2—C14 | −3.4 (10) |
| C16—N2—C14—C15 | 72.0 (10) | O3—C13—N2—C16 | −2.2 (9) |
| C13—N2—C16—C17 | 81.2 (9) | C11—C13—N2—C16 | 173.5 (5) |
| C14—N2—C16—C17 | −101.6 (9) | O3—C13—C11—C10 | 114.1 (6) |
| C3—C2—C7—C6 | 2.0 (8) | N2—C13—C11—C10 | −61.6 (8) |
| C1—C2—C7—C6 | −178.8 (5) | O3—C13—C11—C12 | −55.9 (7) |
| C3—C2—C1—O2 | −178.0 (4) | N2—C13—C11—C12 | 128.3 (6) |
Symmetry codes: (i) −x, −y+2, −z.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O4—H41···O2i | 0.99 (4) | 1.71 (5) | 2.670 (6) | 162 (7) |
| O4—H42···O3ii | 0.93 (5) | 1.85 (5) | 2.766 (6) | 168 (7) |
Symmetry codes: (i) −x, −y+2, −z; (ii) −x−1, −y+2, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HY2120).
References
- Adiwidjaja, G., Rossmanith, E. & Küppers, H. (1978). Acta Cryst. B34, 3079–3083.
- Altomare, A., Cascarano, G., Giacovazzo, C., Guagliardi, A., Burla, M. C., Polidori, G. & Camalli, M. (1994). J. Appl. Cryst.27, 435.
- Amiraslanov, I. R., Mamedov, Kh. S., Movsumov, E. M., Musaev, F. N. & Nadzhafov, G. N. (1979). Zh. Strukt. Khim.20, 1075–1080.
- Antolini, L., Battaglia, L. P., Corradi, A. B., Marcotrigiano, G., Menabue, L., Pellacani, G. C. & Saladini, M. (1982). Inorg. Chem.21, 1391–1395.
- Antsyshkina, A. S., Chiragov, F. M. & Poray-Koshits, M. A. (1980). Koord. Khim.15, 1098–1103.
- Enraf–Nonius (1989). CAD-4 EXPRESS Enraf–Nonius, Delft, The Netherlands.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Harms, K. & Wocadlo, S. (1995). XCAD4 University of Marburg, Germany.
- Hökelek, T., Budak, K. & Necefoglu, H. (1997). Acta Cryst. C53, 1049–1051.
- Hökelek, T., Çaylak, N. & Necefoğlu, H. (2007). Acta Cryst. E63, m2561–m2562.
- Hökelek, T. & Necefouglu, H. (1996). Acta Cryst. C52, 1128–1131.
- Hökelek, T. & Necefouglu, H. (1997). Acta Cryst. C53, 187–189.
- Hökelek, T. & Necefoğlu, H. (2007). Acta Cryst. E63, m821–m823.
- Hökelek, T., Necefouglu, H. & Balcı, M. (1995). Acta Cryst. C51, 2020–2023.
- Nadzhafov, G. N., Shnulin, A. N. & Mamedov, Kh. S. (1981). Zh. Strukt. Khim.22, 124–128.
- North, A. C. T., Phillips, D. C. & Mathews, F. S. (1968). Acta Cryst. A24, 351–359.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Shnulin, A. N., Nadzhafov, G. N., Amiraslanov, I. R., Usubaliev, B. T. & Mamedov, Kh. S. (1981). Koord. Khim.7, 1409–1416.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808005540/hy2120sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808005540/hy2120Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




