Abstract
In the title compound, [Fe(C5H5)(C16H14N3O3)], the pyrazoline ring and the substituted cyclopentadienyl ring are nearly coplanar, with a dihedral angle of 8.17 (2)°, while the nitro-substituted benzene ring is twisted out of the pyrazoline ring plane by 70.76 (1)°. The molecules in the crystal structure are held together by three intermolecular C—H⋯O hydrogen bonds. There is also an intramolecular C—H⋯N hydrogen bond. The H atoms of the methyl group are disordered equally over two positions.
Related literature
For related literature, see: Amr et al. (2006 ▶); Biot et al. (2004 ▶); Cremer & Pople (1975 ▶); Fang et al. (2003 ▶); Fouda et al. (2007 ▶); Guirado et al. (2004 ▶); Jaouen et al. (2004 ▶); Karthikeyan et al. (2007 ▶); Küçükgüzel et al. (2000 ▶); Kudar et al. (2005 ▶); Özdemir et al. (2007 ▶); Shi et al. (2006a
▶,b
▶); Shi et al. (2006 ▶); Zora et al. (2006 ▶, 2008 ▶).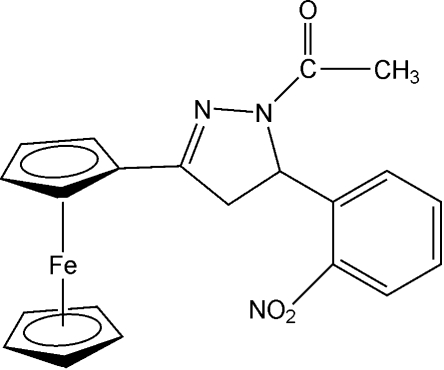
Experimental
Crystal data
[Fe(C5H5)(C16H14N3O3)]
M r = 417.24
Orthorhombic,
a = 8.6691 (6) Å
b = 13.4779 (7) Å
c = 31.4930 (15) Å
V = 3679.7 (4) Å3
Z = 8
Mo Kα radiation
μ = 0.85 mm−1
T = 296 K
0.50 × 0.31 × 0.06 mm
Data collection
Stoe IPDSII diffractometer
Absorption correction: integration (X-RED32; Stoe & Cie, 2002 ▶) T min = 0.636, T max = 0.862
17799 measured reflections
3613 independent reflections
2409 reflections with I > 2σ(I)
R int = 0.061
Refinement
R[F 2 > 2σ(F 2)] = 0.037
wR(F 2) = 0.086
S = 0.94
3613 reflections
253 parameters
H-atom parameters constrained
Δρmax = 0.19 e Å−3
Δρmin = −0.29 e Å−3
Data collection: X-AREA (Stoe & Cie, 2002 ▶); cell refinement: X-AREA; data reduction: X-RED32 (Stoe & Cie, 2002 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶) and PLATON (Spek, 2003 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808004236/hy2116sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808004236/hy2116Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected bond lengths (Å).
| C12—Fe1 | 2.035 (3) |
| C13—Fe1 | 2.044 (3) |
| C14—Fe1 | 2.051 (3) |
| C15—Fe1 | 2.034 (3) |
| C16—Fe1 | 2.016 (3) |
| C17—Fe1 | 2.047 (3) |
| C18—Fe1 | 2.033 (3) |
| C19—Fe1 | 2.036 (3) |
| C20—Fe1 | 2.035 (3) |
| C21—Fe1 | 2.045 (3) |
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C11—H11A⋯N3 | 0.96 | 2.29 | 2.788 (4) | 112 |
| C8—H8A⋯O3i | 0.97 | 2.57 | 3.476 (3) | 154 |
| C8—H8B⋯O2ii | 0.97 | 2.63 | 3.551 (4) | 158 |
| C21—H21⋯O2ii | 0.93 | 2.46 | 3.136 (4) | 130 |
Symmetry codes: (i) 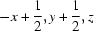 ; (ii)
; (ii) 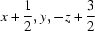 .
.
Acknowledgments
The authors acknowledge the Research Board of Akdeniz University (grant No. BAP-2007.01.0105.001) for financial support and the Faculty of Arts and Sciences, Ondokuz Mayıs University, for the Stoe IPDSII diffractometer purchased under grant F.279 of the University Research Fund.
supplementary crystallographic information
Comment
Pyrazolines are widely studied as five-membered heterocyclic compounds. Condensation of nitrogen-containing binucleophilic agents with α-β unsaturated ketones is one of the most suitable synthetic pathways for 2-pyrazolines (Kudar et al., 2005), which possess widespread pharmaceutical properties such as antimicrobial (Küçükgüzel et al., 2000), anticonvulsant (Karthikeyan et al., 2007), antidepressant Özdemir et al., 2007), antiandrogenic (Amr et al., 2006), antifungal and anti-inflammatory (Guirado et al., 2004) activities. Metallocenes are also known to exhibit a wide range of biological activity. Among them ferrocenyl compounds display interesting antibacterial (Fouda et al., 2007), antitumor (Jaouen et al., 2004), antimalarial and antifungal (Biot et al., 2004) activities. Therefore, incorporation of a ferrocene fragment into a heterocyclic ring may enhance their biological activities or generate new medicinal properties (Fang et al., 2003). As a part of an ongoing investigation of the chemistry of ferrocenyl pyrazolines, the title compound was synthesized and its crystal structure was determined.
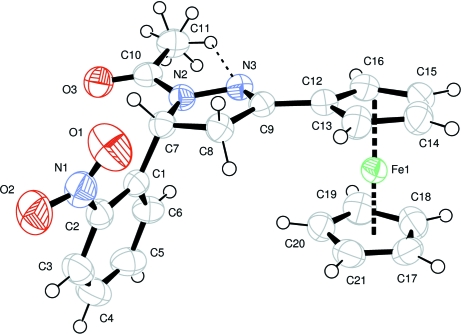
The molecular structure of the title compound is shown in Fig. 1. The puckering parameters (Cremer & Pople, 1975) are q2=0.037 (3)Å and φ=43.3 (8)° for the pyrazoline ring, q2=0.008 (3)Å and φ=156.3 (2)° for the substituted cyclopentadienyl (Cp) ring and q2=0.004 (3)Å and φ=69.6 (5)° for the unsubstituted Cp ring. The dihedral angle of 8.17 (2)° between the pyrazoline ring and the substituted Cp ring indicates that they are conjugated with each other; this is in accord with the C9—C12 bond [1.456 (4) Å], showing a double-bond character (Shi et al., 2006a). Furthermore, N3—C9 bond length [1.284 (3) Å] increases as a result of this conjugation. This observation is in good agreement with those reported for 2,6-bis(3-ferrocenyl-5-methyl-1H-pyrazol-1-ylcarbonyl)pyridine (Shi et al., 2006b) and 1,3-bis(3-ferrocenyl-5-methyl-1H-pyrazol-1-ylcarbonyl)benzene (Shi et al., 2006).
