Abstract
The title compound, C36H27NOP2, has been reported as a ligand on rhodium for the catalysis of hydroformylation reactions. The key feature of the compound is the intramolecular P⋯P distance of 4.255 (2) Å. The bond angles at the P atoms range from 99.93 (10) to 103.02 (10)°. The phenoxazine ring system is essentially planar and a non-crystallographic mirror plane through the N⋯O vector bisects the molecule. The C—O bond lengths range from 1.388 (2) to 1.392 (2) Å and the C—N bond lengths range from 1.398 (3) to 1.403 (3) Å.
Related literature
For related literature, see: Antonio et al. (1989 ▶); Claver & van Leeuwen (2000 ▶); Deprele & Montchamp (2004 ▶); van Leeuwen et al. (2002 ▶); Osiński et al. (2005 ▶); Petrassi et al. (2000 ▶); Ricken et al. (2006a
▶,b
▶,c
▶); Sandee et al. (1999 ▶, 2001 ▶); Tolman (1977 ▶); van der Veen et al. (2000 ▶).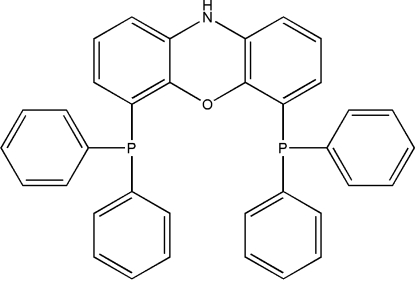
Experimental
Crystal data
C36H27NOP2
M r = 551.53
Triclinic,
a = 10.4233 (3) Å
b = 10.9113 (3) Å
c = 12.9940 (4) Å
α = 104.055 (2)°
β = 102.555 (2)°
γ = 97.459 (2)°
V = 1373.04 (7) Å3
Z = 2
Mo Kα radiation
μ = 0.19 mm−1
T = 173 (2) K
0.40 × 0.18 × 0.12 mm
Data collection
Bruker APEXII CCD area-detector diffractometer
Absorption correction: none
15968 measured reflections
5396 independent reflections
3646 reflections with I > 2σ(I)
R int = 0.055
Refinement
R[F 2 > 2σ(F 2)] = 0.043
wR(F 2) = 0.105
S = 0.95
5396 reflections
365 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.38 e Å−3
Δρmin = −0.29 e Å−3
Data collection: APEX2 (Bruker, 2005 ▶); cell refinement: SAINT-NT (Bruker, 2005 ▶); data reduction: SAINT-NT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: PLATON (Spek, 2003 ▶) and ORTEP-3 (Farrugia, 1997 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808006648/dn2322sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808006648/dn2322Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
We thank Dr Manuel Fernandez for the data collection, and SASOL, THRIP and the University of KwaZulu-Natal for financial support.
supplementary crystallographic information
Comment
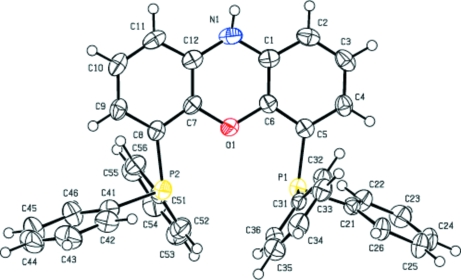
The titled compound, (1) (Fig. 1), is a xanthene based diphenylphosphine ligand. The synthesis of the ligand has been reported in literature (van der Veen et al., 2000; Petrassi et al., 2000; Antonio et al., 1989), in addition it is commercially available and has been used extensively in synthesis and as a precursor for the synthesis of substituted bis(diphenylphoshino)phenoxazine ligands (Osiński et al., 2005; Ricken et al., 2006a,b). However, this is the first time that the crystal structure is being reported. This ligand and similar xantphos based ligands have been used on Rh as catalysts for the regioselective hydroformylation of 1-octene to octanal (Claver & van Leeuwen, 2000; van der Veen et al., 2000). Moreover, (1) has been successfully immobilized on silica (Sandee et al., 2001, 1999; van Leeuwen et al., 2002), polystyrene (Deprele & Montchamp, 2004), and dendritic supports (Ricken et al., 2006a).
The title compound (1) was prepared following literature procedures (Antonio et al., 1989; Petrassi et al., 2000) as part of our ongoing investigation of scorpionate-type ligands by the alkylation of the amine. The structural elucidation of this compound allows for the determination of important ligand factors such as the cone angle (Tolman, 1977), and the flexibility range of the natural bite angle (van der Veen et al., 2000). It is also useful for studies of the coordination chemistry and catalytic applications of xantphos-type ligands. For example, the intramolecular P···P distance of 4.255 Å for (1) is similar to values reported for nixantphos-type ligands functionalized at the nitrogen (Osiński, et al., 2005; Ricken et al., 2006a,c) indicating that a functionality at N has little influence on the bite angle of the ligand.
