Abstract
Rochelle salt, K+·Na+·C4H4O6 2−·4H2O, is known for its remarkable ferroelectric state between 255 and 297 K. The current investigation, based on data collected at 105 K, provides very accurate structural information for the low-temperature paraelectric form. Unlike the ferroelectric form, there is only one tartrate molecule in the asymmetric unit, and the structure displays no disorder to large anisotropic atomic displacements.
Related literature
For previous and related structures, see: Beevers & Hughes (1941 ▶); Iwata et al. (1989 ▶); Solans et al. (1997 ▶); Ottenz et al. (1998 ▶); Hinazumi & Mitsui (1972 ▶); Kay (1978 ▶); Kuroda & Mason (1981 ▶); Brożek & Stadnicka (1994 ▶); Suzuki et al. (1996a
▶,b
▶); Ambady & Kartha (1968 ▶); Boese et al. (1995 ▶). For irradiation studies, see: Suzuki (1974 ▶); Treeck, van & Windsch (1977 ▶). For a description of the Cambridge Structural Database, see: Allen (2002 ▶).
Experimental
Crystal data
K+·Na+·C4H4O6 2−·4H2O
M r = 282.23
Orthorhombic,

a = 11.7859 (6) Å
b = 14.1972 (7) Å
c = 6.1875 (3) Å
V = 1035.33 (9) Å3
Z = 4
Mo Kα radiation
μ = 0.60 mm−1
T = 105 (2) K
0.5 mm (radius)
Data collection
Siemens SMART CCD diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.398, T max = 0.551 (expected range = 0.722–1.000)
33523 measured reflections
10040 independent reflections
8947 reflections with I > 2σ(I)
R int = 0.037
Refinement
R[F 2 > 2σ(F 2)] = 0.029
wR(F 2) = 0.069
S = 1.06
10040 reflections
195 parameters
12 restraints
All H-atom parameters refined
Δρmax = 0.50 e Å−3
Δρmin = −0.73 e Å−3
Absolute structure: Flack, 1983 ▶, 4266 Friedel pairs
Flack parameter: 0.044 (14)
Data collection: SMART (Bruker, 1998 ▶); cell refinement: SAINT-Plus (Bruker, 2001 ▶); data reduction: SAINT-Plus; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808005266/bg2163sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808005266/bg2163Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O5—H5⋯O2 | 0.789 (17) | 2.031 (16) | 2.5946 (6) | 128.2 (14) |
| O6—H6⋯O4Wi | 0.861 (16) | 1.968 (16) | 2.8119 (7) | 166.5 (16) |
| O1W—H11W⋯O6 | 0.824 (8) | 1.960 (8) | 2.7832 (6) | 176.8 (15) |
| O1W—H12W⋯O4ii | 0.843 (9) | 2.010 (9) | 2.8500 (7) | 174.8 (18) |
| O2W—H21W⋯O3iii | 0.868 (9) | 1.830 (9) | 2.6941 (7) | 173.4 (19) |
| O2W—H22W⋯O2iv | 0.862 (9) | 1.890 (9) | 2.7505 (7) | 175.5 (19) |
| O3W—H31W⋯O6v | 0.843 (9) | 2.391 (15) | 3.1029 (7) | 142.5 (19) |
| O3W—H31W⋯O2vi | 0.843 (9) | 2.499 (17) | 3.1181 (7) | 131.0 (17) |
| O3W—H31W⋯O3v | 0.843 (9) | 2.584 (14) | 3.1569 (8) | 126.2 (15) |
| O3W—H32W⋯O4vii | 0.862 (8) | 1.926 (8) | 2.7842 (8) | 173.8 (16) |
| O4W—H41W⋯O1viii | 0.858 (9) | 1.888 (10) | 2.7124 (6) | 160.4 (19) |
| O4W—H42W⋯O3Wiv | 0.836 (8) | 1.939 (9) | 2.7532 (8) | 164.4 (16) |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii) 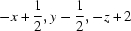 ; (iv)
; (iv)  ; (v)
; (v)  ; (vi)
; (vi)  ; (vii)
; (vii) 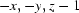 ; (viii)
; (viii)  .
