Abstract
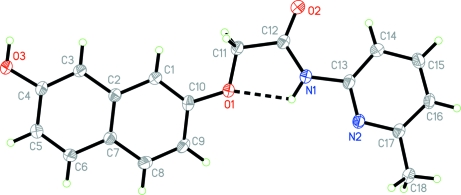
In the title compound, C18H16N2O3, the dihedral angle between the naphthalene ring system and the pyridyl ring is 18.1 (8)°. The molecules are interconnected via C—H⋯O and O—H⋯O hydrogen bonds. Inversion-related molecules are linked by O—H⋯O hydrogen bonds into cyclic centrosymmetric R 2 2(22) dimers. Intramolecular N—H⋯O hydrogen bonding produces an S(5) ring motif. The crystal structure is further stabilized by weak C—H—π interactions.
Related literature
For related literature on the applications; see: Atwood et al. (1996 ▶); Garcia-Tellado et al. (1990 ▶); Ghosh & Masanta (2006 ▶). For comparison bond lengths and angles see: Jin & Jin (2005 ▶); Liu & Li (2004 ▶); Rozycka-Sokolowska et al. (2004 ▶).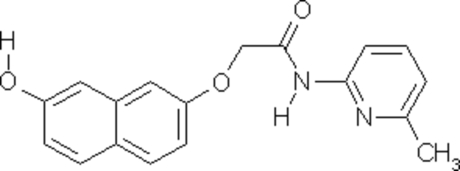
Experimental
Crystal data
C18H16N2O3
M r = 308.33
Triclinic,
a = 5.3676 (3) Å
b = 11.6991 (7) Å
c = 12.2915 (6) Å
α = 104.994 (4)°
β = 94.777 (3)°
γ = 94.877 (4)°
V = 738.42 (7) Å3
Z = 2
Mo Kα radiation
μ = 0.10 mm−1
T = 100.0 (1) K
0.4 × 0.16 × 0.09 mm
Data collection
Bruker SMART APEXII CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2005 ▶) T min = 0.963, T max = 0.992
12299 measured reflections
3340 independent reflections
2480 reflections with I > 2σ(I)
R int = 0.046
Refinement
R[F 2 > 2σ(F 2)] = 0.045
wR(F 2) = 0.130
S = 1.08
3340 reflections
217 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.24 e Å−3
Δρmin = −0.31 e Å−3
Data collection: APEX2 (Bruker, 2005 ▶); cell refinement: APEX2; data reduction: SAINT (Bruker, 2005 ▶); program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL and PLATON (Spek, 2003 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808006211/ng2430sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808006211/ng2430Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C11—H11B⋯O3i | 0.97 | 2.45 | 3.410 (2) | 168 |
| N1—H1N1⋯O1 | 0.88 (2) | 2.11 (2) | 2.5688 (18) | 111.9 (16) |
| O3—H1O3⋯O2ii | 0.88 (3) | 1.85 (2) | 2.6575 (17) | 152 (2) |
| C11—H11A⋯Cg1iii | 0.97 | 2.63 | 3.438 | 141 |
| C18—H18A⋯Cg2iv | 0.97 | 2.92 | 3.805 | 153 |
Symmetry codes: (i) 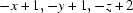 ; (ii)
; (ii)  ; (iii)
; (iii) 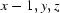 ; (iv)
; (iv) 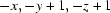 . Cg1 is the centroid of the C1,C2,C7–C10 ring and Cg2 is the centroid of C2–C7 ring.
. Cg1 is the centroid of the C1,C2,C7–C10 ring and Cg2 is the centroid of C2–C7 ring.
Acknowledgments
FHK and SRJ thank the Malaysian Government and Universiti Sains Malaysia for the Science Fund grant No. 305/PFIZIK/613312. SRJ thanks the Universiti Sains Malaysia for the awarding of a post-doctoral research fellowship. SG thanks the CSIR and DST for financial support. RC thanks the CSIR for a research fellowship.
supplementary crystallographic information
Comment
Pyridine amide moiety is widely used for the recognition of carboxylic acid functional group due to its complementary donor-acceptor arrangement (Garcia-Tellado et al., 1990). This group attached with different spacer having photo physical properties is the current interest for the recognition studies of both mono/di carboxylic acids (Ghosh & Masanta, 2006).This type of compounds is also important for its unique supramolecular arrangement (Atwood et al., 1996).
