Abstract
The title compound, C30H30N4O2, has a non-planar conformation, the dihedral angles formed by the pyridazinone ring plane and the three phenyl rings being 54.61 (7), 51.10 (7) and 59.53 (8)°. The piperazine ring adopts a chair conformation. Inter- and intramolecular C—H⋯O contacts are found in the crystal structure and these consolidate the three-dimensional packing.
Related literature
For related structures, see: Doğruer et al. (2007 ▶); Swenson et al. (1997 ▶); Yüksektepe et al. (2004 ▶). For structure analysis, see: Allen et al. (1987 ▶); Cremer & Pople (1975 ▶).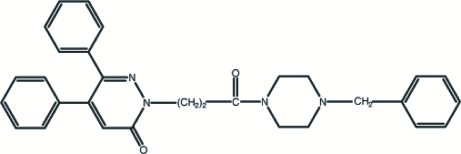
Experimental
Crystal data
C30H30N4O2
M r = 478.58
Monoclinic,

a = 15.6725 (12) Å
b = 9.1139 (5) Å
c = 17.6743 (12) Å
β = 90.553 (6)°
V = 2524.4 (3) Å3
Z = 4
Mo Kα radiation
μ = 0.08 mm−1
T = 296 K
0.73 × 0.51 × 0.11 mm
Data collection
Stoe IPDS-2 diffractometer
Absorption correction: integration (X-RED32; Stoe & Cie, 2002 ▶) T min = 0.477, T max = 0.907
30083 measured reflections
4966 independent reflections
3394 reflections with I > 2σ(I)
R int = 0.062
Refinement
R[F 2 > 2σ(F 2)] = 0.041
wR(F 2) = 0.094
S = 1.03
4966 reflections
325 parameters
H-atom parameters constrained
Δρmax = 0.14 e Å−3
Δρmin = −0.15 e Å−3
Data collection: X-AREA (Stoe & Cie, 2002 ▶); cell refinement: X-AREA; data reduction: X-RED32 (Stoe & Cie, 2002 ▶); program(s) used to solve structure: SIR97 (Altomare et al., 1999 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808013159/tk2267sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808013159/tk2267Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C2—H2⋯O2i | 0.93 | 2.47 | 3.3470 (19) | 157 |
| C18—H18B⋯O1 | 0.97 | 2.56 | 3.0755 (18) | 113 |
| C20—H20B⋯O2 | 0.97 | 2.35 | 2.759 (2) | 105 |
Symmetry code: (i)  .
.
Acknowledgments
The authors acknowledge the Faculty of Arts and Sciences, Ondokuz Mayıs University, Turkey, for the use of the Stoe IPDS-2 diffractometer (purchased under grant F.279 of the University Research Fund).
supplementary crystallographic information
Comment
The title compound (I) was prepared recently (Doğruer et al., 2007) and displays analgesic and anti-inflammatory effects. In the crystal structure of (I), Fig. 1, the bond lengths and angles are within their normal ranges (Allen et al., 1987). The piperazine bridge has a normal chair conformation, with puckering parameters (Cremer & Pople, 1975) QT = 0.562 (2) Å, θ = 176.2 (2) ° and φ = 354 (3) °. Relevant literature values for the puckering of the cyclobutane ring are 29.0 (1)° (Yüksektepe et al., 2004) and 23.5° (Swenson et al., 1997). In this study, the N1/N2/C1—C4 pyridazinone ring plane forms dihedral angles of 54.61 (7), 51.10 (7) and 59.53 (8)°, respectively, with the planes of the (A: C5—C10), (B: C11—C16) and (C: C25 –C30) phenyl rings. The dihedral angles between the phenyl rings are A/B = 50.77 (8), A/C = 83.01 (9) and B/C = 70.15 (9)°.
The crystal structure is stabilized by inter and intramolecular C—H···O contacts that stabilize the three-dimensional network (Table 1, Fig. 2).
Experimental
[3-(5,6-Diphenyl-3(2H)-pyridazinone-2-yl)propanoic acid (0.01 mol) in dichloromethane (40 ml) at 273 K (ice-bath) was treated with triethylamine (1 ml) and ethyl chloroformate (0.01 mol). After stirring the reaction mixture at 273 K for 15 min, benzylpiperazine (0.011 mol) was added. The final mixture was stirred at 273–298 K for 24 h and evaporated to dryness. The product was solidified with ice-cold water and crystallized from ethanol (yield 25%, m.p. 422 K). IR vmax(cm-1) (KBr): 1660 (CO ring), 1635 (CO amide) (Doğruer et al., 2007).
Refinement
All H atoms were then placed in geometrically idealized positions and constrained to ride on their parent atoms, with Csp3—H = 0.97 Å and Csp2—H = 0.93 Å, and with Uiso(H) = 1.2Ueq(C).