Experimental
The compound was synthesized via a three step procedure adapted from literature (Antonio et al., 1989; Petrassi et al., 2000; van der Veen et al., 2000). Yield: 70% of yellow crystals of (1), m.p. 457–459 K. Spectroscopic analysis: 1H NMR (600 MHz, CDCl3, δ, p.p.m): 5.16 (s, 1H; NH), 5.97 (d, 2H; J(H,H) = 6.4 Hz,), 6.34 (bd, 2H;J(H,H) = 7.3 Hz,), 6.58 (t, 2H J(H,H) = 7.7 Hz), 7.17–7.23 (bs, 20H). 13C NMR (600 MHz, CDCl3, δ, p.p.m): 113.7(CH), 123.7(CH), 125.8(CH), 128.1(CH), 128.2(CH), 128.3(C), 128.3 (C), 131.3(bs,CN), 133.9(CH), 134.0(C), 136.7 (C). 31P NMR (600 MHz, CDCl3, δ, p.p.m): -19.0 MS m/z (%): 552.1633 (M + H) calculated = 552.1648 for C36H27NOP2 Elemental Analysis: C, 78.01; H, 4.95; N, 2.47. Found: C, 77.61; H, 4.91; N,2.41. FTIR: cm-1 = 3408(w), (NH), 1565(s), 1452(s), 1398(s), 1286, CN,1256(m), 1206(m), 1090(m), 766(m), 739(m), (NH), 723(m), 690(s).
Refinement
All H atoms attached to C atoms were fixed geometrically and treated as riding with C—H = 0.95 Å and Uiso(H) = 1.2Ueq(C). H atom attached to nitrogen was freely refined.
Figures
Fig. 1.
Molecular structure of the title complex with the atom labelling scheme. Ellipsoids are drawn at the 50% probability level.
Crystal data
| C36H27NOP2 | Z = 2 |
| Mr = 551.53 | F000 = 576 |
| Triclinic, P1 | Dx = 1.334 Mg m−3 |
| Hall symbol: -P 1 | Melting point: 457(2) K |
| a = 10.4233 (3) Å | Mo Kα radiation λ = 0.71073 Å |
| b = 10.9113 (3) Å | Cell parameters from 3152 reflections |
| c = 12.9940 (4) Å | θ = 2.2–25.5º |
| α = 104.055 (2)º | µ = 0.19 mm−1 |
| β = 102.555 (2)º | T = 173 (2) K |
| γ = 97.459 (2)º | Triangular, yellow |
| V = 1373.04 (7) Å3 | 0.40 × 0.18 × 0.12 mm |
Data collection
| Bruker SMART CCD area-detector diffractometer | 3646 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.055 |
| Monochromator: graphite | θmax = 26.0º |
| T = 173(2) K | θmin = 1.7º |
| φ and ω scans | h = −10→12 |
| Absorption correction: none | k = −13→13 |
| 15968 measured reflections | l = −16→16 |
| 5396 independent reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.043 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.106 | w = 1/[σ2(Fo2) + (0.048P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 0.95 | (Δ/σ)max = 0.001 |
| 5396 reflections | Δρmax = 0.38 e Å−3 |
| 365 parameters | Δρmin = −0.29 e Å−3 |
| Primary atom site location: structure-invariant direct methods | Extinction correction: none |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.0632 (2) | 0.3597 (2) | 0.56267 (18) | 0.0339 (5) | |
| C2 | 0.1275 (2) | 0.4755 (2) | 0.63818 (19) | 0.0391 (6) | |
| H2 | 0.1111 | 0.4968 | 0.7089 | 0.047* | |
| C3 | 0.2159 (2) | 0.5608 (2) | 0.61113 (19) | 0.0401 (6) | |
| H3 | 0.2593 | 0.6407 | 0.6633 | 0.048* | |