The Fe—Cgs and Fe—Cgas distances are 1.6398 (14) and 1.6544 (14) Å, respectively, and the Cgs—Fe—Cgas angle is 177.78 (7)°, where Cgs and Cgas are the centroids of the substituted and unsubstituted Cp rings. The small dihedral angle of 3.68 (2)° between the unsubstituted and substituted Cp rings indicates that the two Cp rings are parallel to each other (Shi et al., 2006a). The C12—Cgs—Cgas—C20 torsion angle of 3.71 (2)° indicates that the two Cp rings of the ferrocenyl group is nearly in an eclipsed conformation, as was previously observed in a ferrocene-containing compound (Zora et al., 2008). The C—C bond distances in the Cp rings range from 1.389 (4) to 1.434 (4) Å, while Fe—C bond lengths range between 2.016 (3) and 2.051 (3)Å (Table 1), all of which are as expected (Zora et al., 2006).
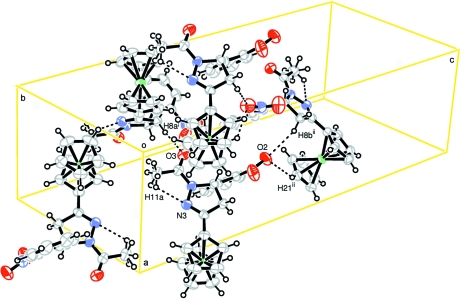
The pyrazoline ring and the nitro substituted phenyl group make a dihedral angle of 70.76 (1)°. The dihedral angle between the nitro plane and pheny ring is 18.30 (5)°. The dihedral angles between the phenyl ring and the substituted and unsubstituted Cp planes are 76.16 (1) and 76.12 (1)°, respectively. The molecules in crystal are held together by three intermolecular C—H···O hydrogen bonds (Table 2; Fig. 2). There is one intramolecular C—H···N hydrogen bond.
Experimental
A mixture of 1-ferrocenyl-3-(2-nitrophenyl)-2-propenone (0.09 g, 0.24 mmol), 80% hydrazine hydrate (0.264 g, 5.28 mmol) and glacial acetic acid (10 ml) was refluxed under nitrogen atmosphere for 5 h. TLC indicated the formation of the reaction product. It was poured into ice-water to give dark orange solid. The participate was separated by filtration and washed with water. The solid product was dried at room temperature. Single crystals of the title compound suitable for X-ray measurements were obtained by recrystallization from methanol at room temperature (yield 50.5%; m.p. 425–426 K). IR (KBr, cm-1): 1653 (C?O), 1601 (C?C), 1574 (C?N), 1527 (asym N?O), 1333 (sym N?O), 1107 (C—N). 1H-NMR (CDCl3, p.p.m.): 2.33 (s, 1H, CH3), 2.89 (dd, 1H, pyrazoline H4), 3.84 (dd, 1H, pyrazoline H4), 4.04 (s, 5H, ferrocene), 4.36 (s, 2H, ferrocene), 4.53 (s, 1H, ferrocene), 4.60 (s, 1H, ferrocene), 6.03 (dd, 1H, pyrazoline H5), 7.23–8.05 (m, 4H, aromatic). 13C-NMR (CDCl3, p.p.m.): 21.75 (CH3), 43.83 (C4), 56.52 (C5), 67.30, 67.78 (C2', C5'), 69.46 (C6'–C10'), 70.61, 71.10 (C3', C4'), 74.86 (C1'), 125.56 (C8), 126.37 (C9), 128.50 (C11), 134.28 (C10), 137.33 (C6), 147.08 (C7), 156.63 (C3), 168.30 (C?O).
Refinement
H atoms were positioned geometrically and refined as riding atoms, with C—H = 0.93 (aromatic), 0.98 (CH) and 0.97 Å (CH2) and with Uiso(H) = 1.2Ueq(C). H atoms of the methyl group (C11) show rotational disorder from a difference Fourier map. These H atoms were refined as riding atoms, with C—H = 0.96 Å and Uiso(H) = 1.5Ueq(C).
Figures
Fig. 1.
The molecular structure of the title compound. Displacement ellipsoids are drawn at the 50% probability level.
Fig. 2.
A view of the hydrogen-bonding network in the title compound. The dashed lines denote hydrogen bonds. [Symmetry codes: (i) -x + 1/2, y + 1/2, z; (ii) x + 1/2, y, -z + 3/2.]