Figures
Fig. 1.
The molecular structure of (I) showing the atom numbering scheme. Displacement ellipsoids for non-H atoms are drawn at the 30% probability level.
Fig. 2.
View of the hydrogen bonding interactions (dashed lines) in (I) down b axis. H atoms not involved in hydrogen bonding interactions have been omitted for clarity.
Crystal data
| C30H30N4O2 | F000 = 1016 |
| Mr = 478.58 | Dx = 1.259 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 32998 reflections |
| a = 15.6725 (12) Å | θ = 1.8–28.0º |
| b = 9.1139 (5) Å | µ = 0.08 mm−1 |
| c = 17.6743 (12) Å | T = 296 K |
| β = 90.553 (6)º | Plate, colourless |
| V = 2524.4 (3) Å3 | 0.73 × 0.51 × 0.11 mm |
| Z = 4 |
Data collection
| Stoe IPDS-2 diffractometer | 4966 independent reflections |
| Monochromator: plane graphite | 3394 reflections with I > 2σ(I) |
| Detector resolution: 6.67 pixels mm-1 | Rint = 0.062 |
| T = 296 K | θmax = 26.0º |
| rotation method scans | θmin = 2.3º |
| Absorption correction: integration(XRED32; Stoe & Cie, 2002) | h = −19→19 |
| Tmin = 0.477, Tmax = 0.907 | k = −11→11 |
| 30083 measured reflections | l = −21→21 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.041 | H-atom parameters constrained |
| wR(F2) = 0.094 | w = 1/[σ2(Fo2) + (0.0452P)2 + 0.0381P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.03 | (Δ/σ)max < 0.001 |
| 4966 reflections | Δρmax = 0.14 e Å−3 |
| 325 parameters | Δρmin = −0.15 e Å−3 |
| Primary atom site location: structure-invariant direct methods | Extinction correction: none |
Special details
| Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell e.s.d.'s are taken into account in the estimation of distances, angles and torsion angles |
| Refinement. Refinement on F2 for ALL reflections except those flagged by the user for potential systematic errors. Weighted R-factors wR and all goodnesses of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The observed criterion of F2 > σ(F2) is used only for calculating -R-factor-obs etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.77910 (7) | 0.86642 (13) | 0.78316 (6) | 0.0564 (4) | |
| O2 | 0.75320 (8) | 0.76212 (12) | 0.50119 (6) | 0.0545 (4) | |
| N1 | 0.65740 (8) | 0.85831 (14) | 0.71319 (7) | 0.0435 (4) | |
| N2 | 0.57156 (8) | 0.85469 (13) | 0.70467 (7) | 0.0429 (4) | |
| N3 | 0.83628 (9) | 0.57588 (14) | 0.54110 (7) | 0.0483 (4) | |
| N4 | 0.89677 (9) | 0.28978 (14) | 0.50320 (7) | 0.0512 (5) | |
| C1 | 0.70100 (10) | 0.85822 (16) | 0.78161 (8) | 0.0430 (5) | |
| C2 | 0.64657 (10) | 0.84702 (16) | 0.84615 (8) | 0.0429 (5) | |
| C3 | 0.56060 (9) | 0.84191 (14) | 0.84000 (8) | 0.0380 (4) | |
| C4 | 0.52408 (9) | 0.84847 (15) | 0.76493 (8) | 0.0378 (4) | |
| C5 | 0.43077 (9) | 0.84729 (15) | 0.74879 (8) | 0.0383 (4) | |