| C4 | 0.2414 (2) | 0.5308 (2) | 0.50922 (19) | 0.0347 (5) | |
| H4 | 0.3029 | 0.5900 | 0.4921 | 0.042* | |
| C5 | 0.1780 (2) | 0.41454 (19) | 0.43100 (17) | 0.0288 (5) | |
| C6 | 0.0885 (2) | 0.33272 (19) | 0.45990 (18) | 0.0300 (5) | |
| C7 | −0.0601 (2) | 0.1287 (2) | 0.40544 (18) | 0.0307 (5) | |
| C8 | −0.1125 (2) | 0.0139 (2) | 0.32431 (18) | 0.0311 (5) | |
| C9 | −0.1946 (2) | −0.0809 (2) | 0.34914 (19) | 0.0350 (5) | |
| H9 | −0.2323 | −0.1612 | 0.2954 | 0.042* | |
| C10 | −0.2214 (2) | −0.0594 (2) | 0.4502 (2) | 0.0393 (6) | |
| H10 | −0.2765 | −0.1250 | 0.4660 | 0.047* | |
| C11 | −0.1690 (2) | 0.0565 (2) | 0.5287 (2) | 0.0395 (6) | |
| H11 | −0.1897 | 0.0709 | 0.5978 | 0.047* | |
| C12 | −0.0863 (2) | 0.1525 (2) | 0.50796 (19) | 0.0341 (5) | |
| C21 | 0.3147 (2) | 0.50031 (19) | 0.28652 (17) | 0.0283 (5) | |
| C22 | 0.2595 (2) | 0.6054 (2) | 0.2703 (2) | 0.0390 (6) | |
| H22 | 0.1680 | 0.6049 | 0.2692 | 0.047* | |
| C23 | 0.3343 (2) | 0.7100 (2) | 0.2558 (2) | 0.0415 (6) | |
| H23 | 0.2950 | 0.7818 | 0.2470 | 0.050* | |
| C24 | 0.4657 (2) | 0.7113 (2) | 0.25396 (19) | 0.0400 (6) | |
| H24 | 0.5171 | 0.7829 | 0.2424 | 0.048* | |
| C25 | 0.5217 (2) | 0.6081 (2) | 0.2690 (2) | 0.0423 (6) | |
| H25 | 0.6126 | 0.6084 | 0.2679 | 0.051* | |
| C26 | 0.4479 (2) | 0.5037 (2) | 0.28585 (19) | 0.0351 (5) | |
| H26 | 0.4888 | 0.4335 | 0.2971 | 0.042* | |
| C31 | 0.3144 (2) | 0.24846 (18) | 0.31367 (17) | 0.0280 (5) | |
| C32 | 0.4063 (2) | 0.26320 (19) | 0.41242 (18) | 0.0329 (5) | |
| H32 | 0.4126 | 0.3345 | 0.4734 | 0.040* | |
| C33 | 0.4891 (2) | 0.1757 (2) | 0.4233 (2) | 0.0401 (6) | |
| H33 | 0.5516 | 0.1867 | 0.4916 | 0.048* | |
| C34 | 0.4812 (2) | 0.0733 (2) | 0.3362 (2) | 0.0450 (6) | |
| H34 | 0.5383 | 0.0131 | 0.3440 | 0.054* | |
| C35 | 0.3916 (3) | 0.0568 (2) | 0.2377 (2) | 0.0479 (7) | |
| H35 | 0.3872 | −0.0142 | 0.1770 | 0.057* | |
| C36 | 0.3075 (2) | 0.1434 (2) | 0.2262 (2) | 0.0385 (6) | |
| H36 | 0.2445 | 0.1309 | 0.1579 | 0.046* | |
| C41 | −0.1440 (2) | −0.1688 (2) | 0.12171 (18) | 0.0346 (5) | |
| C42 | −0.0664 (3) | −0.2619 (2) | 0.1300 (2) | 0.0457 (6) | |
| H42 | 0.0234 | −0.2367 | 0.1738 | 0.055* | |
| C43 | −0.1170 (3) | −0.3900 (2) | 0.0759 (2) | 0.0570 (8) | |
| H43 | −0.0627 | −0.4525 | 0.0831 | 0.068* | |
| C44 | −0.2466 (3) | −0.4270 (2) | 0.0113 (2) | 0.0568 (8) | |
| H44 | −0.2820 | −0.5152 | −0.0263 | 0.068* | |
| C45 | −0.3243 (3) | −0.3372 (2) | 0.0015 (2) | 0.0567 (7) | |
| H45 | −0.4137 | −0.3629 | −0.0432 | 0.068* | |
| C46 | −0.2737 (2) | −0.2084 (2) | 0.0563 (2) | 0.0465 (6) | |
| H46 | −0.3288 | −0.1467 | 0.0488 | 0.056* | |
| C51 | −0.1711 (2) | 0.09115 (19) | 0.13194 (18) | 0.0325 (5) | |