Crystal data
| [Fe(C5H5)(C16H14N3O3)] | F000 = 1728 |
| Mr = 417.24 | Dx = 1.506 Mg m−3 |
| Orthorhombic, Pbca | Mo Kα radiation λ = 0.71073 Å |
| Hall symbol: -P 2ac 2ab | Cell parameters from 16565 reflections |
| a = 8.6691 (6) Å | θ = 1.6–27.9º |
| b = 13.4779 (7) Å | µ = 0.85 mm−1 |
| c = 31.4930 (15) Å | T = 296 K |
| V = 3679.7 (4) Å3 | Prism, red |
| Z = 8 | 0.50 × 0.31 × 0.06 mm |
Data collection
| Stoe IPDSII diffractometer | 3613 independent reflections |
| Radiation source: fine-focus sealed tube | 2409 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.061 |
| T = 296(2) K | θmax = 26.0º |
| ω scans | θmin = 2.6º |
| Absorption correction: integration(X-RED32; Stoe & Cie, 2002) | h = −10→10 |
| Tmin = 0.636, Tmax = 0.862 | k = −16→13 |
| 17799 measured reflections | l = −38→38 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.037 | H-atom parameters constrained |
| wR(F2) = 0.086 | w = 1/[σ2(Fo2) + (0.044P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 0.94 | (Δ/σ)max = 0.001 |
| 3613 reflections | Δρmax = 0.19 e Å−3 |
| 253 parameters | Δρmin = −0.29 e Å−3 |
| Primary atom site location: structure-invariant direct methods | Extinction correction: none |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| C1 | 0.4226 (3) | 0.55162 (19) | 0.68996 (7) | 0.0419 (6) | |
| C2 | 0.3958 (3) | 0.5738 (2) | 0.73262 (8) | 0.0481 (7) | |
| C3 | 0.4637 (4) | 0.5207 (2) | 0.76547 (9) | 0.0617 (8) | |
| H3 | 0.4434 | 0.5376 | 0.7935 | 0.074* | |
| C4 | 0.5601 (4) | 0.4437 (2) | 0.75645 (9) | 0.0674 (9) | |
| H4 | 0.6058 | 0.4078 | 0.7783 | 0.081* | |
| C5 | 0.5895 (4) | 0.4195 (2) | 0.71485 (10) | 0.0636 (8) | |
| H5 | 0.6561 | 0.3675 | 0.7085 | 0.076* | |
| C6 | 0.5201 (3) | 0.4723 (2) | 0.68231 (9) | 0.0518 (7) | |
| H6 | 0.5398 | 0.4539 | 0.6544 | 0.062* | |
| C7 | 0.3550 (3) | 0.61147 (18) | 0.65354 (7) | 0.0413 (6) | |
| H7 | 0.2483 | 0.6299 | 0.6601 | 0.050* | |
| C8 | 0.4494 (3) | 0.70502 (18) | 0.64348 (7) | 0.0434 (6) | |
| H8A | 0.3860 | 0.7642 | 0.6452 | 0.052* | |
| H8B | 0.5363 | 0.7118 | 0.6627 | 0.052* | |
| C9 | 0.5024 (3) | 0.68659 (19) | 0.59887 (8) | 0.0409 (6) | |
| C10 | 0.2617 (3) | 0.48364 (19) | 0.60260 (8) | 0.0461 (6) | |
| C11 | 0.2627 (4) | 0.4488 (2) | 0.55716 (9) | 0.0591 (8) | |
| H11A | 0.3349 | 0.4877 | 0.5411 | 0.089* | 0.50 |
| H11B | 0.1614 | 0.4562 | 0.5453 | 0.089* | 0.50 |
| H11C | 0.2925 | 0.3802 | 0.5562 | 0.089* | 0.50 |
| H11D | 0.1910 | 0.3950 | 0.5539 | 0.089* | 0.50 |
| H11E | 0.3644 | 0.4265 | 0.5498 | 0.089* | 0.50 |
| H11F | 0.2334 | 0.5025 | 0.5389 | 0.089* | 0.50 |
| C12 | 0.6069 (3) | 0.7528 (2) | 0.57636 (8) | 0.0456 (6) | |
| C13 | 0.6708 (3) | 0.84249 (19) | 0.59343 (9) | 0.0523 (7) | |
| H13 | 0.6467 | 0.8705 | 0.6196 | 0.063* | |
| C14 | 0.7769 (4) | 0.8809 (2) | 0.56346 (10) | 0.0593 (8) | |
| H14 | 0.8346 | 0.9387 | 0.5665 | 0.071* | |
| C15 | 0.7799 (4) | 0.8165 (3) | 0.52835 (9) | 0.0615 (8) | |
| H15 | 0.8397 | 0.8247 | 0.5041 | 0.074* | |
| C16 | 0.6772 (3) | 0.7373 (2) | 0.53603 (8) | 0.0558 (7) | |
| H16 | 0.6586 | 0.6841 | 0.5179 | 0.067* | |
| C17 | 1.0530 (4) | 0.7425 (2) | 0.60978 (10) | 0.0635 (8) | |
| H17 | 1.1130 | 0.7984 | 0.6149 | 0.076* | |
| C18 | 1.0552 (4) | 0.6843 (3) | 0.57284 (10) | 0.0665 (9) | |
| H18 | 1.1166 | 0.6948 | 0.5491 | 0.080* | |
| C19 | 0.9461 (4) | 0.6060 (2) | 0.57837 (10) | 0.0628 (8) | |
| H19 | 0.9238 | 0.5560 | 0.5590 | 0.075* | |
| C20 | 0.8795 (4) | 0.6185 (2) | 0.61815 (9) | 0.0560 (8) | |
| H20 | 0.8039 | 0.5782 | 0.6300 | 0.067* | |
| C21 | 0.9452 (4) | 0.7019 (2) | 0.63740 (9) | 0.0581 (8) | |
| H21 | 0.9208 | 0.7262 | 0.6642 | 0.070* | |
| N1 | 0.2950 (3) | 0.6566 (2) | 0.74518 (7) | 0.0580 (7) | |
