Abstract
All atoms of the title molecule, C8H7NO3S, except the two oxide O atoms and two H atoms of the methyl group, lie on a crystallographic mirror plane. The crystal structure is stabilized by weak inter- and intramolecular C—H⋯O hydrogen bonds.
Related literature
For related literature, see: Hu et al. (2004 ▶); Kap-Sun & Nicholas (1998 ▶); Liang et al. (2006 ▶); Masashi et al. (1999 ▶); Nagasawa et al. (1995 ▶); Siddiqui et al. (2006 ▶, 2007a
▶,b
▶,c
▶); Siddiqui, Ahmad, Khan & Siddiqui (2007 ▶); Siddiqui, Ahmad, Khan, Siddiqui & Ahmad (2007 ▶); Siddiqui, Ahmad, Khan, Siddiqui & Parvez (2007 ▶).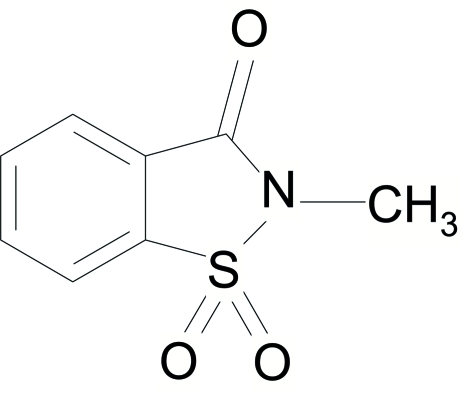
Experimental
Crystal data
C8H7NO3S
M r = 197.21
Monoclinic,

a = 7.463 (7) Å
b = 6.761 (6) Å
c = 8.748 (8) Å
β = 103.78 (3)°
V = 428.7 (7) Å3
Z = 2
Mo Kα radiation
μ = 0.35 mm−1
T = 173 (2) K
0.12 × 0.08 × 0.07 mm
Data collection
Nonius KappaCCD diffractometer
Absorption correction: multi-scan (SORTAV; Blessing, 1997 ▶) T min = 0.960, T max = 0.976
1724 measured reflections
1045 independent reflections
889 reflections with I > 2σ(I)
R int = 0.023
Refinement
R[F 2 > 2σ(F 2)] = 0.041
wR(F 2) = 0.106
S = 1.03
1045 reflections
76 parameters
H-atom parameters constrained
Δρmax = 0.41 e Å−3
Δρmin = −0.42 e Å−3
Data collection: COLLECT (Hooft, 1998 ▶); cell refinement: DENZO (Otwinowski & Minor, 1997 ▶); data reduction: SCALEPACK (Otwinowski & Minor, 1997 ▶); program(s) used to solve structure: SAPI91 (Fan, 1991 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEPII (Johnson, 1976 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808004637/lh2597sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808004637/lh2597Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C8—H8A⋯O1 | 0.96 | 2.49 | 2.869 (4) | 104 |
| C2—H2⋯O1i | 0.95 | 2.29 | 3.227 (4) | 169 |
| C8—H8B⋯O2ii | 0.96 | 2.49 | 3.358 (3) | 151 |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
supplementary crystallographic information
Comment
Benzisothiazolone-1,1-dioxide is part of a class of heterocycles which has been investigated in pharmaceutical research (Kap-Sun & Nicholas, 1998). 1,2-benzisothiazole-3-one 1,1-dioxide (saccharin) has been widely incorporated into a variety of biologically active compounds. It has been identified as an important molecular component in various classes of 5-HTla antagonists, analgesics and human mast cell tryptase inhibitors (Liang et al., 2006). In particular, N-substituted derivatives, e.g with N-hydroxy and N-alkyl substituents, have shown important biological activites (Nagasawa et al., 1995). Among N-alkyl derivatives, various synthetic routes have been reported for the synthesis of the title compound involving ionic liquids and free radical mechanisms (Hu et al., 2004; Masashi et al., 1999). In continuation of our research on the synthesis of 1,2-benzothiazine 1,1-dioxide derivatives, we have in addtion, embarked on the synthesis of benzisothiazole derivatives (Siddiqui et al., 2006; Siddiqui et al., 2007a,b,c; Siddiqui, Ahmad, Khan & Siddiqui, 2007; Siddiqui, Ahmad, Khan, Siddiqui & Ahmad, 2007; Siddiqui, Ahmad, Khan, Siddiqui & Parvez, 2007). Herein, we report the synthesis and crystal structure of the title compound, (I).