| C52 | −0.1280 (2) | 0.1525 (2) | 0.0605 (2) | 0.0459 (6) | |
| H52 | −0.0439 | 0.1441 | 0.0455 | 0.055* | |
| C53 | −0.2058 (3) | 0.2257 (3) | 0.0107 (2) | 0.0557 (7) | |
| H53 | −0.1746 | 0.2680 | −0.0375 | 0.067* | |
| C54 | −0.3276 (3) | 0.2372 (2) | 0.0307 (2) | 0.0525 (7) | |
| H54 | −0.3819 | 0.2858 | −0.0052 | 0.063* | |
| C55 | −0.3718 (3) | 0.1792 (2) | 0.1020 (2) | 0.0453 (6) | |
| H55 | −0.4559 | 0.1887 | 0.1166 | 0.054* | |
| C56 | −0.2939 (2) | 0.1070 (2) | 0.15255 (19) | 0.0386 (6) | |
| H56 | −0.3249 | 0.0674 | 0.2025 | 0.046* | |
| N1 | −0.0284 (2) | 0.27159 (19) | 0.58580 (18) | 0.0414 (5) | |
| H1 | −0.018 (3) | 0.277 (3) | 0.655 (2) | 0.079 (11)* | |
| O1 | 0.02287 (15) | 0.21948 (13) | 0.37894 (12) | 0.0384 (4) | |
| P1 | 0.20112 (5) | 0.36083 (5) | 0.29234 (5) | 0.03016 (16) | |
| P2 | −0.06220 (6) | −0.00273 (5) | 0.19630 (5) | 0.03407 (16) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0333 (13) | 0.0373 (13) | 0.0362 (14) | 0.0149 (10) | 0.0118 (11) | 0.0126 (10) |
| C2 | 0.0404 (14) | 0.0482 (14) | 0.0305 (14) | 0.0187 (11) | 0.0101 (11) | 0.0078 (11) |
| C3 | 0.0358 (13) | 0.0364 (13) | 0.0387 (15) | 0.0118 (11) | 0.0014 (11) | −0.0018 (11) |
| C4 | 0.0260 (12) | 0.0330 (12) | 0.0406 (14) | 0.0056 (9) | 0.0035 (10) | 0.0066 (10) |
| C5 | 0.0252 (11) | 0.0298 (11) | 0.0323 (13) | 0.0114 (9) | 0.0042 (10) | 0.0100 (9) |
| C6 | 0.0275 (11) | 0.0285 (11) | 0.0325 (13) | 0.0092 (9) | 0.0058 (10) | 0.0057 (9) |
| C7 | 0.0253 (11) | 0.0354 (12) | 0.0376 (14) | 0.0088 (9) | 0.0108 (10) | 0.0177 (10) |
| C8 | 0.0231 (11) | 0.0348 (12) | 0.0363 (13) | 0.0068 (9) | 0.0041 (10) | 0.0142 (10) |
| C9 | 0.0246 (12) | 0.0394 (13) | 0.0404 (14) | 0.0024 (9) | 0.0038 (10) | 0.0161 (10) |
| C10 | 0.0285 (12) | 0.0452 (14) | 0.0503 (16) | 0.0036 (10) | 0.0099 (11) | 0.0267 (12) |
| C11 | 0.0325 (13) | 0.0562 (16) | 0.0419 (15) | 0.0157 (11) | 0.0179 (11) | 0.0251 (12) |
| C12 | 0.0312 (12) | 0.0396 (13) | 0.0378 (14) | 0.0139 (10) | 0.0113 (11) | 0.0167 (11) |
| C21 | 0.0295 (12) | 0.0278 (11) | 0.0261 (12) | 0.0030 (9) | 0.0050 (9) | 0.0077 (9) |
| C22 | 0.0348 (13) | 0.0386 (13) | 0.0498 (16) | 0.0118 (10) | 0.0137 (11) | 0.0188 (11) |
| C23 | 0.0503 (15) | 0.0314 (13) | 0.0462 (15) | 0.0139 (11) | 0.0113 (12) | 0.0151 (11) |
| C24 | 0.0403 (14) | 0.0307 (12) | 0.0446 (15) | −0.0036 (10) | 0.0051 (11) | 0.0130 (11) |
| C25 | 0.0273 (12) | 0.0406 (14) | 0.0569 (17) | 0.0009 (10) | 0.0057 (11) | 0.0174 (12) |
| C26 | 0.0293 (12) | 0.0290 (12) | 0.0462 (15) | 0.0044 (9) | 0.0051 (11) | 0.0141 (10) |
| C31 | 0.0272 (11) | 0.0237 (11) | 0.0336 (13) | 0.0002 (8) | 0.0110 (10) | 0.0085 (9) |
| C32 | 0.0359 (13) | 0.0277 (11) | 0.0355 (14) | 0.0068 (9) | 0.0093 (11) | 0.0091 (9) |