| N2 | 0.3604 (3) | 0.55775 (15) | 0.61266 (6) | 0.0421 (5) | |
| N3 | 0.4499 (2) | 0.60674 (15) | 0.58194 (6) | 0.0446 (5) | |
| O1 | 0.2640 (4) | 0.72062 (19) | 0.71980 (7) | 0.0857 (8) | |
| O2 | 0.2464 (3) | 0.6580 (2) | 0.78155 (7) | 0.0818 (7) | |
| O3 | 0.1803 (2) | 0.44698 (15) | 0.63000 (6) | 0.0583 (5) | |
| Fe1 | 0.84006 (4) | 0.74057 (2) | 0.581679 (10) | 0.04091 (12) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0399 (16) | 0.0440 (15) | 0.0416 (13) | −0.0072 (12) | −0.0011 (11) | 0.0002 (10) |
| C2 | 0.0490 (17) | 0.0539 (16) | 0.0413 (13) | −0.0120 (13) | 0.0013 (12) | −0.0019 (12) |
| C3 | 0.074 (2) | 0.068 (2) | 0.0428 (14) | −0.0177 (18) | −0.0069 (15) | 0.0037 (14) |
| C4 | 0.082 (3) | 0.062 (2) | 0.0585 (19) | −0.0102 (18) | −0.0196 (17) | 0.0154 (15) |
| C5 | 0.066 (2) | 0.0507 (17) | 0.074 (2) | 0.0045 (15) | −0.0186 (17) | 0.0042 (14) |
| C6 | 0.0549 (19) | 0.0486 (16) | 0.0519 (14) | 0.0029 (13) | −0.0086 (13) | −0.0040 (12) |
| C7 | 0.0393 (15) | 0.0431 (13) | 0.0415 (12) | 0.0030 (12) | 0.0012 (11) | −0.0015 (10) |
| C8 | 0.0465 (17) | 0.0377 (14) | 0.0459 (13) | −0.0015 (11) | −0.0004 (12) | −0.0004 (10) |
| C9 | 0.0366 (15) | 0.0453 (14) | 0.0410 (12) | 0.0036 (12) | 0.0000 (11) | 0.0027 (11) |
| C10 | 0.0453 (17) | 0.0407 (15) | 0.0523 (14) | 0.0038 (12) | −0.0092 (14) | −0.0006 (12) |
| C11 | 0.065 (2) | 0.0535 (17) | 0.0591 (17) | −0.0014 (15) | −0.0124 (15) | −0.0099 (14) |
| C12 | 0.0397 (13) | 0.0507 (15) | 0.0463 (13) | 0.0012 (12) | −0.0023 (10) | 0.0069 (12) |
| C13 | 0.0491 (17) | 0.0425 (14) | 0.0654 (17) | 0.0040 (13) | 0.0066 (14) | 0.0035 (12) |
| C14 | 0.0485 (18) | 0.0491 (17) | 0.080 (2) | 0.0027 (14) | 0.0036 (16) | 0.0182 (15) |
| C15 | 0.0476 (18) | 0.081 (2) | 0.0553 (16) | −0.0004 (16) | 0.0005 (14) | 0.0281 (16) |
| C16 | 0.0494 (17) | 0.0726 (19) | 0.0453 (13) | −0.0041 (15) | −0.0064 (12) | 0.0084 (13) |
| C17 | 0.0484 (18) | 0.0617 (19) | 0.080 (2) | −0.0082 (16) | −0.0178 (15) | 0.0164 (17) |
| C18 | 0.051 (2) | 0.077 (2) | 0.072 (2) | 0.0194 (16) | 0.0159 (15) | 0.0255 (17) |
| C19 | 0.066 (2) | 0.0487 (17) | 0.0741 (19) | 0.0129 (14) | 0.0016 (18) | −0.0015 (15) |
| C20 | 0.0488 (19) | 0.0531 (17) | 0.0661 (18) | 0.0028 (13) | 0.0005 (14) | 0.0162 (14) |
| C21 | 0.063 (2) | 0.0604 (19) | 0.0511 (15) | 0.0017 (15) | −0.0096 (15) | 0.0077 (13) |
| N1 | 0.0575 (17) | 0.0695 (17) | 0.0470 (13) | −0.0097 (13) | 0.0061 (12) | −0.0141 (12) |
| N2 | 0.0446 (14) | 0.0448 (12) | 0.0370 (10) | −0.0040 (10) | −0.0023 (9) | −0.0012 (8) |
| N3 | 0.0421 (13) | 0.0491 (12) | 0.0427 (10) | −0.0009 (10) | −0.0021 (11) | 0.0011 (10) |
| O1 | 0.118 (2) | 0.0697 (16) | 0.0695 (14) | 0.0264 (15) | 0.0219 (14) | −0.0057 (12) |
| O2 | 0.0805 (18) | 0.114 (2) | 0.0514 (12) | −0.0059 (15) | 0.0203 (12) | −0.0183 (12) |
| O3 | 0.0565 (14) | 0.0540 (12) | 0.0644 (12) | −0.0097 (10) | −0.0030 (10) | 0.0044 (9) |
| Fe1 | 0.0390 (2) | 0.0419 (2) | 0.04183 (17) | 0.00169 (17) | 0.00009 (16) | 0.00513 (16) |
Geometric parameters (Å, °)
| C1—C6 | 1.384 (4) | C12—Fe1 | 2.035 (3) |
| C1—C2 | 1.396 (3) | C13—C14 | 1.416 (4) |
| C1—C7 | 1.519 (3) | C13—Fe1 | 2.044 (3) |
| C2—C3 | 1.389 (4) | C13—H13 | 0.9300 |
| C2—N1 | 1.472 (4) | C14—C15 | 1.407 (4) |
| C3—C4 | 1.362 (5) | C14—Fe1 | 2.051 (3) |
| C3—H3 | 0.9300 | C14—H14 | 0.9300 |
| C4—C5 | 1.374 (4) | C15—C16 | 1.411 (4) |
| C4—H4 | 0.9300 | C15—Fe1 | 2.034 (3) |
| C5—C6 | 1.385 (4) | C15—H15 | 0.9300 |
| C5—H5 | 0.9300 | C16—Fe1 | 2.016 (3) |
| C6—H6 | 0.9300 | C16—H16 | 0.9300 |
| C7—N2 | 1.478 (3) | C17—C21 | 1.389 (4) |
| C7—C8 | 1.536 (3) | C17—C18 | 1.403 (5) |
| C7—H7 | 0.9800 | C17—Fe1 | 2.047 (3) |
| C8—C9 | 1.499 (3) | C17—H17 | 0.9300 |
| C8—H8A | 0.9700 | C18—C19 | 1.428 (5) |