| C6 | 0.37801 (11) | 0.73728 (17) | 0.77581 (9) | 0.0490 (5) | |
| C7 | 0.29174 (12) | 0.73806 (19) | 0.75865 (10) | 0.0568 (6) | |
| C8 | 0.25695 (11) | 0.84874 (19) | 0.71553 (10) | 0.0568 (6) | |
| C9 | 0.30880 (12) | 0.95805 (19) | 0.68845 (10) | 0.0570 (6) | |
| C10 | 0.39510 (11) | 0.95668 (17) | 0.70496 (9) | 0.0481 (5) | |
| C11 | 0.50675 (9) | 0.82763 (15) | 0.90846 (8) | 0.0391 (4) | |
| C12 | 0.52513 (11) | 0.72082 (17) | 0.96183 (9) | 0.0498 (5) | |
| C13 | 0.47574 (13) | 0.7070 (2) | 1.02585 (9) | 0.0591 (6) | |
| C14 | 0.40832 (12) | 0.8005 (2) | 1.03718 (10) | 0.0605 (6) | |
| C15 | 0.39035 (11) | 0.90860 (19) | 0.98546 (9) | 0.0538 (6) | |
| C16 | 0.43914 (10) | 0.92255 (17) | 0.92135 (8) | 0.0454 (5) | |
| C17 | 0.70489 (11) | 0.85361 (17) | 0.64203 (8) | 0.0490 (5) | |
| C18 | 0.74754 (11) | 0.70578 (16) | 0.63244 (8) | 0.0481 (5) | |
| C19 | 0.78017 (10) | 0.68481 (16) | 0.55269 (8) | 0.0410 (5) | |
| C20 | 0.86743 (11) | 0.54334 (17) | 0.46501 (8) | 0.0486 (5) | |
| C21 | 0.85466 (11) | 0.38316 (17) | 0.44691 (9) | 0.0485 (5) | |
| C22 | 0.86159 (14) | 0.32222 (19) | 0.57767 (10) | 0.0624 (6) | |
| C23 | 0.87401 (13) | 0.4804 (2) | 0.59858 (9) | 0.0624 (7) | |
| C24 | 0.88493 (13) | 0.13378 (18) | 0.48756 (11) | 0.0628 (7) | |
| C25 | 0.93064 (11) | 0.07611 (16) | 0.41898 (9) | 0.0476 (5) | |
| C26 | 1.00083 (12) | 0.14359 (18) | 0.38788 (10) | 0.0566 (6) | |
| C27 | 1.04082 (13) | 0.0836 (2) | 0.32604 (11) | 0.0674 (7) | |
| C28 | 1.01149 (16) | −0.0452 (2) | 0.29468 (11) | 0.0732 (8) | |
| C29 | 0.94303 (15) | −0.1147 (2) | 0.32600 (12) | 0.0724 (8) | |
| C30 | 0.90291 (12) | −0.05462 (18) | 0.38750 (11) | 0.0580 (6) | |
| H2 | 0.67140 | 0.84310 | 0.89410 | 0.0510* | |
| H6 | 0.40080 | 0.66260 | 0.80560 | 0.0590* | |
| H7 | 0.25700 | 0.66310 | 0.77640 | 0.0680* | |
| H8 | 0.19880 | 0.84970 | 0.70470 | 0.0680* | |
| H9 | 0.28570 | 1.03300 | 0.65900 | 0.0680* | |
| H10 | 0.42970 | 1.03080 | 0.68620 | 0.0580* | |
| H12 | 0.57100 | 0.65790 | 0.95460 | 0.0600* | |
| H13 | 0.48820 | 0.63440 | 1.06120 | 0.0710* | |
| H14 | 0.37480 | 0.79040 | 1.08000 | 0.0730* | |
| H15 | 0.34520 | 0.97260 | 0.99360 | 0.0650* | |
| H16 | 0.42670 | 0.99610 | 0.88650 | 0.0540* | |
| H17A | 0.74780 | 0.93030 | 0.64210 | 0.0590* | |
| H17B | 0.66620 | 0.87120 | 0.59990 | 0.0590* | |
| H18A | 0.70700 | 0.62870 | 0.64390 | 0.0580* | |
| H18B | 0.79480 | 0.69770 | 0.66800 | 0.0580* | |
| H20A | 0.92760 | 0.56750 | 0.46210 | 0.0580* | |
| H20B | 0.83690 | 0.60290 | 0.42820 | 0.0580* | |
| H21A | 0.79410 | 0.36130 | 0.44560 | 0.0580* | |
| H21B | 0.87760 | 0.36230 | 0.39730 | 0.0580* | |
| H22A | 0.88930 | 0.26060 | 0.61530 | 0.0750* | |
| H22B | 0.80110 | 0.29940 | 0.57750 | 0.0750* | |