With the exception atoms O2 and H8B, all atoms of the molecule of (I) (Fig. 1) lie on a crystallographic mirror plane. The benzisothiazole moiety is exactly planar. The molecular dimensions are in accord with the corresponding dimensions reported in similar structures (Siddiqui et al., 2007a-c; Siddiqui, Ahmad, Khan, Siddiqui & Parvez, 2007). The structure is stabilized by one intramolecular and two intermolecular interactions of the type C—H···O (details are in Table).
Experimental
Saccharin (2.0 g, 11.0 mmol.) was added to a solution of sodium hydroxide (0.875 g, 22.0 mmol.) in distilled water (25 ml) under constant stirring to give a transparent solution. A solution of dimethylsulfate (2.08 ml, 22.0 mmol.) in methanol (10.0 ml) was then added dropwise over 2 minutes. Precipitates started appearing within 5 minutes and stirring was continued for 20 min. at room temperature. The precipitates were filtered, washed with cold water and dried (343 K) to get 1.75 g of (I) (8.9 mmol. 81%). Recrystallization Solvent: CHCl3. The solution was subjected to slow evaporation at 313 K to obtain colourless crystals.
Refinement
H-atoms bonded were included in the refinements at geometrically idealized positions with aromatic and methyl C—H distances 0.95 and 0.96 Å, respectively, and Uiso = 1.2 times Ueq of the atoms to which they were bonded. The final difference map was free of any chemically significant features.
Figures
Fig. 1.
ORTEPII (Johnson, 1976) drawing of (I) with displacement ellipsoids plotted at 50% probability level. Symmetry code: (iii) x, -y + 1/2, z.
Crystal data
| C8H7NO3S | F000 = 204 |
| Mr = 197.21 | Dx = 1.528 Mg m−3 |
| Monoclinic, P21/m | Mo Kα radiation λ = 0.71073 Å |
| Hall symbol: -P 2yb | Cell parameters from 1724 reflections |
| a = 7.463 (7) Å | θ = 3.2–27.4º |
| b = 6.761 (6) Å | µ = 0.35 mm−1 |
| c = 8.748 (8) Å | T = 173 (2) K |
| β = 103.78 (3)º | Prism, colorless |
| V = 428.7 (7) Å3 | 0.12 × 0.08 × 0.07 mm |
| Z = 2 |
Data collection
| Nonius KappaCCD diffractometer | 1045 independent reflections |
| Radiation source: fine-focus sealed tube | 889 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.023 |
| T = 173(2) K | θmax = 27.4º |
| ω and φ scans | θmin = 3.2º |
| Absorption correction: multi-scan(SORTAV; Blessing, 1997) | h = −9→9 |
| Tmin = 0.960, Tmax = 0.976 | k = −8→8 |
| 1724 measured reflections | l = −11→11 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.041 | H-atom parameters constrained |
| wR(F2) = 0.106 | w = 1/[σ2(Fo2) + (0.0455P)2 + 0.2966P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.03 | (Δ/σ)max < 0.001 |
| 1045 reflections | Δρmax = 0.41 e Å−3 |
| 76 parameters | Δρmin = −0.42 e Å−3 |
| Primary atom site location: structure-invariant direct methods | Extinction correction: none |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.68037 (10) | 0.2500 | 0.26611 (7) | 0.0314 (2) | |
| O1 | 0.2425 (3) | 0.2500 | 0.4038 (3) | 0.0416 (5) | |