| C8—H8B | 0.9700 | C18—Fe1 | 2.033 (3) |
| C9—N3 | 1.284 (3) | C18—H18 | 0.9300 |
| C9—C12 | 1.456 (4) | C19—C20 | 1.390 (4) |
| C10—O3 | 1.219 (3) | C19—Fe1 | 2.036 (3) |
| C10—N2 | 1.353 (3) | C19—H19 | 0.9300 |
| C10—C11 | 1.506 (4) | C20—C21 | 1.398 (4) |
| C11—H11A | 0.9600 | C20—Fe1 | 2.035 (3) |
| C11—H11B | 0.9600 | C20—H20 | 0.9300 |
| C11—H11C | 0.9600 | C21—Fe1 | 2.045 (3) |
| C11—H11D | 0.9600 | C21—H21 | 0.9300 |
| C11—H11E | 0.9600 | N1—O1 | 1.206 (3) |
| C11—H11F | 0.9600 | N1—O2 | 1.221 (3) |
| C12—C16 | 1.424 (4) | N2—N3 | 1.405 (3) |
| C12—C13 | 1.434 (4) | ||
| C6—C1—C2 | 115.7 (2) | C12—C16—H16 | 125.9 |
| C6—C1—C7 | 120.9 (2) | Fe1—C16—H16 | 125.2 |
| C2—C1—C7 | 123.3 (2) | C21—C17—C18 | 108.0 (3) |
| C3—C2—C1 | 122.4 (3) | C21—C17—Fe1 | 70.08 (17) |
| C3—C2—N1 | 116.3 (3) | C18—C17—Fe1 | 69.33 (18) |
| C1—C2—N1 | 121.3 (2) | C21—C17—H17 | 126.0 |
| C4—C3—C2 | 119.8 (3) | C18—C17—H17 | 126.0 |
| C4—C3—H3 | 120.1 | Fe1—C17—H17 | 126.1 |
| C2—C3—H3 | 120.1 | C17—C18—C19 | 107.6 (3) |
| C3—C4—C5 | 119.6 (3) | C17—C18—Fe1 | 70.44 (18) |
| C3—C4—H4 | 120.2 | C19—C18—Fe1 | 69.60 (18) |
| C5—C4—H4 | 120.2 | C17—C18—H18 | 126.2 |
| C4—C5—C6 | 120.2 (3) | C19—C18—H18 | 126.2 |
| C4—C5—H5 | 119.9 | Fe1—C18—H18 | 125.4 |
| C6—C5—H5 | 119.9 | C20—C19—C18 | 107.2 (3) |
| C1—C6—C5 | 122.2 (3) | C20—C19—Fe1 | 70.00 (17) |
| C1—C6—H6 | 118.9 | C18—C19—Fe1 | 69.32 (17) |
| C5—C6—H6 | 118.9 | C20—C19—H19 | 126.4 |
| N2—C7—C1 | 112.7 (2) | C18—C19—H19 | 126.4 |
| N2—C7—C8 | 101.87 (19) | Fe1—C19—H19 | 125.8 |
| C1—C7—C8 | 112.7 (2) | C19—C20—C21 | 108.6 (3) |
| N2—C7—H7 | 109.8 | C19—C20—Fe1 | 70.08 (16) |
| C1—C7—H7 | 109.8 | C21—C20—Fe1 | 70.33 (16) |
| C8—C7—H7 | 109.8 | C19—C20—H20 | 125.7 |
| C9—C8—C7 | 102.7 (2) | C21—C20—H20 | 125.7 |
| C9—C8—H8A | 111.2 | Fe1—C20—H20 | 125.5 |
| C7—C8—H8A | 111.2 | C17—C21—C20 | 108.6 (3) |
| C9—C8—H8B | 111.2 | C17—C21—Fe1 | 70.24 (16) |
| C7—C8—H8B | 111.2 | C20—C21—Fe1 | 69.58 (16) |
| H8A—C8—H8B | 109.1 | C17—C21—H21 | 125.7 |
| N3—C9—C12 | 122.1 (2) | C20—C21—H21 | 125.7 |
| N3—C9—C8 | 114.8 (2) | Fe1—C21—H21 | 126.1 |
| C12—C9—C8 | 123.1 (2) | O1—N1—O2 | 122.3 (3) |
| O3—C10—N2 | 120.0 (2) | O1—N1—C2 | 119.8 (2) |
| O3—C10—C11 | 123.3 (3) | O2—N1—C2 | 117.9 (3) |
| N2—C10—C11 | 116.7 (3) | C10—N2—N3 | 122.3 (2) |
| C10—C11—H11A | 109.5 | C10—N2—C7 | 123.1 (2) |
| C10—C11—H11B | 109.5 | N3—N2—C7 | 112.77 (19) |
| H11A—C11—H11B | 109.5 | C9—N3—N2 | 107.67 (19) |
| C10—C11—H11C | 109.5 | C16—Fe1—C18 | 122.47 (13) |
| H11A—C11—H11C | 109.5 | C16—Fe1—C15 | 40.78 (12) |
| H11B—C11—H11C | 109.5 | C18—Fe1—C15 | 108.04 (13) |
| C10—C11—H11D | 109.5 | C16—Fe1—C12 | 41.17 (11) |
| H11A—C11—H11D | 141.1 | C18—Fe1—C12 | 158.50 (14) |
| H11B—C11—H11D | 56.3 | C15—Fe1—C12 | 68.71 (11) |
| H11C—C11—H11D | 56.3 | C16—Fe1—C20 | 120.16 (13) |
| C10—C11—H11E | 109.5 | C18—Fe1—C20 | 67.76 (12) |
| H11A—C11—H11E | 56.3 | C15—Fe1—C20 | 156.25 (14) |
| H11B—C11—H11E | 141.1 | C12—Fe1—C20 | 106.19 (11) |
| H11C—C11—H11E | 56.3 | C16—Fe1—C19 | 105.08 (13) |
| H11D—C11—H11E | 109.5 | C18—Fe1—C19 | 41.08 (13) |
| C10—C11—H11F | 109.5 | C15—Fe1—C19 | 121.42 (14) |
| H11A—C11—H11F | 56.3 | C12—Fe1—C19 | 121.10 (13) |
| H11B—C11—H11F | 56.3 | C20—Fe1—C19 | 39.92 (12) |
| H11C—C11—H11F | 141.1 | C16—Fe1—C13 | 68.97 (12) |
| H11D—C11—H11F | 109.5 | C18—Fe1—C13 | 159.13 (14) |
| H11E—C11—H11F | 109.5 | C15—Fe1—C13 | 68.14 (12) |
| C16—C12—C13 | 107.0 (2) | C12—Fe1—C13 | 41.18 (11) |
| C16—C12—C9 | 127.6 (3) | C20—Fe1—C13 | 124.17 (12) |
| C13—C12—C9 | 125.1 (2) | C19—Fe1—C13 | 158.78 (13) |
| C16—C12—Fe1 | 68.67 (15) | C16—Fe1—C21 | 156.74 (12) |
| C13—C12—Fe1 | 69.73 (16) | C18—Fe1—C21 | 67.26 (13) |