The asymmetric unit of (I) contains one molecule of 2–(7-hydroxy– naphthalene-2-yloxy)-N-(6-methyl-pyridine–2–yl) –acetamide. The dihedral angle between the naphthalene ring and the pyridine rings being 18.03 (8)°. The bond lengths and bond angles are comparable with the values reported in the literature (Rozycka-Sokolowska et al., 2004; Jin & Jin, 2005). The bond distance of C12=O2 is 1.226 (2) Å, which is typical for double bonds (Liu & Li., 2004). The naphthalene ring is planar, the maximum deviation from the least squares plane being -0.011 (2) Å for atom C10. The pyridine ring is planar with the maximum deviation from planarity being -0.010 (2) Å for atom C17.
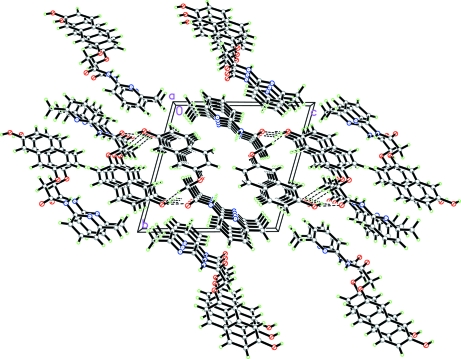
The molecules are stacked into layers parallel to the bc-plane by C11—H11B—O3i and O3—H1O3—O2ii hydrogen bonds (Fig. 2). In the crystal structure of (I), inversion-related molecules at (x,y,z) and (2 - x,1 - y,3 - z) are linked by O3—H1O3—O2 hydrogen bonds into cyclic centrosymmetric R22(22) dimers. The crystal structure is further stabilized by weak C—H—π interactions involving rings C11—H11A—Cg1 (where Cg1 is the centroid of the C1,C2,C7—C10 ring) and C18—H18A—Cg2 (where Cg2 is the centroid of C2—C7 ring). The molecular conformation is stabilized by a N1—H1N1—O1 intramolecular interaction generating a ring motif S(5).
Experimental
2,7-Dihydroxynaphthalene (160 mg, 1.0 mmol) and N–picolylchloroacetamide (185 mg, 1.0 mmol) were stirred with K2CO3 (345 mg, 2.5 mmol) and tBu4N+Br- (50 mg, 0.16 mmol) in dry acetone (10 ml) for 7 h at room temperature. Acetone was then distilled off and the crude product was extracted with CHCl3 (4 x 20 ml) after washing with water. The product (I) was purified by column chromatography (Silica gel 100–200 mesh) using 20% ethyl acetate in pet ether as eluent to afford an off-white coloured solid compound (Yield 61%). Single crystals were grown by slow evaporation of CHCl3/MeOH/Xylene solution (v/v 1:1:3) (Mp. 178–80 °C).
Refinement
H atoms were placed in calculated positions, with C—H=0.93 Å,and O—H=0.86 Å, N—H=0.86 Å, and refined using a riding model, with Uiso(H)=1.2Uequ(C,N,O).
Figures
Fig. 1.
The molecular structure of the title compound, showing 50% probability displacement ellipsoids and the atomic numbering scheme. Hydrogen bonds are shown as dashed lines.
Fig. 2.
The crystal packing of the title compound, viewed along the a axis. Hydrogen bonds are shown as dashed lines.