| C33 | 0.0360 (13) | 0.0389 (13) | 0.0486 (16) | 0.0067 (10) | 0.0092 (12) | 0.0201 (12) |
| C34 | 0.0409 (14) | 0.0321 (13) | 0.0689 (19) | 0.0132 (11) | 0.0205 (14) | 0.0182 (12) |
| C35 | 0.0511 (16) | 0.0307 (13) | 0.0604 (19) | 0.0103 (11) | 0.0233 (14) | 0.0007 (12) |
| C36 | 0.0361 (13) | 0.0352 (13) | 0.0388 (14) | 0.0006 (10) | 0.0091 (11) | 0.0040 (10) |
| C41 | 0.0373 (13) | 0.0336 (12) | 0.0352 (14) | 0.0092 (10) | 0.0109 (11) | 0.0112 (10) |
| C42 | 0.0488 (15) | 0.0455 (15) | 0.0458 (16) | 0.0169 (12) | 0.0128 (13) | 0.0140 (12) |
| C43 | 0.080 (2) | 0.0410 (16) | 0.0562 (19) | 0.0263 (14) | 0.0215 (16) | 0.0142 (13) |
| C44 | 0.088 (2) | 0.0299 (14) | 0.0496 (18) | 0.0051 (14) | 0.0203 (16) | 0.0068 (12) |
| C45 | 0.0572 (17) | 0.0420 (15) | 0.0559 (18) | 0.0010 (13) | 0.0041 (14) | 0.0000 (13) |
| C46 | 0.0469 (15) | 0.0356 (14) | 0.0483 (16) | 0.0065 (11) | 0.0024 (13) | 0.0055 (11) |
| C51 | 0.0355 (13) | 0.0271 (11) | 0.0303 (13) | −0.0020 (9) | 0.0055 (10) | 0.0062 (9) |
| C52 | 0.0444 (15) | 0.0511 (15) | 0.0436 (16) | 0.0021 (12) | 0.0138 (12) | 0.0174 (12) |
| C53 | 0.0628 (19) | 0.0604 (18) | 0.0529 (18) | 0.0077 (14) | 0.0145 (15) | 0.0351 (14) |
| C54 | 0.0589 (18) | 0.0488 (16) | 0.0542 (18) | 0.0128 (13) | 0.0074 (14) | 0.0275 (13) |
| C55 | 0.0464 (15) | 0.0447 (14) | 0.0494 (17) | 0.0136 (12) | 0.0121 (13) | 0.0192 (12) |
| C56 | 0.0408 (14) | 0.0406 (13) | 0.0399 (14) | 0.0094 (11) | 0.0126 (11) | 0.0189 (11) |
| N1 | 0.0534 (13) | 0.0434 (12) | 0.0354 (13) | 0.0145 (10) | 0.0226 (11) | 0.0132 (10) |
| O1 | 0.0460 (10) | 0.0326 (8) | 0.0359 (9) | −0.0022 (7) | 0.0180 (8) | 0.0069 (7) |
| P1 | 0.0261 (3) | 0.0298 (3) | 0.0338 (3) | 0.0032 (2) | 0.0063 (3) | 0.0100 (2) |
| P2 | 0.0288 (3) | 0.0356 (3) | 0.0365 (4) | 0.0035 (2) | 0.0078 (3) | 0.0096 (3) |
Geometric parameters (Å, °)
| C1—C2 | 1.379 (3) | C31—C36 | 1.386 (3) |
| C1—C6 | 1.386 (3) | C31—P1 | 1.836 (2) |
| C1—N1 | 1.398 (3) | C32—C33 | 1.379 (3) |
| C2—C3 | 1.384 (3) | C32—H32 | 0.9500 |
| C2—H2 | 0.9500 | C33—C34 | 1.363 (3) |
| C3—C4 | 1.377 (3) | C33—H33 | 0.9500 |
| C3—H3 | 0.9500 | C34—C35 | 1.366 (3) |
| C4—C5 | 1.394 (3) | C34—H34 | 0.9500 |
| C4—H4 | 0.9500 | C35—C36 | 1.383 (3) |
| C5—C6 | 1.381 (3) | C35—H35 | 0.9500 |
| C5—P1 | 1.833 (2) | C36—H36 | 0.9500 |
| C6—O1 | 1.392 (2) | C41—C46 | 1.381 (3) |
| C7—C8 | 1.382 (3) | C41—C42 | 1.387 (3) |
| C7—O1 | 1.386 (2) | C41—P2 | 1.826 (2) |
| C7—C12 | 1.387 (3) | C42—C43 | 1.377 (3) |
| C8—C9 | 1.400 (3) | C42—H42 | 0.9500 |
| C8—P2 | 1.825 (2) | C43—C44 | 1.376 (4) |
| C9—C10 | 1.372 (3) | C43—H43 | 0.9500 |
| C9—H9 | 0.9500 | C44—C45 | 1.362 (3) |
| C10—C11 | 1.374 (3) | C44—H44 | 0.9500 |
| C10—H10 | 0.9500 | C45—C46 | 1.384 (3) |
| C11—C12 | 1.384 (3) | C45—H45 | 0.9500 |