| O2 | 0.7324 (2) | 0.0698 (2) | 0.20266 (16) | 0.0445 (4) | |
| N1 | 0.4534 (3) | 0.2500 | 0.2520 (3) | 0.0314 (5) | |
| C1 | 0.7314 (4) | 0.2500 | 0.4722 (3) | 0.0254 (5) | |
| C2 | 0.9043 (4) | 0.2500 | 0.5750 (3) | 0.0337 (6) | |
| H2 | 1.0141 | 0.2500 | 0.5381 | 0.040* | |
| C3 | 0.9090 (4) | 0.2500 | 0.7337 (3) | 0.0403 (7) | |
| H3 | 1.0249 | 0.2500 | 0.8079 | 0.048* | |
| C4 | 0.7486 (4) | 0.2500 | 0.7873 (3) | 0.0379 (7) | |
| H4 | 0.7566 | 0.2500 | 0.8974 | 0.045* | |
| C5 | 0.5767 (4) | 0.2500 | 0.6832 (3) | 0.0308 (6) | |
| H5 | 0.4670 | 0.2500 | 0.7203 | 0.037* | |
| C6 | 0.5690 (3) | 0.2500 | 0.5235 (3) | 0.0251 (5) | |
| C7 | 0.4012 (4) | 0.2500 | 0.3931 (3) | 0.0289 (6) | |
| C8 | 0.3218 (5) | 0.2500 | 0.0986 (3) | 0.0455 (8) | |
| H8A | 0.1982 | 0.2500 | 0.1130 | 0.055* | |
| H8B | 0.3405 | 0.1341 | 0.0410 | 0.055* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0415 (4) | 0.0319 (4) | 0.0237 (3) | 0.000 | 0.0133 (3) | 0.000 |
| O1 | 0.0284 (10) | 0.0449 (13) | 0.0521 (13) | 0.000 | 0.0107 (9) | 0.000 |
| O2 | 0.0587 (10) | 0.0435 (9) | 0.0369 (8) | 0.0058 (7) | 0.0223 (7) | −0.0101 (7) |
| N1 | 0.0355 (12) | 0.0301 (12) | 0.0266 (11) | 0.000 | 0.0031 (9) | 0.000 |
| C1 | 0.0319 (13) | 0.0222 (12) | 0.0237 (12) | 0.000 | 0.0099 (10) | 0.000 |
| C2 | 0.0276 (13) | 0.0367 (16) | 0.0371 (14) | 0.000 | 0.0085 (11) | 0.000 |
| C3 | 0.0402 (16) | 0.0417 (17) | 0.0340 (15) | 0.000 | −0.0010 (12) | 0.000 |
| C4 | 0.0553 (18) | 0.0343 (16) | 0.0238 (13) | 0.000 | 0.0087 (12) | 0.000 |
| C5 | 0.0392 (15) | 0.0257 (13) | 0.0324 (14) | 0.000 | 0.0181 (12) | 0.000 |
| C6 | 0.0280 (12) | 0.0189 (12) | 0.0297 (13) | 0.000 | 0.0093 (10) | 0.000 |
| C7 | 0.0330 (14) | 0.0221 (13) | 0.0320 (13) | 0.000 | 0.0086 (11) | 0.000 |
| C8 | 0.0551 (19) | 0.0452 (19) | 0.0284 (15) | 0.000 | −0.0053 (13) | 0.000 |
Geometric parameters (Å, °)
| S1—O2i | 1.430 (2) | C2—H2 | 0.9500 |
| S1—O2 | 1.430 (2) | C3—C4 | 1.386 (4) |
| S1—N1 | 1.668 (3) | C3—H3 | 0.9500 |
| S1—C1 | 1.752 (3) | C4—C5 | 1.385 (4) |
| O1—C7 | 1.211 (3) | C4—H4 | 0.9500 |
| N1—C7 | 1.380 (4) | C5—C6 | 1.384 (4) |
| N1—C8 | 1.462 (4) | C5—H5 | 0.9500 |
| C1—C2 | 1.386 (4) | C6—C7 | 1.479 (4) |
| C1—C6 | 1.389 (4) | C8—H8A | 0.9600 |
| C2—C3 | 1.380 (4) | C8—H8B | 0.9600 |
| O2i—S1—O2 | 116.79 (14) | C4—C3—H3 | 119.2 |
| O2i—S1—N1 | 109.63 (8) | C5—C4—C3 | 121.1 (3) |
| O2—S1—N1 | 109.63 (8) | C5—C4—H4 | 119.5 |
| O2i—S1—C1 | 112.76 (8) | C3—C4—H4 | 119.5 |
| O2—S1—C1 | 112.76 (8) | C6—C5—C4 | 118.2 (2) |
| N1—S1—C1 | 92.54 (12) | C6—C5—H5 | 120.9 |
| C7—N1—C8 | 123.3 (2) | C4—C5—H5 | 120.9 |
| C7—N1—S1 | 115.6 (2) | C5—C6—C1 | 119.7 (2) |
| C8—N1—S1 | 121.1 (2) | C5—C6—C7 | 127.0 (2) |
| C2—C1—C6 | 122.7 (2) | C1—C6—C7 | 113.2 (2) |
| C2—C1—S1 | 127.5 (2) | O1—C7—N1 | 124.0 (3) |
| C6—C1—S1 | 109.9 (2) | O1—C7—C6 | 127.2 (3) |
| C3—C2—C1 | 116.7 (3) | N1—C7—C6 | 108.8 (2) |
| C3—C2—H2 | 121.6 | N1—C8—H8A | 109.6 |
| C1—C2—H2 | 121.6 | N1—C8—H8B | 109.4 |