| C9—C12—Fe1 | 121.89 (18) | C15—Fe1—C21 | 161.94 (14) |
| C14—C13—C12 | 108.0 (3) | C12—Fe1—C21 | 122.28 (12) |
| C14—C13—Fe1 | 70.04 (17) | C20—Fe1—C21 | 40.08 (12) |
| C12—C13—Fe1 | 69.09 (15) | C19—Fe1—C21 | 67.40 (13) |
| C14—C13—H13 | 126.0 | C13—Fe1—C21 | 109.62 (12) |
| C12—C13—H13 | 126.0 | C16—Fe1—C17 | 160.09 (13) |
| Fe1—C13—H13 | 126.5 | C18—Fe1—C17 | 40.24 (13) |
| C15—C14—C13 | 108.1 (3) | C15—Fe1—C17 | 125.55 (13) |
| C15—C14—Fe1 | 69.23 (17) | C12—Fe1—C17 | 158.46 (12) |
| C13—C14—Fe1 | 69.50 (16) | C20—Fe1—C17 | 67.35 (12) |
| C15—C14—H14 | 126.0 | C19—Fe1—C17 | 68.06 (14) |
| C13—C14—H14 | 126.0 | C13—Fe1—C17 | 124.09 (13) |
| Fe1—C14—H14 | 126.9 | C21—Fe1—C17 | 39.68 (12) |
| C14—C15—C16 | 108.7 (3) | C16—Fe1—C14 | 68.53 (13) |
| C14—C15—Fe1 | 70.50 (16) | C18—Fe1—C14 | 123.41 (13) |
| C16—C15—Fe1 | 68.89 (15) | C15—Fe1—C14 | 40.27 (13) |
| C14—C15—H15 | 125.6 | C12—Fe1—C14 | 68.73 (12) |
| C16—C15—H15 | 125.6 | C20—Fe1—C14 | 161.46 (12) |
| Fe1—C15—H15 | 126.5 | C19—Fe1—C14 | 158.08 (13) |
| C15—C16—C12 | 108.2 (3) | C13—Fe1—C14 | 40.47 (11) |
| C15—C16—Fe1 | 70.33 (16) | C21—Fe1—C14 | 126.43 (13) |
| C12—C16—Fe1 | 70.16 (15) | C17—Fe1—C14 | 110.47 (13) |
| C15—C16—H16 | 125.9 | ||
| C6—C1—C2—C3 | 0.7 (4) | C16—C15—Fe1—C14 | −120.1 (3) |
| C7—C1—C2—C3 | −177.3 (3) | C13—C12—Fe1—C16 | −118.6 (2) |
| C6—C1—C2—N1 | 179.6 (3) | C9—C12—Fe1—C16 | 121.9 (3) |
| C7—C1—C2—N1 | 1.7 (4) | C16—C12—Fe1—C18 | −47.3 (4) |
| C1—C2—C3—C4 | −0.1 (5) | C13—C12—Fe1—C18 | −166.0 (3) |
| N1—C2—C3—C4 | −179.1 (3) | C9—C12—Fe1—C18 | 74.6 (4) |
| C2—C3—C4—C5 | 0.1 (5) | C16—C12—Fe1—C15 | 37.92 (18) |
| C3—C4—C5—C6 | −0.7 (5) | C13—C12—Fe1—C15 | −80.71 (18) |
| C2—C1—C6—C5 | −1.3 (4) | C9—C12—Fe1—C15 | 159.9 (3) |
| C7—C1—C6—C5 | 176.7 (3) | C16—C12—Fe1—C20 | −117.56 (18) |
| C4—C5—C6—C1 | 1.3 (5) | C13—C12—Fe1—C20 | 123.81 (17) |
| C6—C1—C7—N2 | 19.3 (3) | C9—C12—Fe1—C20 | 4.4 (2) |
| C2—C1—C7—N2 | −162.9 (2) | C16—C12—Fe1—C19 | −76.8 (2) |
| C6—C1—C7—C8 | −95.4 (3) | C13—C12—Fe1—C19 | 164.57 (17) |
| C2—C1—C7—C8 | 82.5 (3) | C9—C12—Fe1—C19 | 45.1 (3) |
| N2—C7—C8—C9 | −3.4 (3) | C16—C12—Fe1—C13 | 118.6 (2) |
| C1—C7—C8—C9 | 117.6 (2) | C9—C12—Fe1—C13 | −119.4 (3) |
| C7—C8—C9—N3 | 3.9 (3) | C16—C12—Fe1—C21 | −158.19 (18) |
| C7—C8—C9—C12 | −175.3 (2) | C13—C12—Fe1—C21 | 83.18 (18) |
| N3—C9—C12—C16 | −6.5 (4) | C9—C12—Fe1—C21 | −36.3 (3) |
| C8—C9—C12—C16 | 172.7 (3) | C16—C12—Fe1—C17 | 173.3 (3) |
| N3—C9—C12—C13 | −179.4 (3) | C13—C12—Fe1—C17 | 54.7 (4) |
| C8—C9—C12—C13 | −0.3 (4) | C9—C12—Fe1—C17 | −64.7 (4) |
| N3—C9—C12—Fe1 | −93.0 (3) | C16—C12—Fe1—C14 | 81.28 (19) |
| C8—C9—C12—Fe1 | 86.2 (3) | C13—C12—Fe1—C14 | −37.35 (16) |
| C16—C12—C13—C14 | 0.7 (3) | C9—C12—Fe1—C14 | −156.8 (2) |
| C9—C12—C13—C14 | 174.8 (3) | C19—C20—Fe1—C16 | 77.0 (2) |
| Fe1—C12—C13—C14 | 59.4 (2) | C21—C20—Fe1—C16 | −163.69 (18) |
| C16—C12—C13—Fe1 | −58.77 (19) | C19—C20—Fe1—C18 | −38.7 (2) |
| C9—C12—C13—Fe1 | 115.4 (3) | C21—C20—Fe1—C18 | 80.6 (2) |
| C12—C13—C14—C15 | −0.2 (3) | C19—C20—Fe1—C15 | 45.6 (4) |
| Fe1—C13—C14—C15 | 58.6 (2) | C21—C20—Fe1—C15 | 165.0 (3) |
| C12—C13—C14—Fe1 | −58.83 (19) | C19—C20—Fe1—C12 | 119.41 (19) |
| C13—C14—C15—C16 | −0.4 (3) | C21—C20—Fe1—C12 | −121.23 (19) |
| Fe1—C14—C15—C16 | 58.4 (2) | C21—C20—Fe1—C19 | 119.4 (3) |
| C13—C14—C15—Fe1 | −58.8 (2) | C19—C20—Fe1—C13 | 160.80 (19) |
| C14—C15—C16—C12 | 0.8 (3) | C21—C20—Fe1—C13 | −79.8 (2) |
| Fe1—C15—C16—C12 | 60.21 (19) | C19—C20—Fe1—C21 | −119.4 (3) |
| C14—C15—C16—Fe1 | −59.4 (2) | C19—C20—Fe1—C17 | −82.4 (2) |
| C13—C12—C16—C15 | −0.9 (3) | C21—C20—Fe1—C17 | 36.95 (19) |
| C9—C12—C16—C15 | −174.8 (3) | C19—C20—Fe1—C14 | −169.4 (4) |
| Fe1—C12—C16—C15 | −60.3 (2) | C21—C20—Fe1—C14 | −50.0 (5) |