| H23A | 0.84760 | 0.49950 | 0.64700 | 0.0750* | |
| H23B | 0.93450 | 0.50130 | 0.60350 | 0.0750* | |
| H24A | 0.82440 | 0.11510 | 0.48110 | 0.0750* | |
| H24B | 0.90420 | 0.07860 | 0.53140 | 0.0750* | |
| H26 | 1.02140 | 0.23040 | 0.40880 | 0.0680* | |
| H27 | 1.08790 | 0.13050 | 0.30540 | 0.0810* | |
| H28 | 1.03800 | −0.08480 | 0.25250 | 0.0880* | |
| H29 | 0.92360 | −0.20280 | 0.30560 | 0.0870* | |
| H30 | 0.85630 | −0.10270 | 0.40830 | 0.0700* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0356 (7) | 0.0821 (8) | 0.0516 (6) | 0.0029 (6) | 0.0057 (5) | −0.0074 (6) |
| O2 | 0.0572 (8) | 0.0642 (7) | 0.0423 (6) | 0.0168 (6) | 0.0084 (5) | −0.0007 (5) |
| N1 | 0.0377 (7) | 0.0554 (7) | 0.0374 (6) | 0.0085 (6) | 0.0074 (5) | −0.0026 (6) |
| N2 | 0.0392 (7) | 0.0508 (7) | 0.0386 (7) | 0.0076 (6) | 0.0029 (6) | −0.0026 (5) |
| N3 | 0.0500 (8) | 0.0562 (7) | 0.0388 (7) | 0.0133 (6) | 0.0059 (6) | −0.0037 (6) |
| N4 | 0.0533 (9) | 0.0515 (7) | 0.0488 (8) | 0.0122 (6) | 0.0070 (6) | 0.0015 (6) |
| C1 | 0.0388 (9) | 0.0487 (8) | 0.0416 (8) | 0.0071 (7) | 0.0022 (7) | −0.0051 (7) |
| C2 | 0.0416 (9) | 0.0529 (8) | 0.0341 (7) | 0.0067 (7) | 0.0008 (6) | −0.0043 (6) |
| C3 | 0.0402 (9) | 0.0367 (7) | 0.0371 (7) | 0.0055 (6) | 0.0033 (6) | −0.0021 (6) |
| C4 | 0.0396 (8) | 0.0372 (7) | 0.0366 (8) | 0.0051 (6) | 0.0037 (6) | −0.0012 (6) |
| C5 | 0.0388 (8) | 0.0420 (7) | 0.0342 (7) | 0.0034 (6) | 0.0022 (6) | −0.0048 (6) |
| C6 | 0.0466 (10) | 0.0452 (8) | 0.0551 (9) | 0.0012 (7) | −0.0026 (7) | 0.0055 (7) |
| C7 | 0.0461 (10) | 0.0561 (10) | 0.0683 (11) | −0.0091 (8) | 0.0007 (8) | 0.0012 (9) |
| C8 | 0.0397 (10) | 0.0632 (10) | 0.0674 (11) | 0.0031 (8) | −0.0076 (8) | −0.0086 (9) |
| C9 | 0.0513 (11) | 0.0553 (9) | 0.0640 (11) | 0.0079 (8) | −0.0126 (9) | 0.0052 (8) |
| C10 | 0.0446 (9) | 0.0486 (9) | 0.0509 (9) | 0.0012 (7) | −0.0028 (7) | 0.0039 (7) |
| C11 | 0.0379 (8) | 0.0458 (8) | 0.0337 (7) | 0.0012 (6) | 0.0020 (6) | −0.0024 (6) |
| C12 | 0.0521 (10) | 0.0534 (9) | 0.0440 (9) | 0.0074 (8) | 0.0029 (7) | 0.0014 (7) |
| C13 | 0.0657 (12) | 0.0676 (10) | 0.0441 (9) | 0.0003 (9) | 0.0070 (8) | 0.0124 (8) |
| C14 | 0.0550 (11) | 0.0813 (12) | 0.0455 (9) | −0.0051 (10) | 0.0153 (8) | 0.0001 (9) |
| C15 | 0.0413 (10) | 0.0713 (10) | 0.0489 (9) | 0.0076 (8) | 0.0083 (8) | −0.0077 (8) |
| C16 | 0.0421 (9) | 0.0524 (8) | 0.0417 (8) | 0.0069 (7) | 0.0016 (7) | −0.0003 (7) |
| C17 | 0.0487 (10) | 0.0600 (9) | 0.0385 (8) | 0.0092 (8) | 0.0117 (7) | −0.0013 (7) |
| C18 | 0.0501 (10) | 0.0519 (9) | 0.0426 (8) | 0.0015 (7) | 0.0098 (7) | −0.0071 (7) |
| C19 | 0.0350 (8) | 0.0467 (8) | 0.0413 (8) | −0.0018 (6) | 0.0038 (6) | −0.0056 (7) |
| C20 | 0.0472 (10) | 0.0557 (9) | 0.0432 (8) | 0.0099 (7) | 0.0115 (7) | −0.0024 (7) |
