Abstract
In the title compound, C13H12N2O2S, the dihedral angle between the two aromatic ring planes is 87.52 (12)°. The molecule shows an intramolecular N—H⋯O hydrogen bond. The crystal structure is stabilized by intermolecular N—H⋯S and C—H⋯O hydrogen bonding.
Related literature
For general background, see: Estévez-Hernández et al. (2007 ▶); Otazo et al. (2001 ▶). For related structures, see: Arslan et al. (2004 ▶); Khawar Rauf et al. (2007 ▶). For the synthesis, see: Otazo et al. (2001 ▶).
Experimental
Crystal data
C13H12N2O2S
M r = 260.31
Tetragonal,

a = 9.445 (3) Å
c = 27.107 (6) Å
V = 2418.2 (12) Å3
Z = 8
Mo Kα radiation
μ = 0.26 mm−1
T = 150 (2) K
0.3 × 0.1 × 0.08 mm
Data collection
Nonius KappaCCD diffractometer
Absorption correction: none
12492 measured reflections
2120 independent reflections
1922 reflections with I > 2σ(I)
R int = 0.092
Refinement
R[F 2 > 2σ(F 2)] = 0.035
wR(F 2) = 0.085
S = 1.06
2120 reflections
163 parameters
H-atom parameters constrained
Δρmax = 0.17 e Å−3
Δρmin = −0.19 e Å−3
Absolute structure: Flack (1983 ▶), 802 Friedel pairs
Flack parameter: −0.16 (10)
Data collection: COLLECT (Nonius, 2000 ▶); cell refinement: SCALEPACK (Otwinowski & Minor, 1997 ▶); data reduction: DENZO (Otwinowski & Minor, 1997 ▶) and SCALEPACK; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808006181/xu2401sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808006181/xu2401Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1⋯O1 | 0.88 | 2.00 | 2.697 (3) | 135 |
| N2—H2⋯S1i | 0.88 | 2.70 | 3.578 (2) | 174 |
| C7—H7⋯O1ii | 0.95 | 2.58 | 3.423 (3) | 148 |
Symmetry codes: (i) 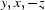 ; (ii)
; (ii)  .
.
Acknowledgments
The authors thank the Crystallography Group, São Carlos Physics Institute, USP, Brazil, for allowing the X-ray data collection. The authors acknowledge financial support from the Brazilian agency CAPES (Project 018/05).
supplementary crystallographic information
Comment
Substituted N-acylthioureas have been a subject of investigations, due to their ability to form stable metal complexes and as ionophores in potenciometric and amperometric sensors for Cd(II), Hg(II) and Pb(II) (Otazo et al., 2001; Estévez-Hernández et al., 2007). The title compound, (I) (Fig. 1), is another example of our newly synthesized furoylthiourea derivatives, which show outstanding complexation properties.
Compound (I) is a typical N,N'-disubstituted thiourea derivative with normal geometric parameters. The C2—S1 and C3—O1 bonds (Table 1) both show the expected double-bond character. The short values of the C2—N1, C2—N2 and C3—N2 bonds indicate partial double bond character.
The dihedral angle between the aromatic rings is 87.52 (12)°, and the angles with the thiourea plane are 86.67 (19)° for the benzene ring and 4.81 (12)° for the furan ring. An intramolecular N–H···O hydrogen bond is present (Table 2), forming a six-membered ring commonly observed in this type of compounds (Arslan et al., 2004; Khawar Rauf et al., 2007). The crystal structure of (I) is stabilized by intermolecular N—H···S and C—H···O hydrogen bonding (Table 2).
Experimental
The title compound, (I), was synthesized according to a procedure described by Otazo et al. (2001) by converting furoyl chloride into furoyl isothiocyanate and then condensing with the appropriate amine. The resulting solid product was crystallized from a dichlorometane-methanol (1:1) mixture yielding X-ray quality single crystals. Elemental analysis for C13H12N2O2S found: C 67.73, H 4.75, N 8.23, S 9.34%; calculated: C 67.86, H 4.46, N 8.33, S 9.52%
Refinement
H atoms were placed in calculated positions with C–H = 0.95 Å (aromatic), N–H = 0.88 Å and C–H = 0.99 Å (methylene), and refined in riding model, Uiso(H) = 1.2Ueq(C,N).
Figures
Fig. 1.
The molecular structure of title compound. Displacement ellipsoids are drawn at the 50% probability level. The intramolecular N—H···O hydrogen bond is shown as a dashed line.