.
Acknowledgments
The purchase of the Siemens SMART CCD diffractometer was made possible through support from the Research Council of Norway (NFR)
supplementary crystallographic information
Comment
The radiation-induced free radical chemistry of dicarboxylic acids and their salts has received attention for several decades. The Rochelle salt, is of particular interest as it as it exhibits a ferroelectric phase between 255 and 297 K, where the structure is monoclinic, space group P21; outside this temperature range the compound is paraelectric and presents orthorhombic phases in space group P21212. The nature of the radicals formed in Rochelle salt is currently investigated in order to understand the mechanisms producing changes in the ferroelectric properties of this compound upon irradiation (Suzuki, 1974; Treeck, van & Windsch, 1977). For the analysis of the electron magnetic resonance data, precise knowledge of the low-temperature orthorhombic form is necessary. Structural data for the high-temperature orthorhombic form were first provided by Beevers & Hughes (1941). Iwata et al. (1989) carried out a neutron diffraction study for both orthorhombic forms; more accurate X-ray diffraction studies were later presented by Solans et al. (1997), who concluded that differences between the two P21212 states are "small but significant". None of these structures are, however, available in the Cambridge Structural Database (Version 5.29 of November 2007; Allen, 2002). A high-precision redetermination of Rochelle salt at low temperature has therefore been executed.
The molecular structure of (I) is shown in Fig. 1. The crystal packing arrangement, illustrated in Fig. 2, is very similar to those found in the P21212 structures of other salts of tartaric acid in which Na+ is replaced by Li+ and/or K+ by NH4+ or Tl+ [Li+/K+: Ottenz et al., 1998; Li+/NH4+: Hinazumi & Mitsui, 1972; Li+/Tl+: Kay, 1978; Na+/NH4+ (II): Kuroda & Mason, 1981; Brożek & Stadnicka, 1994; Suzuki et al., 1996a] as well as in salts where K+ has been only partly replaced by NH4+ (Suzuki et al., 1996a; Suzuki et al., 1996b). The pure sodium (Ambady & Kartha, 1968) or potassium salts (Boese et al., 1995) on the other hand, have completely different structures.
Hydrogen bonds are listed in Table 1, the most unusual feature is the almost symmetric four-center interaction involving H31W.
When K+ is replaced by NH4+ [as, for instance, in II] the four shortest K2···O contacts are converted into hydrogen bonds, while only the two K1···O4 interactions are transformed into short hydrogen bonds, the K1···O1W and K1···O2W contacts being replaced by a three-center hydrogen bond.
Experimental
Rochelle salt was obtained from Sigma-Aldrich and tetrahydrate crystals were grown from saturated aqueous solutions. A large block-shaped speciemen was ground into a sphere in a mill and used for data collection.
Refinement
Full isotropic refinement was carried out for all H atoms.
Figures
Fig. 1.
: The molecular structure of (I). Displacement ellipsoids are shown at the 50% probability level. Metal ccordination has been indicated by dashed lines.
Fig. 2.
: Crystal packing arrangement viewed approximately along the c axis. H atoms bonded to C have been left out for clarity. Na+ is yellow, K+ is light blue with K1 at the centre of the unit cell and K2 at the cell edge. Hydrogen bonds are shown as black dotted lines while ligand coordination is indicated in orange for three selected metal ions. The arrow points to H31W, which is involved in a four-center hydrogen bond.