| C21 | 0.0462 (10) | 0.0566 (9) | 0.0427 (8) | 0.0080 (7) | 0.0054 (7) | −0.0028 (7) |
| C22 | 0.0721 (13) | 0.0687 (11) | 0.0465 (9) | 0.0236 (9) | 0.0087 (9) | 0.0087 (8) |
| C23 | 0.0660 (13) | 0.0771 (12) | 0.0439 (9) | 0.0254 (10) | −0.0028 (8) | −0.0051 (8) |
| C24 | 0.0664 (12) | 0.0523 (10) | 0.0699 (12) | 0.0077 (8) | 0.0195 (10) | 0.0062 (9) |
| C25 | 0.0438 (9) | 0.0430 (8) | 0.0560 (9) | 0.0066 (7) | 0.0039 (7) | 0.0052 (7) |
| C26 | 0.0504 (11) | 0.0491 (9) | 0.0704 (11) | −0.0015 (8) | 0.0078 (9) | −0.0006 (8) |
| C27 | 0.0567 (12) | 0.0738 (12) | 0.0721 (12) | 0.0094 (10) | 0.0182 (10) | 0.0148 (10) |
| C28 | 0.0857 (16) | 0.0784 (13) | 0.0557 (11) | 0.0254 (12) | 0.0058 (11) | −0.0058 (10) |
| C29 | 0.0785 (16) | 0.0632 (11) | 0.0754 (13) | 0.0039 (10) | −0.0073 (12) | −0.0143 (10) |
| C30 | 0.0475 (11) | 0.0512 (9) | 0.0751 (12) | −0.0004 (8) | −0.0013 (9) | 0.0040 (9) |
Geometric parameters (Å, °)
| O1—C1 | 1.2263 (19) | C25—C30 | 1.383 (2) |
| O2—C19 | 1.2232 (18) | C26—C27 | 1.378 (3) |
| N1—N2 | 1.3527 (18) | C27—C28 | 1.375 (3) |
| N1—C1 | 1.3832 (19) | C28—C29 | 1.368 (3) |
| N1—C17 | 1.468 (2) | C29—C30 | 1.375 (3) |
| N2—C4 | 1.3064 (19) | C2—H2 | 0.9300 |
| N3—C19 | 1.343 (2) | C6—H6 | 0.9300 |
| N3—C20 | 1.4655 (19) | C7—H7 | 0.9300 |
| N3—C23 | 1.459 (2) | C8—H8 | 0.9300 |
| N4—C21 | 1.462 (2) | C9—H9 | 0.9300 |
| N4—C22 | 1.462 (2) | C10—H10 | 0.9300 |
| N4—C24 | 1.460 (2) | C12—H12 | 0.9300 |
| C1—C2 | 1.435 (2) | C13—H13 | 0.9300 |
| C2—C3 | 1.352 (2) | C14—H14 | 0.9300 |
| C3—C4 | 1.441 (2) | C15—H15 | 0.9300 |
| C3—C11 | 1.488 (2) | C16—H16 | 0.9300 |
| C4—C5 | 1.487 (2) | C17—H17A | 0.9700 |
| C5—C6 | 1.387 (2) | C17—H17B | 0.9700 |
| C5—C10 | 1.378 (2) | C18—H18A | 0.9700 |
| C6—C7 | 1.383 (3) | C18—H18B | 0.9700 |
| C7—C8 | 1.374 (2) | C20—H20A | 0.9700 |
| C8—C9 | 1.375 (2) | C20—H20B | 0.9700 |
| C9—C10 | 1.381 (3) | C21—H21A | 0.9700 |
| C11—C12 | 1.384 (2) | C21—H21B | 0.9700 |
| C11—C16 | 1.388 (2) | C22—H22A | 0.9700 |
| C12—C13 | 1.383 (2) | C22—H22B | 0.9700 |
| C13—C14 | 1.374 (3) | C23—H23A | 0.9700 |
| C14—C15 | 1.371 (2) | C23—H23B | 0.9700 |
| C15—C16 | 1.379 (2) | C24—H24A | 0.9700 |
| C17—C18 | 1.514 (2) | C24—H24B | 0.9700 |
| C18—C19 | 1.516 (2) | C26—H26 | 0.9300 |
| C20—C21 | 1.507 (2) | C27—H27 | 0.9300 |
| C22—C23 | 1.501 (3) | C28—H28 | 0.9300 |
| C24—C25 | 1.509 (3) | C29—H29 | 0.9300 |
| C25—C26 | 1.379 (2) | C30—H30 | 0.9300 |
| O1···C18 | 3.0755 (18) | H6···N2vii | 2.8500 |
| O2···C2i | 3.3470 (19) | H7···C1vii | 3.0400 |
| O1···H17A | 2.6000 | H7···H17Avii | 2.5700 |
| O1···H27ii | 2.6200 | H8···C25ix | 3.0400 |
| O1···H20Biii | 2.7200 | H8···C30ix | 2.9400 |
| O1···H29iv | 2.7400 | H9···C2vi | 3.0500 |
| O1···H18B | 2.5600 | H9···C30ix | 3.0700 |