| C11—H11 | 0.9500 | C46—H46 | 0.9500 |
| C12—N1 | 1.403 (3) | C51—C52 | 1.384 (3) |
| C21—C26 | 1.385 (3) | C51—C56 | 1.388 (3) |
| C21—C22 | 1.388 (3) | C51—P2 | 1.828 (2) |
| C21—P1 | 1.831 (2) | C52—C53 | 1.380 (3) |
| C22—C23 | 1.372 (3) | C52—H52 | 0.9500 |
| C22—H22 | 0.9500 | C53—C54 | 1.365 (3) |
| C23—C24 | 1.374 (3) | C53—H53 | 0.9500 |
| C23—H23 | 0.9500 | C54—C55 | 1.366 (3) |
| C24—C25 | 1.369 (3) | C54—H54 | 0.9500 |
| C24—H24 | 0.9500 | C55—C56 | 1.377 (3) |
| C25—C26 | 1.380 (3) | C55—H55 | 0.9500 |
| C25—H25 | 0.9500 | C56—H56 | 0.9500 |
| C26—H26 | 0.9500 | N1—H1 | 0.86 (3) |
| C31—C32 | 1.384 (3) | ||
| C2—C1—C6 | 118.3 (2) | C31—C32—H32 | 119.6 |
| C2—C1—N1 | 122.0 (2) | C34—C33—C32 | 120.0 (2) |
| C6—C1—N1 | 119.7 (2) | C34—C33—H33 | 120.0 |
| C1—C2—C3 | 120.1 (2) | C32—C33—H33 | 120.0 |
| C1—C2—H2 | 119.9 | C33—C34—C35 | 120.3 (2) |
| C3—C2—H2 | 119.9 | C33—C34—H34 | 119.8 |
| C4—C3—C2 | 120.6 (2) | C35—C34—H34 | 119.8 |
| C4—C3—H3 | 119.7 | C34—C35—C36 | 120.1 (2) |
| C2—C3—H3 | 119.7 | C34—C35—H35 | 120.0 |
| C3—C4—C5 | 120.7 (2) | C36—C35—H35 | 120.0 |
| C3—C4—H4 | 119.7 | C35—C36—C31 | 120.5 (2) |
| C5—C4—H4 | 119.7 | C35—C36—H36 | 119.8 |
| C6—C5—C4 | 117.2 (2) | C31—C36—H36 | 119.8 |
| C6—C5—P1 | 116.86 (15) | C46—C41—C42 | 117.8 (2) |
| C4—C5—P1 | 125.91 (18) | C46—C41—P2 | 125.83 (17) |
| C5—C6—C1 | 123.1 (2) | C42—C41—P2 | 116.31 (18) |
| C5—C6—O1 | 116.04 (19) | C43—C42—C41 | 121.4 (2) |
| C1—C6—O1 | 120.89 (19) | C43—C42—H42 | 119.3 |
| C8—C7—O1 | 115.78 (19) | C41—C42—H42 | 119.3 |
| C8—C7—C12 | 122.8 (2) | C44—C43—C42 | 119.7 (2) |
| O1—C7—C12 | 121.38 (19) | C44—C43—H43 | 120.2 |
| C7—C8—C9 | 117.3 (2) | C42—C43—H43 | 120.2 |
| C7—C8—P2 | 116.80 (16) | C45—C44—C43 | 120.0 (2) |
| C9—C8—P2 | 125.87 (17) | C45—C44—H44 | 120.0 |
| C10—C9—C8 | 120.8 (2) | C43—C44—H44 | 120.0 |
| C10—C9—H9 | 119.6 | C44—C45—C46 | 120.4 (3) |
| C8—C9—H9 | 119.6 | C44—C45—H45 | 119.8 |
| C9—C10—C11 | 120.5 (2) | C46—C45—H45 | 119.8 |
| C9—C10—H10 | 119.7 | C41—C46—C45 | 120.8 (2) |
| C11—C10—H10 | 119.7 | C41—C46—H46 | 119.6 |
| C10—C11—C12 | 120.6 (2) | C45—C46—H46 | 119.6 |
| C10—C11—H11 | 119.7 | C52—C51—C56 | 117.8 (2) |
| C12—C11—H11 | 119.7 | C52—C51—P2 | 118.61 (18) |
| C11—C12—C7 | 118.0 (2) | C56—C51—P2 | 123.61 (18) |
| C11—C12—N1 | 122.9 (2) | C53—C52—C51 | 120.9 (2) |
| C7—C12—N1 | 119.1 (2) | C53—C52—H52 | 119.6 |
| C26—C21—C22 | 117.61 (19) | C51—C52—H52 | 119.6 |
| C26—C21—P1 | 124.86 (16) | C54—C53—C52 | 120.0 (2) |
| C22—C21—P1 | 117.19 (16) | C54—C53—H53 | 120.0 |
| C23—C22—C21 | 121.4 (2) | C52—C53—H53 | 120.0 |
| C23—C22—H22 | 119.3 | C53—C54—C55 | 120.4 (2) |
| C21—C22—H22 | 119.3 | C53—C54—H54 | 119.8 |