| C13—C12—C16—Fe1 | 59.44 (19) | C20—C19—Fe1—C16 | −119.27 (19) |
| C9—C12—C16—Fe1 | −114.5 (3) | C18—C19—Fe1—C16 | 122.48 (19) |
| C21—C17—C18—C19 | −0.3 (4) | C20—C19—Fe1—C18 | 118.2 (3) |
| Fe1—C17—C18—C19 | −59.9 (2) | C20—C19—Fe1—C15 | −160.29 (19) |
| C21—C17—C18—Fe1 | 59.7 (2) | C18—C19—Fe1—C15 | 81.5 (2) |
| C17—C18—C19—C20 | 0.4 (3) | C20—C19—Fe1—C12 | −77.7 (2) |
| Fe1—C18—C19—C20 | −60.0 (2) | C18—C19—Fe1—C12 | 164.07 (18) |
| C17—C18—C19—Fe1 | 60.4 (2) | C18—C19—Fe1—C20 | −118.2 (3) |
| C18—C19—C20—C21 | −0.4 (3) | C20—C19—Fe1—C13 | −48.7 (4) |
| Fe1—C19—C20—C21 | −60.0 (2) | C18—C19—Fe1—C13 | −167.0 (3) |
| C18—C19—C20—Fe1 | 59.6 (2) | C20—C19—Fe1—C21 | 37.44 (19) |
| C18—C17—C21—C20 | 0.0 (4) | C18—C19—Fe1—C21 | −80.8 (2) |
| Fe1—C17—C21—C20 | 59.2 (2) | C20—C19—Fe1—C17 | 80.5 (2) |
| C18—C17—C21—Fe1 | −59.2 (2) | C18—C19—Fe1—C17 | −37.78 (19) |
| C19—C20—C21—C17 | 0.2 (3) | C20—C19—Fe1—C14 | 171.0 (3) |
| Fe1—C20—C21—C17 | −59.6 (2) | C18—C19—Fe1—C14 | 52.7 (4) |
| C19—C20—C21—Fe1 | 59.9 (2) | C14—C13—Fe1—C16 | −81.2 (2) |
| C3—C2—N1—O1 | 161.2 (3) | C12—C13—Fe1—C16 | 38.25 (15) |
| C1—C2—N1—O1 | −17.8 (4) | C14—C13—Fe1—C18 | 46.1 (4) |
| C3—C2—N1—O2 | −18.3 (4) | C12—C13—Fe1—C18 | 165.5 (3) |
| C1—C2—N1—O2 | 162.7 (3) | C14—C13—Fe1—C15 | −37.22 (19) |
| O3—C10—N2—N3 | −176.0 (2) | C12—C13—Fe1—C15 | 82.21 (17) |
| C11—C10—N2—N3 | 5.6 (4) | C14—C13—Fe1—C12 | −119.4 (2) |
| O3—C10—N2—C7 | −12.4 (4) | C14—C13—Fe1—C20 | 165.90 (19) |
| C11—C10—N2—C7 | 169.3 (2) | C12—C13—Fe1—C20 | −74.68 (19) |
| C1—C7—N2—C10 | 76.4 (3) | C14—C13—Fe1—C19 | −158.4 (3) |
| C8—C7—N2—C10 | −162.6 (2) | C12—C13—Fe1—C19 | −39.0 (4) |
| C1—C7—N2—N3 | −118.6 (2) | C14—C13—Fe1—C21 | 123.6 (2) |
| C8—C7—N2—N3 | 2.4 (3) | C12—C13—Fe1—C21 | −116.97 (16) |
| C12—C9—N3—N2 | 176.7 (2) | C14—C13—Fe1—C17 | 81.8 (2) |
| C8—C9—N3—N2 | −2.5 (3) | C12—C13—Fe1—C17 | −158.78 (15) |
| C10—N2—N3—C9 | 165.0 (2) | C12—C13—Fe1—C14 | 119.4 (2) |
| C7—N2—N3—C9 | −0.1 (3) | C17—C21—Fe1—C16 | 157.6 (3) |
| C15—C16—Fe1—C18 | −79.9 (2) | C20—C21—Fe1—C16 | 37.9 (4) |
| C12—C16—Fe1—C18 | 161.37 (17) | C17—C21—Fe1—C18 | 37.7 (2) |
| C12—C16—Fe1—C15 | −118.8 (3) | C20—C21—Fe1—C18 | −82.0 (2) |
| C15—C16—Fe1—C12 | 118.8 (3) | C17—C21—Fe1—C15 | −40.6 (5) |
| C15—C16—Fe1—C20 | −161.29 (19) | C20—C21—Fe1—C15 | −160.3 (4) |
| C12—C16—Fe1—C20 | 80.0 (2) | C17—C21—Fe1—C12 | −164.09 (19) |
| C15—C16—Fe1—C19 | −120.9 (2) | C20—C21—Fe1—C12 | 76.2 (2) |
| C12—C16—Fe1—C19 | 120.30 (18) | C17—C21—Fe1—C20 | 119.7 (3) |
| C15—C16—Fe1—C13 | 80.5 (2) | C17—C21—Fe1—C19 | 82.4 (2) |
| C12—C16—Fe1—C13 | −38.25 (16) | C20—C21—Fe1—C19 | −37.29 (18) |
| C15—C16—Fe1—C21 | 171.5 (3) | C17—C21—Fe1—C13 | −120.1 (2) |
| C12—C16—Fe1—C21 | 52.7 (4) | C20—C21—Fe1—C13 | 120.17 (19) |
| C15—C16—Fe1—C17 | −54.1 (5) | C20—C21—Fe1—C17 | −119.7 (3) |
| C12—C16—Fe1—C17 | −172.8 (3) | C17—C21—Fe1—C14 | −77.9 (2) |
| C15—C16—Fe1—C14 | 36.94 (19) | C20—C21—Fe1—C14 | 162.37 (18) |
| C12—C16—Fe1—C14 | −81.82 (18) | C21—C17—Fe1—C16 | −153.8 (3) |
| C17—C18—Fe1—C16 | 166.73 (17) | C18—C17—Fe1—C16 | −34.7 (5) |
| C19—C18—Fe1—C16 | −74.9 (2) | C21—C17—Fe1—C18 | −119.2 (3) |
| C17—C18—Fe1—C15 | 124.18 (19) | C21—C17—Fe1—C15 | 165.6 (2) |
| C19—C18—Fe1—C15 | −117.4 (2) | C18—C17—Fe1—C15 | −75.2 (2) |
| C17—C18—Fe1—C12 | −158.3 (3) | C21—C17—Fe1—C12 | 39.1 (4) |
| C19—C18—Fe1—C12 | −39.9 (4) | C18—C17—Fe1—C12 | 158.3 (3) |
| C17—C18—Fe1—C20 | −80.74 (19) | C21—C17—Fe1—C20 | −37.31 (18) |
| C19—C18—Fe1—C20 | 37.64 (18) | C18—C17—Fe1—C20 | 81.9 (2) |
| C17—C18—Fe1—C19 | −118.4 (3) | C21—C17—Fe1—C19 | −80.6 (2) |
| C17—C18—Fe1—C13 | 48.4 (4) | C18—C17—Fe1—C19 | 38.56 (18) |
| C19—C18—Fe1—C13 | 166.8 (3) | C21—C17—Fe1—C13 | 79.6 (2) |