Crystal data
| K+·Na+·C4H4O62–·4H2O | Dx = 1.811 Mg m−3 |
| Mr = 282.23 | Mo Kα radiation λ = 0.71073 Å |
| Orthorhombic, P21212 | Cell parameters from 10000 reflections |
| a = 11.7859 (6) Å | θ = 2.9–49.7º |
| b = 14.1972 (7) Å | µ = 0.60 mm−1 |
| c = 6.1875 (3) Å | T = 105 (2) K |
| V = 1035.33 (9) Å3 | Sphere, colourless |
| Z = 4 | 0.5 mm (radius) |
| F000 = 584 |
Data collection
| Siemens SMART CCD diffractometer | 10040 independent reflections |
| Radiation source: fine-focus sealed tube | 8947 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.037 |
| Detector resolution: 8.3 pixels mm-1 | θmax = 49.7º |
| T = 105(2) K | θmin = 2.9º |
| sets of exposures each taken over 0.3° ω rotation scans | h = −25→25 |
| Absorption correction: multi-scan(SADABS; Sheldrick, 1996) | k = −28→30 |
| Tmin = 0.398, Tmax = 0.551 | l = −12→12 |
| 33523 measured reflections |
Refinement
| Refinement on F2 | All H-atom parameters refined |
| Least-squares matrix: full | w = 1/[σ2(Fo2) + (0.0324P)2 + 0.0088P] where P = (Fo2 + 2Fc2)/3 |
| R[F2 > 2σ(F2)] = 0.029 | (Δ/σ)max = 0.002 |
| wR(F2) = 0.069 | Δρmax = 0.50 e Å−3 |
| S = 1.06 | Δρmin = −0.73 e Å−3 |
| 10040 reflections | Extinction correction: SHELXTL (Bruker, 2000), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 195 parameters | Extinction coefficient: 0.132 (3) |
| 12 restraints | Absolute structure: Flack, 1983, 4266 Friedel pairs |
| Primary atom site location: structure-invariant direct methods | Flack parameter: 0.044 (14) |
| Hydrogen site location: difference Fourier map |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Data were collected by measuring six sets of exposures with the detector set at 2θ = 29° and 65°, crystal-to-detector distance 5.00 cm. Refinement of F2 against ALL reflections. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| K1 | 0.0000 | 0.0000 | 0.04255 (4) | 0.02054 (4) | |
| K2 | 0.5000 | 0.0000 | 0.83902 (3) | 0.01318 (3) | |
| Na | 0.23248 (2) | −0.007143 (18) | 0.51526 (4) | 0.01041 (4) | |
| O1 | 0.12000 (3) | 0.10859 (3) | 0.34799 (7) | 0.01016 (5) | |
| O2 | 0.21269 (4) | 0.20379 (3) | 0.11755 (7) | 0.01211 (6) | |
| O3 | 0.22830 (4) | 0.40729 (3) | 0.82011 (8) | 0.01585 (7) | |
| O4 | 0.04765 (4) | 0.35891 (3) | 0.84893 (8) | 0.01439 (7) | |
| O5 | 0.16547 (4) | 0.35790 (3) | 0.32421 (7) | 0.01060 (5) | |
| H5 | 0.1932 (12) | 0.3393 (11) | 0.216 (3) | 0.021 (3)* | |
| O6 | 0.29638 (3) | 0.24888 (3) | 0.63394 (7) | 0.01132 (6) | |
| H6 | 0.3284 (13) | 0.2989 (11) | 0.584 (3) | 0.025 (3)* | |