Crystal data
| C13H12N2O2S | Z = 8 |
| Mr = 260.31 | F000 = 1088 |
| Tetragonal, P41212 | Dx = 1.43 Mg m−3 |
| Hall symbol: P 4abw 2nw | Melting point: 402.5 K |
| a = 9.445 (3) Å | Mo Kα radiation λ = 0.71073 Å |
| b = 9.445 (3) Å | Cell parameters from 9761 reflections |
| c = 27.107 (6) Å | θ = 2.9–26.0º |
| α = 90º | µ = 0.26 mm−1 |
| β = 90º | T = 150 (2) K |
| γ = 90º | Block, colourless |
| V = 2418.2 (12) Å3 | 0.3 × 0.1 × 0.08 mm |
Data collection
| Nonius KappaCCD diffractometer | Rint = 0.092 |
| ω scans | θmax = 25.0º |
| Absorption correction: none | θmin = 3.7º |
| 12492 measured reflections | h = −11→9 |
| 2120 independent reflections | k = −8→11 |
| 1922 reflections with I > 2σ(I) | l = −32→24 |
Refinement
| Refinement on F2 | w = 1/[σ2(Fo2) + (0.0402P)2 + 0.4478P] where P = (Fo2 + 2Fc2)/3 |
| Least-squares matrix: full | (Δ/σ)max < 0.001 |
| R[F2 > 2σ(F2)] = 0.035 | Δρmax = 0.17 e Å−3 |
| wR(F2) = 0.085 | Δρmin = −0.19 e Å−3 |
| S = 1.06 | Extinction correction: none |
| 2120 reflections | Absolute structure: Flack (1983), 802 Friedel pairs |
| 163 parameters | Flack parameter: −0.16 (10) |
| H-atom parameters constrained |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.7270 (2) | 0.4402 (2) | 0.16180 (8) | 0.0311 (6) | |
| H1A | 0.7104 | 0.3494 | 0.1791 | 0.037* | |
| H1B | 0.6351 | 0.4904 | 0.1597 | 0.037* | |
| C2 | 0.7259 (2) | 0.4740 (2) | 0.07225 (7) | 0.0251 (5) | |
| C3 | 0.8761 (2) | 0.3236 (2) | 0.01767 (8) | 0.0260 (5) | |
| C4 | 0.9003 (2) | 0.2913 (2) | −0.03448 (8) | 0.0270 (5) | |
| C5 | 0.9858 (2) | 0.1962 (2) | −0.05694 (8) | 0.0316 (5) | |
| H5 | 1.0486 | 0.1313 | −0.0415 | 0.038* | |
| C6 | 0.9623 (2) | 0.2131 (2) | −0.10836 (8) | 0.0336 (6) | |
| H6 | 1.0069 | 0.1616 | −0.1341 | 0.04* | |
| C7 | 0.8651 (2) | 0.3157 (2) | −0.11361 (8) | 0.0330 (6) | |
| H7 | 0.8296 | 0.3482 | −0.1444 | 0.04* | |
| C8 | 0.8284 (2) | 0.5288 (2) | 0.19216 (8) | 0.0272 (5) | |
| C9 | 0.9223 (2) | 0.6243 (2) | 0.17099 (9) | 0.0320 (5) | |
| H9 | 0.9287 | 0.6313 | 0.1361 | 0.038* | |
| C10 | 1.0065 (3) | 0.7093 (3) | 0.20046 (10) | 0.0389 (6) | |
| H10 | 1.0706 | 0.7739 | 0.1855 | 0.047* | |
| C11 | 0.9987 (3) | 0.7014 (3) | 0.25126 (10) | 0.0388 (6) | |
| H11 | 1.0563 | 0.7606 | 0.2713 | 0.047* | |
| C12 | 0.9063 (3) | 0.6063 (3) | 0.27252 (9) | 0.0388 (6) | |
| H12 | 0.9001 | 0.6 | 0.3074 | 0.047* | |
| C13 | 0.8222 (2) | 0.5198 (3) | 0.24330 (8) | 0.0333 (6) | |
| H13 | 0.7598 | 0.4539 | 0.2584 | 0.04* | |
| O1 | 0.94160 (17) | 0.26029 (17) | 0.04999 (6) | 0.0314 (4) | |
| O2 | 0.82422 (16) | 0.36671 (15) | −0.06877 (5) | 0.0309 (4) | |
| N1 | 0.7763 (2) | 0.41033 (19) | 0.11201 (6) | 0.0275 (4) | |
| H1 | 0.8432 | 0.3465 | 0.1081 | 0.033* | |