Crystal data
| C18H16N2O3 | Z = 2 |
| Mr = 308.33 | F000 = 324 |
| Triclinic, P1 | Dx = 1.387 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation λ = 0.71073 Å |
| a = 5.3676 (3) Å | Cell parameters from 2511 reflections |
| b = 11.6991 (7) Å | θ = 3.4–30.4º |
| c = 12.2915 (6) Å | µ = 0.10 mm−1 |
| α = 104.994 (4)º | T = 100.0 (1) K |
| β = 94.777 (3)º | Block, colourless |
| γ = 94.877 (4)º | 0.4 × 0.16 × 0.09 mm |
| V = 738.42 (7) Å3 |
Data collection
| Bruker SMART APEXII CCD area-detector diffractometer | 2480 reflections with I > 2σ(I) |
| Detector resolution: 8.33 pixels mm-1 | Rint = 0.046 |
| T = 100.0(1) K | θmax = 27.5º |
| ω scans | θmin = 1.7º |
| Absorption correction: multi-scan(SADABS; Bruker, 2005) | h = −6→6 |
| Tmin = 0.963, Tmax = 0.992 | k = −15→13 |
| 12299 measured reflections | l = −15→15 |
| 3340 independent reflections |
Refinement
| Refinement on F2 | H atoms treated by a mixture of independent and constrained refinement |
| Least-squares matrix: full | w = 1/[σ2(Fo2) + (0.0577P)2 + 0.2187P] where P = (Fo2 + 2Fc2)/3 |
| R[F2 > 2σ(F2)] = 0.045 | (Δ/σ)max < 0.001 |
| wR(F2) = 0.130 | Δρmax = 0.24 e Å−3 |
| S = 1.08 | Δρmin = −0.31 e Å−3 |
| 3340 reflections | Extinction correction: none |
| 217 parameters |
Special details
| Geometry. Experimental. The low-temperature data was collected with the Oxford Crysosystem Cobra low-temperature attachement.All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | −0.0438 (2) | 0.40705 (11) | 0.65093 (10) | 0.0240 (3) | |
| O2 | −0.5889 (2) | 0.23059 (11) | 0.67844 (10) | 0.0252 (3) | |
| O3 | 0.8081 (2) | 0.77389 (11) | 1.13643 (11) | 0.0245 (3) | |
| N1 | −0.3537 (3) | 0.23114 (14) | 0.53179 (13) | 0.0225 (3) | |
| N2 | −0.3711 (3) | 0.13147 (13) | 0.34601 (12) | 0.0204 (3) | |
| C1 | 0.2212 (3) | 0.52560 (15) | 0.81993 (14) | 0.0202 (4) | |
| H1A | 0.1222 | 0.4975 | 0.8681 | 0.024* | |
| C2 | 0.4376 (3) | 0.60921 (15) | 0.86540 (14) | 0.0188 (4) | |
| C3 | 0.5095 (3) | 0.65141 (15) | 0.98390 (14) | 0.0201 (4) | |
| H3A | 0.4128 | 0.6258 | 1.0342 | 0.024* | |
| C4 | 0.7222 (3) | 0.73013 (15) | 1.02417 (14) | 0.0199 (4) | |
| C5 | 0.8699 (3) | 0.77091 (16) | 0.94921 (15) | 0.0222 (4) | |
| H5A | 1.0129 | 0.8245 | 0.9777 | 0.027* | |
| C6 | 0.8034 (3) | 0.73183 (15) | 0.83490 (15) | 0.0220 (4) | |
| H6A | 0.9019 | 0.759 | 0.786 | 0.026* | |
| C7 | 0.5856 (3) | 0.65024 (15) | 0.79020 (14) | 0.0194 (4) | |
| C8 | 0.5140 (3) | 0.60795 (16) | 0.67167 (15) | 0.0218 (4) | |
| H8A | 0.6104 | 0.6349 | 0.622 | 0.026* | |
| C9 | 0.3063 (3) | 0.52862 (16) | 0.62984 (15) | 0.0219 (4) | |
| H9A | 0.2608 | 0.5019 | 0.5521 | 0.026* | |
| C10 | 0.1598 (3) | 0.48687 (15) | 0.70512 (15) | 0.0212 (4) | |