| C1 | 0.15538 (4) | 0.18798 (3) | 0.28320 (8) | 0.00798 (6) | |
| C2 | 0.12505 (4) | 0.27375 (3) | 0.42269 (8) | 0.00802 (6) | |
| H2 | 0.0351 (13) | 0.2714 (11) | 0.429 (3) | 0.024 (3)* | |
| C3 | 0.17752 (4) | 0.26353 (3) | 0.64784 (8) | 0.00867 (6) | |
| H3 | 0.1368 (13) | 0.2117 (11) | 0.726 (3) | 0.027 (4)* | |
| C4 | 0.14865 (5) | 0.35032 (4) | 0.78496 (9) | 0.01035 (6) | |
| O1W | 0.39615 (4) | 0.08350 (3) | 0.48487 (8) | 0.01405 (7) | |
| H11W | 0.3642 (12) | 0.1317 (8) | 0.527 (2) | 0.023 (3)* | |
| H12W | 0.4433 (14) | 0.0974 (13) | 0.388 (3) | 0.058 (6)* | |
| O2W | 0.23689 (6) | 0.04149 (3) | 0.87925 (8) | 0.02083 (10) | |
| H21W | 0.2524 (16) | 0.0009 (10) | 0.980 (2) | 0.045 (5)* | |
| H22W | 0.2331 (16) | 0.0930 (8) | 0.953 (3) | 0.044 (5)* | |
| O3W | 0.05896 (4) | −0.19201 (4) | −0.03036 (10) | 0.01860 (8) | |
| H31W | 0.1210 (11) | −0.2072 (13) | 0.028 (3) | 0.051 (6)* | |
| H32W | 0.0307 (13) | −0.2458 (8) | −0.066 (3) | 0.037 (5)* | |
| O4W | 0.07835 (4) | −0.10799 (4) | 0.57031 (9) | 0.01684 (8) | |
| H41W | 0.0099 (9) | −0.1009 (14) | 0.526 (3) | 0.054 (6)* | |
| H42W | 0.0734 (12) | −0.1431 (10) | 0.6784 (19) | 0.026 (4)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| K1 | 0.02786 (8) | 0.01565 (7) | 0.01810 (8) | −0.00967 (7) | 0.000 | 0.000 |
| K2 | 0.01563 (5) | 0.01227 (5) | 0.01164 (6) | −0.00229 (5) | 0.000 | 0.000 |
| Na | 0.01174 (8) | 0.00770 (8) | 0.01179 (9) | −0.00031 (6) | −0.00041 (6) | 0.00065 (7) |
| O1 | 0.01294 (12) | 0.00587 (11) | 0.01166 (15) | −0.00070 (9) | −0.00034 (11) | 0.00031 (10) |
| O2 | 0.01710 (14) | 0.00966 (13) | 0.00956 (15) | −0.00019 (11) | 0.00367 (11) | −0.00079 (10) |
| O3 | 0.02292 (18) | 0.01090 (15) | 0.01373 (18) | −0.00454 (13) | −0.00006 (14) | −0.00398 (12) |
| O4 | 0.01600 (14) | 0.01549 (16) | 0.01168 (16) | 0.00482 (12) | 0.00046 (12) | −0.00285 (13) |
| O5 | 0.01694 (14) | 0.00605 (12) | 0.00880 (14) | −0.00054 (10) | −0.00024 (11) | 0.00062 (9) |
| O6 | 0.01094 (12) | 0.00955 (13) | 0.01345 (16) | 0.00182 (10) | −0.00145 (10) | 0.00072 (11) |
| C1 | 0.01007 (13) | 0.00605 (13) | 0.00782 (15) | 0.00050 (11) | −0.00097 (11) | −0.00050 (10) |
| C2 | 0.01034 (13) | 0.00591 (13) | 0.00780 (16) | 0.00042 (11) | −0.00044 (11) | −0.00038 (10) |
| C3 | 0.01152 (13) | 0.00669 (14) | 0.00780 (16) | 0.00042 (11) | −0.00063 (12) | −0.00042 (11) |
| C4 | 0.01571 (16) | 0.00818 (15) | 0.00715 (16) | 0.00110 (13) | −0.00097 (13) | −0.00082 (11) |
| O1W | 0.01351 (14) | 0.01088 (14) | 0.01774 (19) | −0.00051 (11) | 0.00272 (12) | 0.00068 (12) |