| O2···H20B | 2.3500 | H9···H30ix | 2.5900 |
| O2···H30v | 2.6200 | H10···N2 | 2.7600 |
| O2···H17B | 2.4400 | H10···C3vi | 2.8800 |
| O2···H2i | 2.4700 | H12···C2 | 2.8400 |
| N3···N4 | 2.8564 (18) | H12···H2 | 2.5500 |
| N4···N3 | 2.8564 (18) | H13···N2iii | 2.8400 |
| N1···H6vi | 2.9400 | H13···C10iii | 3.0600 |
| N2···H13i | 2.8400 | H15···C19vi | 2.8700 |
| N2···H10 | 2.7600 | H16···C4 | 2.9700 |
| N2···H6vi | 2.8500 | H16···C5 | 2.7900 |
| N4···H26 | 2.6400 | H16···H18Avi | 2.4700 |
| C1···C7vi | 3.536 (2) | H17A···O1 | 2.6000 |
| C2···O2iii | 3.3470 (19) | H17A···H7vi | 2.5700 |
| C5···C16 | 3.128 (2) | H17B···O2 | 2.4400 |
| C6···C16 | 3.215 (2) | H18A···C23 | 3.0600 |
| C6···C11 | 3.187 (2) | H18A···H23A | 2.5000 |
| C7···C1vii | 3.536 (2) | H18A···H16vii | 2.4700 |
| C11···C6 | 3.187 (2) | H18B···O1 | 2.5600 |
| C11···C15viii | 3.441 (2) | H18B···C1 | 2.9000 |
| C14···C16viii | 3.548 (2) | H18B···C23 | 2.6500 |
| C15···C16viii | 3.484 (2) | H18B···H23A | 2.0200 |
| C15···C11viii | 3.441 (2) | H18B···H27ii | 2.4600 |
| C16···C5 | 3.128 (2) | H20A···H23B | 2.5700 |
| C16···C15viii | 3.484 (2) | H20A···H23Bii | 2.5400 |
| C16···C14viii | 3.548 (2) | H20B···O2 | 2.3500 |
| C16···C6 | 3.215 (2) | H20B···O1i | 2.7200 |
| C18···O1 | 3.0755 (18) | H21A···H22B | 2.4000 |
| C21···C26 | 3.339 (2) | H21A···H24A | 2.3800 |
| C26···C21 | 3.339 (2) | H21B···C25 | 2.7600 |
| C1···H18B | 2.9000 | H21B···C26 | 2.7800 |
| C1···H7vi | 3.0400 | H21B···H26 | 2.5600 |
| C2···H12 | 2.8400 | H22A···H24B | 2.2400 |
| C2···H9vii | 3.0500 | H22A···C28x | 2.9600 |
| C3···H10vii | 2.8800 | H22B···H21A | 2.4000 |
| C3···H6 | 3.0500 | H22B···H24A | 2.4200 |
| C4···H16 | 2.9700 | H23A···C18 | 2.4600 |
| C5···H16 | 2.7900 | H23A···H18A | 2.5000 |
| C10···H13i | 3.0600 | H23A···H18B | 2.0200 |
| C11···H6 | 2.8800 | H23B···H20A | 2.5700 |
| C12···H2 | 2.8300 | H23B···H20Aii | 2.5400 |
| C18···H23A | 2.4600 | H23B···H26ii | 2.5500 |
| C19···H15vii | 2.8700 | H24A···H21A | 2.3800 |
| C21···H26 | 3.0400 | H24A···H22B | 2.4200 |
| C23···H18A | 3.0600 | H24A···H30 | 2.4200 |
| C23···H18B | 2.6500 | H24B···H22A | 2.2400 |
| C25···H8ix | 3.0400 | H24B···C25x | 3.0700 |
| C25···H24Bx | 3.0700 | H24B···C26x | 2.8800 |
| C25···H21B | 2.7600 | H24B···C27x | 3.0400 |
| C26···H21B | 2.7800 | H26···N4 | 2.6400 |
| C26···H24Bx | 2.8800 | H26···C21 | 3.0400 |
| C27···H29xi | 3.0900 | H26···H21B | 2.5600 |
| C27···H24Bx | 3.0400 | H26···H23Bii | 2.5500 |
| C28···H22Ax | 2.9600 | H27···H29xi | 2.4900 |
| C30···H8ix | 2.9400 | H27···O1ii | 2.6200 |
| C30···H9ix | 3.0700 | H27···H18Bii | 2.4600 |
| H2···C12 | 2.8300 | H29···C27xii | 3.0900 |
| H2···H12 | 2.5500 | H29···H27xii | 2.4900 |
| H2···O2iii | 2.4700 | H29···O1xiii | 2.7400 |