| N2 | 0.77589 (19) | 0.42680 (19) | 0.02701 (6) | 0.0262 (4) | |
| H2 | 0.739 | 0.4679 | 0.0009 | 0.031* | |
| S1 | 0.60657 (6) | 0.60581 (6) | 0.072955 (19) | 0.03259 (18) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0337 (12) | 0.0375 (13) | 0.0220 (11) | −0.0027 (11) | 0.0039 (9) | 0.0008 (10) |
| C2 | 0.0253 (11) | 0.0269 (11) | 0.0231 (11) | −0.0052 (9) | 0.0015 (9) | −0.0028 (9) |
| C3 | 0.0227 (11) | 0.0265 (11) | 0.0289 (12) | −0.0054 (10) | 0.0013 (9) | −0.0005 (9) |
| C4 | 0.0289 (12) | 0.0274 (12) | 0.0248 (11) | −0.0019 (10) | −0.0021 (9) | 0.0017 (9) |
| C5 | 0.0310 (13) | 0.0319 (12) | 0.0318 (12) | 0.0041 (10) | 0.0005 (10) | −0.0019 (10) |
| C6 | 0.0396 (14) | 0.0353 (13) | 0.0260 (12) | 0.0033 (11) | 0.0041 (10) | −0.0041 (10) |
| C7 | 0.0411 (14) | 0.0380 (13) | 0.0200 (11) | 0.0024 (11) | 0.0016 (10) | −0.0032 (10) |
| C8 | 0.0304 (12) | 0.0266 (12) | 0.0246 (11) | 0.0077 (10) | 0.0000 (9) | −0.0018 (9) |
| C9 | 0.0354 (13) | 0.0324 (12) | 0.0283 (12) | 0.0043 (11) | 0.0009 (10) | 0.0006 (11) |
| C10 | 0.0381 (14) | 0.0358 (13) | 0.0427 (15) | −0.0012 (12) | −0.0005 (11) | −0.0003 (11) |
| C11 | 0.0399 (14) | 0.0381 (13) | 0.0385 (14) | 0.0034 (12) | −0.0058 (12) | −0.0074 (12) |
| C12 | 0.0451 (15) | 0.0452 (15) | 0.0261 (12) | 0.0097 (13) | −0.0045 (11) | −0.0035 (11) |
| C13 | 0.0376 (13) | 0.0347 (13) | 0.0277 (13) | 0.0048 (11) | 0.0023 (11) | 0.0020 (10) |
| O1 | 0.0327 (9) | 0.0354 (9) | 0.0262 (8) | 0.0035 (7) | −0.0005 (7) | 0.0009 (7) |
| O2 | 0.0354 (9) | 0.0319 (9) | 0.0253 (8) | 0.0060 (7) | −0.0011 (7) | −0.0017 (7) |
| N1 | 0.0303 (10) | 0.0287 (10) | 0.0236 (9) | 0.0027 (8) | 0.0016 (8) | −0.0010 (8) |
| N2 | 0.0288 (10) | 0.0291 (10) | 0.0208 (9) | 0.0010 (8) | −0.0008 (8) | −0.0012 (8) |
| S1 | 0.0371 (3) | 0.0324 (3) | 0.0283 (3) | 0.0066 (3) | 0.0013 (3) | −0.0021 (2) |
Geometric parameters (Å, °)
| C1—N1 | 1.456 (3) | C7—O2 | 1.363 (2) |
| C1—C8 | 1.515 (3) | C7—H7 | 0.95 |
| C1—H1A | 0.99 | C8—C9 | 1.389 (3) |
| C1—H1B | 0.99 | C8—C13 | 1.390 (3) |
| C2—N1 | 1.323 (3) | C9—C10 | 1.384 (3) |
| C2—N2 | 1.388 (3) | C9—H9 | 0.95 |
| C2—S1 | 1.679 (2) | C10—C11 | 1.381 (4) |
| C3—O1 | 1.228 (3) | C10—H10 | 0.95 |
| C3—N2 | 1.382 (3) | C11—C12 | 1.379 (4) |
| C3—C4 | 1.464 (3) | C11—H11 | 0.95 |
| C4—C5 | 1.353 (3) | C12—C13 | 1.387 (4) |
| C4—O2 | 1.374 (2) | C12—H12 | 0.95 |
| C5—C6 | 1.420 (3) | C13—H13 | 0.95 |
| C5—H5 | 0.95 | N1—H1 | 0.88 |
| C6—C7 | 1.342 (3) | N2—H2 | 0.88 |
| C6—H6 | 0.95 | ||
| N1—C1—C8 | 114.11 (18) | C9—C8—C1 | 122.5 (2) |
| N1—C1—H1A | 108.7 | C13—C8—C1 | 118.8 (2) |
| C8—C1—H1A | 108.7 | C10—C9—C8 | 120.3 (2) |
| N1—C1—H1B | 108.7 | C10—C9—H9 | 119.8 |
| C8—C1—H1B | 108.7 | C8—C9—H9 | 119.8 |