| C11 | −0.2068 (3) | 0.35993 (16) | 0.71767 (15) | 0.0214 (4) | |
| H11A | −0.2874 | 0.4234 | 0.7641 | 0.026* | |
| H11B | −0.111 | 0.3235 | 0.7675 | 0.026* | |
| C12 | −0.4042 (3) | 0.26785 (15) | 0.64022 (14) | 0.0201 (4) | |
| C13 | −0.4891 (3) | 0.14402 (15) | 0.43903 (15) | 0.0206 (4) | |
| C14 | −0.7171 (3) | 0.08120 (16) | 0.44454 (16) | 0.0241 (4) | |
| H14A | −0.7945 | 0.0941 | 0.511 | 0.029* | |
| C15 | −0.8235 (3) | −0.00193 (17) | 0.34564 (16) | 0.0259 (4) | |
| H15A | −0.9753 | −0.0469 | 0.3448 | 0.031* | |
| C16 | −0.7042 (3) | −0.01777 (16) | 0.24877 (15) | 0.0227 (4) | |
| H16A | −0.7733 | −0.0742 | 0.1826 | 0.027* | |
| C17 | −0.4792 (3) | 0.05155 (15) | 0.25079 (14) | 0.0198 (4) | |
| C18 | −0.3482 (3) | 0.04513 (16) | 0.14692 (15) | 0.0244 (4) | |
| H18D | −0.1719 | 0.0416 | 0.1646 | 0.037* | |
| H18A | −0.3712 | 0.1145 | 0.1209 | 0.037* | |
| H18B | −0.4174 | −0.0248 | 0.0887 | 0.037* | |
| H1N1 | −0.210 (4) | 0.2626 (18) | 0.5165 (17) | 0.026 (5)* | |
| H1O3 | 0.700 (5) | 0.756 (2) | 1.181 (2) | 0.043 (7)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0247 (6) | 0.0271 (7) | 0.0162 (6) | −0.0067 (5) | 0.0001 (5) | 0.0022 (5) |
| O2 | 0.0267 (6) | 0.0274 (7) | 0.0193 (7) | −0.0016 (5) | 0.0039 (5) | 0.0034 (5) |
| O3 | 0.0262 (6) | 0.0291 (7) | 0.0146 (7) | −0.0054 (5) | −0.0010 (5) | 0.0031 (5) |
| N1 | 0.0234 (7) | 0.0243 (8) | 0.0165 (8) | −0.0042 (6) | 0.0005 (6) | 0.0021 (6) |
| N2 | 0.0226 (7) | 0.0195 (7) | 0.0174 (8) | −0.0006 (5) | −0.0010 (6) | 0.0037 (6) |
| C1 | 0.0224 (8) | 0.0214 (9) | 0.0162 (9) | 0.0007 (6) | 0.0028 (7) | 0.0042 (7) |
| C2 | 0.0210 (8) | 0.0170 (8) | 0.0174 (9) | 0.0027 (6) | 0.0020 (7) | 0.0025 (7) |
| C3 | 0.0233 (8) | 0.0205 (9) | 0.0161 (9) | 0.0001 (6) | 0.0028 (7) | 0.0048 (7) |
| C4 | 0.0233 (8) | 0.0186 (9) | 0.0159 (9) | 0.0019 (6) | −0.0002 (7) | 0.0020 (7) |
| C5 | 0.0219 (8) | 0.0213 (9) | 0.0211 (10) | −0.0015 (6) | 0.0010 (7) | 0.0035 (7) |
| C6 | 0.0238 (8) | 0.0210 (9) | 0.0207 (9) | −0.0011 (7) | 0.0047 (7) | 0.0051 (7) |
| C7 | 0.0231 (8) | 0.0177 (8) | 0.0168 (9) | 0.0021 (6) | 0.0022 (7) | 0.0035 (7) |
| C8 | 0.0258 (8) | 0.0216 (9) | 0.0187 (9) | 0.0034 (7) | 0.0050 (7) | 0.0058 (7) |
| C9 | 0.0281 (9) | 0.0226 (9) | 0.0133 (9) | 0.0028 (7) | 0.0008 (7) | 0.0021 (7) |
| C10 | 0.0219 (8) | 0.0182 (9) | 0.0203 (9) | 0.0021 (6) | −0.0012 (7) | 0.0004 (7) |
| C11 | 0.0239 (8) | 0.0219 (9) | 0.0167 (9) | 0.0009 (7) | 0.0003 (7) | 0.0031 (7) |
| C12 | 0.0241 (8) | 0.0196 (9) | 0.0158 (9) | 0.0027 (7) | −0.0001 (7) | 0.0038 (7) |
| C13 | 0.0241 (8) | 0.0195 (9) | 0.0165 (9) | 0.0008 (6) | −0.0013 (7) | 0.0033 (7) |
| C14 | 0.0251 (9) | 0.0277 (10) | 0.0186 (9) | −0.0014 (7) | 0.0022 (7) | 0.0062 (8) |