| C17—C18—Fe1—C21 | −37.20 (18) | C18—C17—Fe1—C13 | −161.24 (18) |
| C19—C18—Fe1—C21 | 81.2 (2) | C18—C17—Fe1—C21 | 119.2 (3) |
| C19—C18—Fe1—C17 | 118.4 (3) | C21—C17—Fe1—C14 | 122.9 (2) |
| C17—C18—Fe1—C14 | 82.5 (2) | C18—C17—Fe1—C14 | −118.0 (2) |
| C19—C18—Fe1—C14 | −159.15 (19) | C15—C14—Fe1—C16 | −37.39 (18) |
| C14—C15—Fe1—C16 | 120.1 (3) | C13—C14—Fe1—C16 | 82.35 (19) |
| C14—C15—Fe1—C18 | −120.8 (2) | C15—C14—Fe1—C18 | 78.2 (2) |
| C16—C15—Fe1—C18 | 119.1 (2) | C13—C14—Fe1—C18 | −162.08 (19) |
| C14—C15—Fe1—C12 | 81.82 (19) | C13—C14—Fe1—C15 | 119.7 (3) |
| C16—C15—Fe1—C12 | −38.28 (18) | C15—C14—Fe1—C12 | −81.75 (19) |
| C14—C15—Fe1—C20 | 163.6 (3) | C13—C14—Fe1—C12 | 37.98 (17) |
| C16—C15—Fe1—C20 | 43.5 (4) | C15—C14—Fe1—C20 | −159.1 (4) |
| C14—C15—Fe1—C19 | −163.88 (18) | C13—C14—Fe1—C20 | −39.3 (5) |
| C16—C15—Fe1—C19 | 76.0 (2) | C15—C14—Fe1—C19 | 39.4 (4) |
| C14—C15—Fe1—C13 | 37.39 (18) | C13—C14—Fe1—C19 | 159.1 (3) |
| C16—C15—Fe1—C13 | −82.7 (2) | C15—C14—Fe1—C13 | −119.7 (3) |
| C14—C15—Fe1—C21 | −49.0 (5) | C15—C14—Fe1—C21 | 163.10 (19) |
| C16—C15—Fe1—C21 | −169.1 (4) | C13—C14—Fe1—C21 | −77.2 (2) |
| C14—C15—Fe1—C17 | −79.7 (2) | C15—C14—Fe1—C17 | 121.3 (2) |
| C16—C15—Fe1—C17 | 160.19 (19) | C13—C14—Fe1—C17 | −118.96 (19) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C11—H11A···N3 | 0.96 | 2.29 | 2.788 (4) | 112 |
| C8—H8A···O3i | 0.97 | 2.57 | 3.476 (3) | 154 |
| C8—H8B···O2ii | 0.97 | 2.63 | 3.551 (4) | 158 |
| C21—H21···O2ii | 0.93 | 2.46 | 3.136 (4) | 130 |
Symmetry codes: (i) −x+1/2, y+1/2, z; (ii) x+1/2, y, −z+3/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HY2116).
References
- Amr, A. E. E., Abdel-Latif, N. A. & Abdalla, M. M. (2006). Bioorg. Med. Chem.14, 373–384. [DOI] [PubMed]
- Biot, C., Dessolin, J., Richard, I. & Dive, D. (2004). J. Organomet. Chem.689, 4678–4682.
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc.97, 1354–1358.
- Fang, J., Jin, Z., Li, Z. & Liu, W. (2003). J. Organomet. Chem.674, 1–9.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Fouda, M. F. R., Abd-Elzaher, M. M., Abdelsamaia, R. A. & Labib, A. A. (2007). Appl. Organomet. Chem.21, 613–625.
- Guirado, A., Martiz, B. & Andreu, R. (2004). Tetrahedron Lett.45, 8523–8526.
- Jaouen, G., Top, S., Vessireres, A., Leclercq, G., Vaissermann, J. & McGlinchey, M. J. (2004). Curr. Med. Chem.11, 2505–2517. [DOI] [PubMed]
- Karthikeyan, M. S., Holla, B. S. & Kumari, N. S. (2007). Eur. J. Med. Chem.42, 30–36. [DOI] [PubMed]
- Küçükgüzel, G. Ş., Rollas, S., Erdeniz, H., Kiraz, M., Ekinci, A. C. & Vidin, A. (2000). Eur. J. Med. Chem.35, 761–771. [DOI] [PubMed]
- Kudar, V., Mady-Zsoldos, V., Simon, K., Csampai, A. & Sohar, P. (2005). J. Organomet. Chem.690, 4018–4026.
- Özdemir, Z., Kandilci, H. B., Gümüşel, B., Çalış, Ü. & Bilgin, A. A. (2007). Eur. J. Med. Chem.42, 373–379. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Shi, Y.-C., Sui, C.-X. & Zhu, B.-B. (2006). Acta Cryst. E62, m1954–m1955.
- Shi, Y.-C., Zhu, B.-B. & Sui, C.-X. (2006a). Acta Cryst. C62, m577–m580. [DOI] [PubMed]
- Shi, Y.-C., Zhu, B.-B. & Sui, C.-X. (2006b). Acta Cryst. E62, m1651–m1653.
- Spek, A. L. (2003). J. Appl. Cryst.36, 7–13.
- Stoe & Cie (2002). X-AREA (Version 1.18) and X-RED32 (Version 1.04). Stoe & Cie, Darmstadt, Germany.
- Zora, M., Açıkgöz, C., Tumay, T. A., Odabaşoğlu, M. & Büyükgüngör, O. (2006). Acta Cryst. C62, m327–m330. [DOI] [PubMed]
- Zora, M., Pınar, A. N., Odabaşoğlu, M., Büyükgüngör, O. & Turgut Cin, G. (2008). J. Organomet. Chem.693, 145–154.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808004236/hy2116sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808004236/hy2116Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report