| O2W | 0.0434 (3) | 0.00952 (15) | 0.00958 (18) | 0.00494 (17) | 0.00155 (17) | −0.00014 (11) |
| O3W | 0.01445 (15) | 0.0230 (2) | 0.0183 (2) | 0.00034 (14) | −0.00277 (14) | 0.00046 (16) |
| O4W | 0.01251 (14) | 0.01583 (17) | 0.0222 (2) | −0.00316 (12) | −0.00284 (13) | 0.00420 (15) |
Geometric parameters (Å, °)
| K1—O1 | 2.8194 (4) | O2—C1 | 1.2479 (6) |
| K1—O1i | 2.8194 (4) | O3—C4 | 1.2581 (7) |
| K1—O3Wi | 2.8491 (6) | O4—C4 | 1.2604 (7) |
| K1—O3W | 2.8491 (6) | O5—C2 | 1.4232 (6) |
| K1—O2Wii | 3.0271 (7) | O5—H5 | 0.789 (17) |
| K1—O2Wiii | 3.0270 (7) | O6—C3 | 1.4188 (6) |
| K2—O1W | 2.7758 (5) | O6—H6 | 0.861 (16) |
| K2—O1Wiv | 2.7758 (5) | C1—C2 | 1.5348 (7) |
| K2—O4v | 2.8383 (5) | C2—C3 | 1.5311 (7) |
| K2—O4vi | 2.8383 (5) | C2—H2 | 1.062 (15) |
| K2—O5vii | 2.9822 (4) | C3—C4 | 1.5342 (7) |
| K2—O5viii | 2.9822 (4) | C3—H3 | 1.004 (17) |
| K2—O2W | 3.1662 (7) | O1W—H11W | 0.824 (8) |
| K2—O2Wiv | 3.1662 (7) | O1W—H12W | 0.843 (9) |
| Na—O1W | 2.3264 (5) | O2W—H21W | 0.868 (9) |
| Na—O4W | 2.3379 (5) | O2W—H22W | 0.862 (9) |
| Na—O1 | 2.3512 (5) | O3W—H31W | 0.843 (9) |
| Na—O2W | 2.3562 (6) | O3W—H32W | 0.862 (8) |
| Na—O3vii | 2.4485 (6) | O4W—H41W | 0.858 (9) |
| Na—O5vii | 2.4707 (5) | O4W—H42W | 0.836 (8) |
| O1—C1 | 1.2668 (6) | ||
| O2—C1—O1 | 126.74 (5) | C2—C3—C4 | 109.72 (4) |
| O2—C1—C2 | 116.44 (4) | O6—C3—H3 | 113.2 (9) |
| O1—C1—C2 | 116.82 (4) | C2—C3—H3 | 108.4 (10) |
| O5—C2—C3 | 109.50 (4) | C4—C3—H3 | 102.5 (10) |
| O5—C2—C1 | 110.32 (4) | O3—C4—O4 | 126.03 (5) |
| C3—C2—C1 | 110.02 (4) | O3—C4—C3 | 116.52 (5) |
| O5—C2—H2 | 112.1 (8) | O4—C4—C3 | 117.44 (5) |
| C3—C2—H2 | 111.5 (9) | H11W—O1W—H12W | 109.6 (12) |
| C1—C2—H2 | 103.2 (9) | H21W—O2W—H22W | 101.2 (11) |
| O6—C3—C2 | 110.95 (4) | H31W—O3W—H32W | 102.7 (11) |
| O6—C3—C4 | 111.73 (4) | H41W—O4W—H42W | 105.2 (11) |
| O2—C1—C2—O5 | 3.05 (6) | O5—C2—C3—C4 | 57.57 (5) |
| O1—C1—C2—O5 | −177.09 (4) | C1—C2—C3—C4 | 178.98 (4) |
| O2—C1—C2—C3 | −117.87 (5) | O6—C3—C4—O3 | 16.43 (7) |
| O1—C1—C2—C3 | 61.98 (5) | C2—C3—C4—O3 | −107.06 (5) |
| O5—C2—C3—O6 | −66.37 (5) | O6—C3—C4—O4 | −164.68 (5) |
| C1—C2—C3—O6 | 55.04 (5) | C2—C3—C4—O4 | 71.84 (6) |
Symmetry codes: (i) −x, −y, z; (ii) −x, −y, z−1; (iii) x, y, z−1; (iv) −x+1, −y, z; (v) x+1/2, −y+1/2, −z+2; (vi) −x+1/2, y−1/2, −z+2; (vii) −x+1/2, y−1/2, −z+1; (viii) x+1/2, −y+1/2, −z+1.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O5—H5···O2 | 0.789 (17) | 2.031 (16) | 2.5946 (6) | 128.2 (14) |
| O6—H6···O4Wix | 0.861 (16) | 1.968 (16) | 2.8119 (7) | 166.5 (16) |