| C2—C3—C4 | 121.6 (3) | H8A—C8—H8B | 109.5 |
| C2—C3—H3 | 119.2 | ||
| O2i—S1—N1—C7 | −115.28 (8) | C3—C4—C5—C6 | 0.000 (1) |
| O2—S1—N1—C7 | 115.28 (8) | C4—C5—C6—C1 | 0.0 |
| C1—S1—N1—C7 | 0.0 | C4—C5—C6—C7 | 180.0 |
| O2i—S1—N1—C8 | 64.72 (8) | C2—C1—C6—C5 | 0.0 |
| O2—S1—N1—C8 | −64.72 (8) | S1—C1—C6—C5 | 180.0 |
| C1—S1—N1—C8 | 180.0 | C2—C1—C6—C7 | 180.0 |
| O2i—S1—C1—C2 | −67.46 (9) | S1—C1—C6—C7 | 0.0 |
| O2—S1—C1—C2 | 67.46 (9) | C8—N1—C7—O1 | 0.0 |
| N1—S1—C1—C2 | 180.0 | S1—N1—C7—O1 | 180.0 |
| O2i—S1—C1—C6 | 112.54 (9) | C8—N1—C7—C6 | 180.0 |
| O2—S1—C1—C6 | −112.54 (9) | S1—N1—C7—C6 | 0.0 |
| N1—S1—C1—C6 | 0.0 | C5—C6—C7—O1 | 0.0 |
| C6—C1—C2—C3 | 0.0 | C1—C6—C7—O1 | 180.0 |
| S1—C1—C2—C3 | 180.0 | C5—C6—C7—N1 | 180.0 |
| C1—C2—C3—C4 | 0.000 (1) | C1—C6—C7—N1 | 0.0 |
| C2—C3—C4—C5 | 0.000 (1) |
Symmetry codes: (i) x, −y+1/2, z.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C8—H8A···O1 | 0.96 | 2.49 | 2.869 (4) | 104 |
| C2—H2···O1ii | 0.95 | 2.29 | 3.227 (4) | 169 |
| C8—H8B···O2iii | 0.96 | 2.49 | 3.358 (3) | 151 |
Symmetry codes: (ii) x+1, y, z; (iii) −x+1, −y, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LH2597).
References
- Blessing, R. H. (1997). J. Appl. Cryst.30, 421–426.
- Fan, H.-F. (1991). SAPI91 Rigaku Corporation, Tokyo, Japan.
- Hooft, R. (1998). COLLECT Nonius BV, Delft, The Netherlands.
- Hu, Y., Chen, Z. C., Le, Z. G. & Zheng, Q. G. (2004). J. Chem. Res.4, 276–278.
- Johnson, C. K. (1976). ORTEPII. Report ORNL-5138. Oak Ridge National Laboratory, Tennessee, USA.
- Kap-Sun, Y. & Nicholas, A. M. (1998). Tetrahedron Lett.39, 5309–5312.
- Liang, X., Hong, S., Ying, L., Suhong, Z. & Mark, L. T. (2006). Tetrahedron, 62, 7902–7910.
- Masashi, K., Hideo, T., Kentaro, Y. & Masataka, Y. (1999). Tetrahedron, 55, 14885–14900.
- Nagasawa, H. T., Kawle, S. P., Elberling, J. A., DeMaster, E. G. & Fukuto, J. M. (1995). J. Med. Chem.38, 1865–1871. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr and R. M. Sweet, pp. 307–326. New York: Academic Press.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Siddiqui, W. A., Ahmad, S., Khan, I. U. & Siddiqui, H. L. (2007). Synth. Commun.37, 767–773.
- Siddiqui, W. A., Ahmad, S., Khan, I. U., Siddiqui, H. L. & Ahmad, V. U. (2007). J. Chem. Soc. Pak.29, 44–47.
- Siddiqui, W. A., Ahmad, S., Khan, I. U., Siddiqui, H. L. & Parvez, M. (2007). Acta Cryst. E63, o4116.
- Siddiqui, W. A., Ahmad, S., Siddiqui, H. L., Tariq, M. I. & Parvez, M. (2007a). Acta Cryst. E63, o4001.
- Siddiqui, W. A., Ahmad, S., Siddiqui, H. L., Tariq, M. I. & Parvez, M. (2007b). Acta Cryst. E63, o4117.
- Siddiqui, W. A., Ahmad, S., Siddiqui, H. L., Tariq, M. I. & Parvez, M. (2007c). Acta Cryst. E63, o4585.
- Siddiqui, W. A., Ahmad, S., Ullah, I. & Malik, A. (2006). J. Chem. Soc. Pak.28, 583–589.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808004637/lh2597sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808004637/lh2597Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