| H6···C3 | 3.0500 | H30···O2xiv | 2.6200 |
| H6···C11 | 2.8800 | H30···H24A | 2.4200 |
| H6···N1vii | 2.9400 | H30···H9ix | 2.5900 |
| N2—N1—C1 | 125.43 (12) | C9—C8—H8 | 120.00 |
| N2—N1—C17 | 114.57 (12) | C8—C9—H9 | 120.00 |
| C1—N1—C17 | 119.91 (13) | C10—C9—H9 | 120.00 |
| N1—N2—C4 | 118.95 (12) | C5—C10—H10 | 119.00 |
| C19—N3—C20 | 120.92 (13) | C9—C10—H10 | 119.00 |
| C19—N3—C23 | 126.61 (13) | C11—C12—H12 | 120.00 |
| C20—N3—C23 | 112.46 (13) | C13—C12—H12 | 120.00 |
| C21—N4—C22 | 108.86 (13) | C12—C13—H13 | 120.00 |
| C21—N4—C24 | 112.48 (13) | C14—C13—H13 | 120.00 |
| C22—N4—C24 | 108.60 (13) | C13—C14—H14 | 120.00 |
| O1—C1—N1 | 120.27 (13) | C15—C14—H14 | 120.00 |
| O1—C1—C2 | 125.99 (14) | C14—C15—H15 | 120.00 |
| N1—C1—C2 | 113.74 (13) | C16—C15—H15 | 120.00 |
| C1—C2—C3 | 122.58 (13) | C11—C16—H16 | 120.00 |
| C2—C3—C4 | 117.35 (13) | C15—C16—H16 | 120.00 |
| C2—C3—C11 | 120.70 (13) | N1—C17—H17A | 110.00 |
| C4—C3—C11 | 121.94 (12) | N1—C17—H17B | 110.00 |
| N2—C4—C3 | 121.88 (13) | C18—C17—H17A | 110.00 |
| N2—C4—C5 | 114.25 (12) | C18—C17—H17B | 110.00 |
| C3—C4—C5 | 123.87 (13) | H17A—C17—H17B | 108.00 |
| C4—C5—C6 | 121.83 (13) | C17—C18—H18A | 109.00 |
| C4—C5—C10 | 119.70 (13) | C17—C18—H18B | 109.00 |
| C6—C5—C10 | 118.46 (14) | C19—C18—H18A | 109.00 |
| C5—C6—C7 | 120.37 (15) | C19—C18—H18B | 109.00 |
| C6—C7—C8 | 120.49 (16) | H18A—C18—H18B | 108.00 |
| C7—C8—C9 | 119.50 (17) | N3—C20—H20A | 110.00 |
| C8—C9—C10 | 120.06 (16) | N3—C20—H20B | 110.00 |
| C5—C10—C9 | 121.11 (15) | C21—C20—H20A | 110.00 |
| C3—C11—C12 | 120.00 (13) | C21—C20—H20B | 110.00 |
| C3—C11—C16 | 121.25 (12) | H20A—C20—H20B | 108.00 |
| C12—C11—C16 | 118.73 (14) | N4—C21—H21A | 109.00 |
| C11—C12—C13 | 120.49 (15) | N4—C21—H21B | 109.00 |
| C12—C13—C14 | 120.02 (16) | C20—C21—H21A | 109.00 |
| C13—C14—C15 | 120.12 (16) | C20—C21—H21B | 109.00 |
| C14—C15—C16 | 120.13 (16) | H21A—C21—H21B | 108.00 |
| C11—C16—C15 | 120.50 (14) | N4—C22—H22A | 109.00 |
| N1—C17—C18 | 110.48 (12) | N4—C22—H22B | 109.00 |
| C17—C18—C19 | 111.68 (12) | C23—C22—H22A | 109.00 |
| O2—C19—N3 | 122.30 (14) | C23—C22—H22B | 109.00 |
| O2—C19—C18 | 120.13 (14) | H22A—C22—H22B | 108.00 |
| N3—C19—C18 | 117.52 (13) | N3—C23—H23A | 110.00 |
| N3—C20—C21 | 110.24 (13) | N3—C23—H23B | 110.00 |
| N4—C21—C20 | 111.18 (13) | C22—C23—H23A | 110.00 |
| N4—C22—C23 | 111.52 (14) | C22—C23—H23B | 110.00 |
| N3—C23—C22 | 110.54 (14) | H23A—C23—H23B | 108.00 |
| N4—C24—C25 | 115.53 (14) | N4—C24—H24A | 108.00 |
| C24—C25—C26 | 123.48 (15) | N4—C24—H24B | 108.00 |
| C24—C25—C30 | 118.26 (15) | C25—C24—H24A | 108.00 |
| C26—C25—C30 | 118.20 (16) | C25—C24—H24B | 108.00 |
| C25—C26—C27 | 120.65 (16) | H24A—C24—H24B | 107.00 |
| C26—C27—C28 | 120.36 (19) | C25—C26—H26 | 120.00 |