| H1A—C1—H1B | 107.6 | C11—C10—C9 | 120.9 (2) |
| N1—C2—N2 | 116.84 (18) | C11—C10—H10 | 119.6 |
| N1—C2—S1 | 124.70 (16) | C9—C10—H10 | 119.6 |
| N2—C2—S1 | 118.46 (15) | C12—C11—C10 | 119.1 (2) |
| O1—C3—N2 | 123.90 (19) | C12—C11—H11 | 120.5 |
| O1—C3—C4 | 120.59 (19) | C10—C11—H11 | 120.5 |
| N2—C3—C4 | 115.51 (18) | C11—C12—C13 | 120.5 (2) |
| C5—C4—O2 | 110.61 (18) | C11—C12—H12 | 119.8 |
| C5—C4—C3 | 131.8 (2) | C13—C12—H12 | 119.8 |
| O2—C4—C3 | 117.63 (18) | C12—C13—C8 | 120.6 (2) |
| C4—C5—C6 | 105.9 (2) | C12—C13—H13 | 119.7 |
| C4—C5—H5 | 127.1 | C8—C13—H13 | 119.7 |
| C6—C5—H5 | 127.1 | C7—O2—C4 | 105.78 (17) |
| C7—C6—C5 | 107.0 (2) | C2—N1—C1 | 123.51 (18) |
| C7—C6—H6 | 126.5 | C2—N1—H1 | 118.2 |
| C5—C6—H6 | 126.5 | C1—N1—H1 | 118.2 |
| C6—C7—O2 | 110.8 (2) | C3—N2—C2 | 128.43 (18) |
| C6—C7—H7 | 124.6 | C3—N2—H2 | 115.8 |
| O2—C7—H7 | 124.6 | C2—N2—H2 | 115.8 |
| C9—C8—C13 | 118.6 (2) | ||
| O1—C3—C4—C5 | 1.4 (4) | C10—C11—C12—C13 | 0.0 (4) |
| N2—C3—C4—C5 | −178.1 (2) | C11—C12—C13—C8 | −0.8 (3) |
| O1—C3—C4—O2 | −179.53 (19) | C9—C8—C13—C12 | 1.1 (3) |
| N2—C3—C4—O2 | 1.0 (3) | C1—C8—C13—C12 | −175.5 (2) |
| O2—C4—C5—C6 | 0.2 (3) | C6—C7—O2—C4 | −0.1 (2) |
| C3—C4—C5—C6 | 179.3 (2) | C5—C4—O2—C7 | −0.1 (2) |
| C4—C5—C6—C7 | −0.2 (3) | C3—C4—O2—C7 | −179.4 (2) |
| C5—C6—C7—O2 | 0.2 (3) | N2—C2—N1—C1 | −175.32 (19) |
| N1—C1—C8—C9 | 27.7 (3) | S1—C2—N1—C1 | 4.4 (3) |
| N1—C1—C8—C13 | −155.8 (2) | C8—C1—N1—C2 | −104.7 (2) |
| C13—C8—C9—C10 | −0.6 (3) | O1—C3—N2—C2 | −3.1 (3) |
| C1—C8—C9—C10 | 175.9 (2) | C4—C3—N2—C2 | 176.4 (2) |
| C8—C9—C10—C11 | −0.3 (4) | N1—C2—N2—C3 | −2.3 (3) |
| C9—C10—C11—C12 | 0.6 (4) | S1—C2—N2—C3 | 178.05 (17) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1···O1 | 0.88 | 2.00 | 2.697 (3) | 135 |
| N2—H2···S1i | 0.88 | 2.70 | 3.578 (2) | 174 |
| C7—H7···O1ii | 0.95 | 2.58 | 3.423 (3) | 148 |
Symmetry codes: (i) y, x, −z; (ii) y+1/2, −x+3/2, z−1/4.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: XU2401).
References
- Arslan, H., Flörke, U. & Külcü, N. (2004). Turk. J. Chem.28, 673–678.
- Estévez-Hernández, O., Hidalgo, J. L., Reguera, E. & Naranjo, I. (2007). Sensors Actuators, B120, 766–772.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Khawar Rauf, M., Badshah, A. & Bolte, M. (2007). Acta Cryst. E63, o1256–o1257.
- Nonius (2000). COLLECT Enraf–Nonius BV, Delft, The Netherlands.
- Otazo, E., Pérez, L., Estévez, O., Rojas, S. & Alonso, J. (2001). J. Chem. Soc. Perkin Trans. 2, pp. 2211–2218.
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808006181/xu2401sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808006181/xu2401Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