| C22—C23—C24 | 120.3 (2) | C55—C54—H54 | 119.8 |
| C22—C23—H23 | 119.8 | C54—C55—C56 | 119.7 (2) |
| C24—C23—H23 | 119.8 | C54—C55—H55 | 120.1 |
| C25—C24—C23 | 119.1 (2) | C56—C55—H55 | 120.1 |
| C25—C24—H24 | 120.4 | C55—C56—C51 | 121.2 (2) |
| C23—C24—H24 | 120.4 | C55—C56—H56 | 119.4 |
| C24—C25—C26 | 120.8 (2) | C51—C56—H56 | 119.4 |
| C24—C25—H25 | 119.6 | C1—N1—C12 | 119.7 (2) |
| C26—C25—H25 | 119.6 | C1—N1—H1 | 115 (2) |
| C25—C26—C21 | 120.7 (2) | C12—N1—H1 | 119.0 (19) |
| C25—C26—H26 | 119.7 | C7—O1—C6 | 118.82 (17) |
| C21—C26—H26 | 119.7 | C21—P1—C5 | 101.96 (10) |
| C32—C31—C36 | 118.29 (19) | C21—P1—C31 | 102.20 (9) |
| C32—C31—P1 | 123.48 (15) | C5—P1—C31 | 99.98 (9) |
| C36—C31—P1 | 118.23 (17) | C8—P2—C41 | 100.81 (10) |
| C33—C32—C31 | 120.8 (2) | C8—P2—C51 | 99.93 (10) |
| C33—C32—H32 | 119.6 | C41—P2—C51 | 103.02 (10) |
| C6—C1—C2—C3 | −0.8 (3) | C41—C42—C43—C44 | 0.6 (4) |
| N1—C1—C2—C3 | −178.9 (2) | C42—C43—C44—C45 | −0.2 (4) |
| C1—C2—C3—C4 | −0.5 (3) | C43—C44—C45—C46 | −0.1 (4) |
| C2—C3—C4—C5 | 0.5 (3) | C42—C41—C46—C45 | 0.4 (4) |
| C3—C4—C5—C6 | 0.7 (3) | P2—C41—C46—C45 | 178.5 (2) |
| C3—C4—C5—P1 | 179.98 (16) | C44—C45—C46—C41 | 0.0 (4) |
| C4—C5—C6—C1 | −2.0 (3) | C56—C51—C52—C53 | −0.7 (3) |
| P1—C5—C6—C1 | 178.60 (16) | P2—C51—C52—C53 | −179.29 (19) |
| C4—C5—C6—O1 | 177.72 (17) | C51—C52—C53—C54 | −0.7 (4) |
| P1—C5—C6—O1 | −1.7 (2) | C52—C53—C54—C55 | 1.6 (4) |
| C2—C1—C6—C5 | 2.1 (3) | C53—C54—C55—C56 | −1.0 (4) |
| N1—C1—C6—C5 | −179.71 (19) | C54—C55—C56—C51 | −0.4 (4) |
| C2—C1—C6—O1 | −177.62 (18) | C52—C51—C56—C55 | 1.3 (3) |
| N1—C1—C6—O1 | 0.6 (3) | P2—C51—C56—C55 | 179.79 (18) |
| O1—C7—C8—C9 | 178.75 (17) | C2—C1—N1—C12 | −177.0 (2) |
| C12—C7—C8—C9 | −0.3 (3) | C6—C1—N1—C12 | 4.9 (3) |
| O1—C7—C8—P2 | 1.3 (2) | C11—C12—N1—C1 | 174.5 (2) |
| C12—C7—C8—P2 | −177.74 (16) | C7—C12—N1—C1 | −5.5 (3) |
| C7—C8—C9—C10 | 0.1 (3) | C8—C7—O1—C6 | −174.40 (17) |
| P2—C8—C9—C10 | 177.27 (16) | C12—C7—O1—C6 | 4.7 (3) |
| C8—C9—C10—C11 | 0.7 (3) | C5—C6—O1—C7 | 174.95 (17) |
| C9—C10—C11—C12 | −1.3 (3) | C1—C6—O1—C7 | −5.3 (3) |
| C10—C11—C12—C7 | 1.0 (3) | C26—C21—P1—C5 | 109.83 (19) |
| C10—C11—C12—N1 | −178.9 (2) | C22—C21—P1—C5 | −77.11 (18) |
| C8—C7—C12—C11 | −0.2 (3) | C26—C21—P1—C31 | 6.7 (2) |
| O1—C7—C12—C11 | −179.27 (18) | C22—C21—P1—C31 | 179.77 (17) |
| C8—C7—C12—N1 | 179.68 (19) | C6—C5—P1—C21 | 175.90 (15) |
| O1—C7—C12—N1 | 0.7 (3) | C4—C5—P1—C21 | −3.4 (2) |
| C26—C21—C22—C23 | −1.0 (3) | C6—C5—P1—C31 | −79.23 (17) |
| P1—C21—C22—C23 | −174.60 (19) | C4—C5—P1—C31 | 101.45 (18) |
| C21—C22—C23—C24 | 1.9 (4) | C32—C31—P1—C21 | 73.74 (19) |
| C22—C23—C24—C25 | −1.3 (4) | C36—C31—P1—C21 | −106.18 (18) |
| C23—C24—C25—C26 | 0.0 (4) | C32—C31—P1—C5 | −30.94 (19) |