| O1W—H11W···O6 | 0.824 (8) | 1.960 (8) | 2.7832 (6) | 176.8 (15) |
| O1W—H12W···O4viii | 0.843 (9) | 2.010 (9) | 2.8500 (7) | 174.8 (18) |
| O2W—H21W···O3vi | 0.868 (9) | 1.830 (9) | 2.6941 (7) | 173.4 (19) |
| O2W—H22W···O2x | 0.862 (9) | 1.890 (9) | 2.7505 (7) | 175.5 (19) |
| O3W—H31W···O6vii | 0.843 (9) | 2.391 (15) | 3.1029 (7) | 142.5 (19) |
| O3W—H31W···O2xi | 0.843 (9) | 2.499 (17) | 3.1181 (7) | 131.0 (17) |
| O3W—H31W···O3vii | 0.843 (9) | 2.584 (14) | 3.1569 (8) | 126.2 (15) |
| O3W—H32W···O4ii | 0.862 (8) | 1.926 (8) | 2.7842 (8) | 173.8 (16) |
| O4W—H41W···O1i | 0.858 (9) | 1.888 (10) | 2.7124 (6) | 160.4 (19) |
| O4W—H42W···O3Wx | 0.836 (8) | 1.939 (9) | 2.7532 (8) | 164.4 (16) |
Symmetry codes: (ix) −x+1/2, y+1/2, −z+1; (viii) x+1/2, −y+1/2, −z+1; (vi) −x+1/2, y−1/2, −z+2; (x) x, y, z+1; (vii) −x+1/2, y−1/2, −z+1; (xi) −x+1/2, y−1/2, −z; (ii) −x, −y, z−1; (i) −x, −y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BG2163).
References
- Allen, F. H. (2002). Acta Cryst. B58, 380–388. [DOI] [PubMed]
- Ambady, G. K. & Kartha, G. (1968). Acta Cryst. B24, 1540–1547.
- Beevers, C. A. & Hughes, W. (1941). Proc. R. Soc. London Ser. A, 177, 251–259.
- Boese, R., Bläser, D., Latz, R. & Piennisch, M. (1995). Acta Cryst. C51, 2227–2229.
- Brożek, Z. & Stadnicka, K. (1994). Acta Cryst. B50, 59–68.
- Bruker (1998). SMART Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2001). SAINT-Plus Bruker AXS, Inc., Madison, Wisconsin, USA.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Hinazumi, H. & Mitsui, T. (1972). Acta Cryst. B28, 3299–3305.
- Iwata, Y., Mitani, S. & Shibuya, I. (1989). Ferroelectrics, 96, 215–219.
- Kay, M. I. (1978). Ferroelectrics, 19, 159–164.
- Kuroda, R. & Mason, S. F. (1981). J. Chem. Soc. Dalton Trans. pp. 1261–1273.
- Ottenz, C., Schurmann, M., Preut, H. & Bleckmann, P. (1998). Z. Kristallogr. New Cryst. Struct.213, 166.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Solans, X., Gonzalez-Silgo, C. & Ruiz-Pérez, C. (1997). J. Solid State Chem.131, 350–357.
- Suzuki, I. (1974). J. Phys. Soc. Jpn, 37, 1379–1384.
- Suzuki, E., Kabasawa, H., Honma, T., Nozaki, R. & Shiozaki, Y. (1996b). Acta Cryst. B52, 976–981.
- Suzuki, E., Muta, T., Nozaki, R. & Shiozaki, Y. (1996a). Acta Cryst. B52, 296–302.
- Treeck, E. van & Windsch, W. (1977). J. Magn. Reson.25, 15–23.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808005266/bg2163sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808005266/bg2163Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