| C15 | 0.0240 (8) | 0.0277 (10) | 0.0241 (10) | −0.0060 (7) | −0.0029 (7) | 0.0082 (8) |
| C16 | 0.0257 (8) | 0.0205 (9) | 0.0183 (9) | −0.0032 (7) | −0.0038 (7) | 0.0028 (7) |
| C17 | 0.0233 (8) | 0.0183 (8) | 0.0168 (9) | 0.0022 (6) | −0.0012 (7) | 0.0039 (7) |
| C18 | 0.0272 (9) | 0.0243 (9) | 0.0183 (9) | 0.0000 (7) | −0.0001 (7) | 0.0016 (7) |
Geometric parameters (Å, °)
| O1—C10 | 1.376 (2) | C6—H6A | 0.93 |
| O1—C11 | 1.419 (2) | C7—C8 | 1.420 (2) |
| O2—C12 | 1.226 (2) | C8—C9 | 1.360 (2) |
| O3—C4 | 1.366 (2) | C8—H8A | 0.93 |
| O3—H1O3 | 0.88 (3) | C9—C10 | 1.415 (3) |
| N1—C12 | 1.348 (2) | C9—H9A | 0.93 |
| N1—C13 | 1.415 (2) | C11—C12 | 1.516 (2) |
| N1—H1N1 | 0.88 (2) | C11—H11A | 0.97 |
| N2—C13 | 1.334 (2) | C11—H11B | 0.97 |
| N2—C17 | 1.345 (2) | C13—C14 | 1.389 (2) |
| C1—C10 | 1.368 (2) | C14—C15 | 1.388 (2) |
| C1—C2 | 1.426 (2) | C14—H14A | 0.93 |
| C1—H1A | 0.93 | C15—C16 | 1.377 (3) |
| C2—C7 | 1.415 (2) | C15—H15A | 0.93 |
| C2—C3 | 1.420 (2) | C16—C17 | 1.391 (2) |
| C3—C4 | 1.374 (2) | C16—H16A | 0.93 |
| C3—H3A | 0.93 | C17—C18 | 1.496 (3) |
| C4—C5 | 1.411 (2) | C18—H18D | 0.96 |
| C5—C6 | 1.366 (2) | C18—H18A | 0.96 |
| C5—H5A | 0.93 | C18—H18B | 0.96 |
| C6—C7 | 1.418 (2) | ||
| C10—O1—C11 | 118.54 (13) | C1—C10—O1 | 125.35 (16) |
| C4—O3—H1O3 | 112.9 (16) | C1—C10—C9 | 121.31 (16) |
| C12—N1—C13 | 129.91 (15) | O1—C10—C9 | 113.33 (15) |
| C12—N1—H1N1 | 115.8 (13) | O1—C11—C12 | 109.16 (14) |
| C13—N1—H1N1 | 114.2 (13) | O1—C11—H11A | 109.8 |
| C13—N2—C17 | 117.79 (14) | C12—C11—H11A | 109.8 |
| C10—C1—C2 | 119.74 (17) | O1—C11—H11B | 109.8 |
| C10—C1—H1A | 120.1 | C12—C11—H11B | 109.8 |
| C2—C1—H1A | 120.1 | H11A—C11—H11B | 108.3 |
| C7—C2—C3 | 119.23 (15) | O2—C12—N1 | 125.19 (16) |
| C7—C2—C1 | 119.02 (15) | O2—C12—C11 | 120.00 (15) |
| C3—C2—C1 | 121.74 (16) | N1—C12—C11 | 114.78 (15) |
| C4—C3—C2 | 119.93 (16) | N2—C13—C14 | 124.72 (16) |
| C4—C3—H3A | 120 | N2—C13—N1 | 111.27 (15) |
| C2—C3—H3A | 120 | C14—C13—N1 | 124.00 (17) |
| O3—C4—C3 | 124.03 (16) | C15—C14—C13 | 116.46 (17) |
| O3—C4—C5 | 115.14 (15) | C15—C14—H14A | 121.8 |
| C3—C4—C5 | 120.83 (16) | C13—C14—H14A | 121.8 |
| C6—C5—C4 | 120.17 (15) | C16—C15—C14 | 120.03 (16) |
| C6—C5—H5A | 119.9 | C16—C15—H15A | 120 |
| C4—C5—H5A | 119.9 | C14—C15—H15A | 120 |
| C5—C6—C7 | 120.59 (17) | C15—C16—C17 | 119.32 (16) |
| C5—C6—H6A | 119.7 | C15—C16—H16A | 120.3 |
| C7—C6—H6A | 119.7 | C17—C16—H16A | 120.3 |
| C2—C7—C6 | 119.24 (15) | N2—C17—C16 | 121.65 (16) |
| C2—C7—C8 | 119.26 (15) | N2—C17—C18 | 116.05 (15) |
| C6—C7—C8 | 121.50 (16) | C16—C17—C18 | 122.26 (15) |
| C9—C8—C7 | 120.91 (17) | C17—C18—H18D | 109.5 |
| C9—C8—H8A | 119.5 | C17—C18—H18A | 109.5 |
| C7—C8—H8A | 119.5 | H18D—C18—H18A | 109.5 |