| C24—C25—C26—C21 | 0.8 (4) | C36—C31—P1—C5 | 149.14 (17) |
| C22—C21—C26—C25 | −0.3 (3) | C7—C8—P2—C41 | 176.03 (16) |
| P1—C21—C26—C25 | 172.73 (18) | C9—C8—P2—C41 | −1.1 (2) |
| C36—C31—C32—C33 | 0.1 (3) | C7—C8—P2—C51 | −78.53 (17) |
| P1—C31—C32—C33 | −179.82 (17) | C9—C8—P2—C51 | 104.31 (19) |
| C31—C32—C33—C34 | 0.2 (3) | C46—C41—P2—C8 | 87.2 (2) |
| C32—C33—C34—C35 | 0.1 (4) | C42—C41—P2—C8 | −94.69 (19) |
| C33—C34—C35—C36 | −0.6 (4) | C46—C41—P2—C51 | −15.8 (2) |
| C34—C35—C36—C31 | 0.9 (4) | C42—C41—P2—C51 | 162.34 (18) |
| C32—C31—C36—C35 | −0.7 (3) | C52—C51—P2—C8 | 151.05 (18) |
| P1—C31—C36—C35 | 179.27 (18) | C56—C51—P2—C8 | −27.4 (2) |
| C46—C41—C42—C43 | −0.7 (4) | C52—C51—P2—C41 | −105.31 (19) |
| P2—C41—C42—C43 | −179.0 (2) | C56—C51—P2—C41 | 76.2 (2) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: DN2322).
References
- Antonio, Y., Barrera, P., Contreas, O., Verlarde, E. & Muchowski, J. M. (1989). J. Am. Chem. Soc.54, 2159–2165.
- Bruker (2005). APEX2 and SAINT-NT Bruker AXS Inc., Madison, Wisconsin, USA.
- Claver, C. & van Leeuwen, P. W. N. M. (2000). Rhodium Catalyzed Hydroformylation Dordrecht: Kluwer Academic Publishers.
- Deprele, S. & Montchamp, J.-L. (2004). Org. Lett.6, 3805–3808. [DOI] [PubMed]
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Leeuwen, P. W. N. M. van, Sandee, A. J., Reek, J. N. H. & Kamer, P. C. J. (2002). J. Mol. Catal. A, 182–183, 107–123.
- Osiński, P. W., Schürmann, M., Preut, H., Haag, R. & Eilbracht, P. (2005). Acta Cryst. E61, o3115–o3116.
- Petrassi, H. M., Klabunde, T., Sacchettini, J. & Kelly, J. W. (2000). J. Am. Chem. Soc.122, 2178–2192.
- Ricken, S., Osiński, P. W., Eilbracht, P. & Haag, R. (2006a). J. Mol. Catal. A.257, 78–88.
- Ricken, S., Osinski, P. W., Schürmann, M., Preut, H. & Eilbracht, P. (2006b). Acta Cryst. E62, o1807–o1808.
- Ricken, S., Schürmann, M., Preut, H. & Eilbracht, P. (2006c). Acta Cryst. E62, o2637–o2638.
- Sandee, A. J., Reek, J. N. H., Kamer, P. C. J. & van Leeuwen, P. W. N. M. (2001). J. Am. Chem. Soc.123, 8468–8476. [DOI] [PubMed]
- Sandee, A. J., van der Veen, L. A., Reek, J. N. H., Kamer, P. C. J., Lutz, M., Spek, A. L. & van Leeuwen, P. W. N. M. (1999). Angew. Chem. Int. Ed.38, 3231–3235. [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2003). J. Appl. Cryst.36, 7–13.
- Tolman, C. A. (1977). Chem. Rev.77, 313–348.
- Veen, L. A. van der, Keeven, P. H., Schoemaker, G. C., Reek, J. N. H., Kamer, P. C. J., van Leeuwen, P., Lutz, M. & Spek, A. L. (2000). Organometallics, 19, 872–883.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808006648/dn2322sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808006648/dn2322Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report