| C8—C9—C10 | 119.75 (16) | C17—C18—H18B | 109.5 |
| C8—C9—H9A | 120.1 | H18D—C18—H18B | 109.5 |
| C10—C9—H9A | 120.1 | H18A—C18—H18B | 109.5 |
| C10—C1—C2—C7 | −0.2 (2) | C11—O1—C10—C9 | −179.27 (14) |
| C10—C1—C2—C3 | −179.63 (16) | C8—C9—C10—C1 | 0.7 (3) |
| C7—C2—C3—C4 | −0.7 (3) | C8—C9—C10—O1 | −179.89 (15) |
| C1—C2—C3—C4 | 178.70 (16) | C10—O1—C11—C12 | −175.79 (14) |
| C2—C3—C4—O3 | −178.86 (15) | C13—N1—C12—O2 | −1.0 (3) |
| C2—C3—C4—C5 | 0.6 (3) | C13—N1—C12—C11 | 177.16 (16) |
| O3—C4—C5—C6 | 179.21 (16) | O1—C11—C12—O2 | −167.56 (15) |
| C3—C4—C5—C6 | −0.3 (3) | O1—C11—C12—N1 | 14.2 (2) |
| C4—C5—C6—C7 | 0.1 (3) | C17—N2—C13—C14 | −0.6 (3) |
| C3—C2—C7—C6 | 0.5 (2) | C17—N2—C13—N1 | −179.81 (14) |
| C1—C2—C7—C6 | −178.93 (15) | C12—N1—C13—N2 | −178.05 (16) |
| C3—C2—C7—C8 | 179.92 (16) | C12—N1—C13—C14 | 2.7 (3) |
| C1—C2—C7—C8 | 0.5 (2) | N2—C13—C14—C15 | 1.4 (3) |
| C5—C6—C7—C2 | −0.2 (3) | N1—C13—C14—C15 | −179.45 (16) |
| C5—C6—C7—C8 | −179.62 (16) | C13—C14—C15—C16 | −0.6 (3) |
| C2—C7—C8—C9 | −0.2 (3) | C14—C15—C16—C17 | −1.0 (3) |
| C6—C7—C8—C9 | 179.21 (16) | C13—N2—C17—C16 | −1.1 (2) |
| C7—C8—C9—C10 | −0.4 (3) | C13—N2—C17—C18 | 176.69 (15) |
| C2—C1—C10—O1 | −179.74 (15) | C15—C16—C17—N2 | 1.9 (3) |
| C2—C1—C10—C9 | −0.4 (3) | C15—C16—C17—C18 | −175.75 (17) |
| C11—O1—C10—C1 | 0.2 (2) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C11—H11B···O3i | 0.97 | 2.45 | 3.410 (2) | 168 |
| N1—H1N1···O1 | 0.88 (2) | 2.11 (2) | 2.5688 (18) | 111.9 (16) |
| O3—H1O3···O2ii | 0.88 (3) | 1.85 (2) | 2.6575 (17) | 152 (2) |
| C11—H11A···Cg1iii | 0.97 | 2.63 | 3.438 | 141 |
| C18—H18A···Cg2iv | 0.97 | 2.93 | 3.805 | 153 |
Symmetry codes: (i) −x+1, −y+1, −z+2; (ii) −x, −y+1, −z+2; (iii) x−1, y, z; (iv) −x, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: NG2430).
References
- Atwood, J. L., Davies, J. E. D., MacNico, D. D. & Vogtle, F. (1996). Editors. Comprehensive Supramolecular Chemistry, Vols. 6, 7, 9. Oxford: Pergamon.
- Bruker (2005). SAINT and SMART Bruker Axs Inc., Madison, Wisconsin, USA.
- Garcia-Tellado, F., Goswami, S., Chang, S. K., Geib, S. J. & Hamilton, A. D. (1990). J. Am. Chem. Soc.112, 7393–7394.
- Ghosh, K. & Masanta, G. (2006). Tetrahedron Lett.47, 2365–2369.
- Jin, C.-Z. & Jin, L.-F. (2005). Acta Cryst. E61, o275–o276.
- Liu, W.-Y. & Li, Y.-Z. (2004). Acta Cryst. E60, o694–o695.
- Rozycka-Sokolowska, E., Marciniak, B. & Pavlyuk, V. (2004). Acta Cryst. E60, o884–o885.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2003). J. Appl. Cryst.36, 7–13.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808006211/ng2430sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808006211/ng2430Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report