| C27—C28—C29 | 119.52 (19) | C27—C26—H26 | 120.00 |
| C28—C29—C30 | 120.14 (18) | C26—C27—H27 | 120.00 |
| C25—C30—C29 | 121.12 (17) | C28—C27—H27 | 120.00 |
| C1—C2—H2 | 119.00 | C27—C28—H28 | 120.00 |
| C3—C2—H2 | 119.00 | C29—C28—H28 | 120.00 |
| C5—C6—H6 | 120.00 | C28—C29—H29 | 120.00 |
| C7—C6—H6 | 120.00 | C30—C29—H29 | 120.00 |
| C6—C7—H7 | 120.00 | C25—C30—H30 | 119.00 |
| C8—C7—H7 | 120.00 | C29—C30—H30 | 119.00 |
| C7—C8—H8 | 120.00 | ||
| C1—N1—N2—C4 | −1.0 (2) | C3—C4—C5—C10 | 126.30 (15) |
| C17—N1—N2—C4 | 175.45 (13) | N2—C4—C5—C10 | −54.31 (18) |
| N2—N1—C1—O1 | −177.67 (14) | N2—C4—C5—C6 | 124.74 (15) |
| C17—N1—C1—O1 | 6.0 (2) | C3—C4—C5—C6 | −54.7 (2) |
| N2—N1—C1—C2 | 2.7 (2) | C6—C5—C10—C9 | 0.4 (2) |
| C17—N1—C1—C2 | −173.61 (13) | C10—C5—C6—C7 | 0.2 (2) |
| N2—N1—C17—C18 | −107.77 (14) | C4—C5—C10—C9 | 179.48 (15) |
| C1—N1—C17—C18 | 68.93 (17) | C4—C5—C6—C7 | −178.86 (14) |
| N1—N2—C4—C3 | −1.5 (2) | C5—C6—C7—C8 | −0.9 (3) |
| N1—N2—C4—C5 | 179.11 (12) | C6—C7—C8—C9 | 1.0 (3) |
| C20—N3—C19—O2 | −0.7 (2) | C7—C8—C9—C10 | −0.4 (3) |
| C23—N3—C19—O2 | 178.20 (16) | C8—C9—C10—C5 | −0.3 (3) |
| C23—N3—C20—C21 | 53.97 (18) | C12—C11—C16—C15 | −1.2 (2) |
| C19—N3—C20—C21 | −127.01 (15) | C3—C11—C16—C15 | −179.40 (14) |
| C23—N3—C19—C18 | −4.4 (2) | C3—C11—C12—C13 | 179.70 (15) |
| C19—N3—C23—C22 | 127.24 (17) | C16—C11—C12—C13 | 1.5 (2) |
| C20—N3—C19—C18 | 176.74 (14) | C11—C12—C13—C14 | −0.6 (3) |
| C20—N3—C23—C22 | −53.8 (2) | C12—C13—C14—C15 | −0.6 (3) |
| C24—N4—C21—C20 | 179.51 (14) | C13—C14—C15—C16 | 0.9 (3) |
| C21—N4—C22—C23 | −58.98 (19) | C14—C15—C16—C11 | 0.0 (3) |
| C22—N4—C21—C20 | 59.10 (17) | N1—C17—C18—C19 | 167.27 (13) |
| C24—N4—C22—C23 | 178.24 (16) | C17—C18—C19—N3 | 164.55 (14) |
| C22—N4—C24—C25 | −170.09 (16) | C17—C18—C19—O2 | −18.0 (2) |
| C21—N4—C24—C25 | 69.35 (19) | N3—C20—C21—N4 | −56.76 (18) |
| N1—C1—C2—C3 | −2.0 (2) | N4—C22—C23—N3 | 56.5 (2) |
| O1—C1—C2—C3 | 178.38 (15) | N4—C24—C25—C26 | 22.2 (2) |
| C1—C2—C3—C11 | 178.92 (13) | N4—C24—C25—C30 | −160.63 (16) |
| C1—C2—C3—C4 | −0.1 (2) | C24—C25—C26—C27 | 178.65 (17) |
| C11—C3—C4—C5 | 2.3 (2) | C30—C25—C26—C27 | 1.4 (3) |
| C2—C3—C4—C5 | −178.64 (13) | C24—C25—C30—C29 | −178.55 (18) |
| C2—C3—C11—C12 | −49.2 (2) | C26—C25—C30—C29 | −1.2 (3) |
| C2—C3—C11—C16 | 128.97 (15) | C25—C26—C27—C28 | −0.4 (3) |
| C4—C3—C11—C12 | 129.80 (15) | C26—C27—C28—C29 | −1.0 (3) |
| C4—C3—C11—C16 | −52.04 (19) | C27—C28—C29—C30 | 1.2 (3) |
| C11—C3—C4—N2 | −177.02 (13) | C28—C29—C30—C25 | −0.1 (3) |
| C2—C3—C4—N2 | 2.0 (2) |
Symmetry codes: (i) x, −y+3/2, z−1/2; (ii) −x+2, −y+1, −z+1; (iii) x, −y+3/2, z+1/2; (iv) x, −y+1/2, z+1/2; (v) x, y+1, z; (vi) −x+1, y+1/2, −z+3/2; (vii) −x+1, y−1/2, −z+3/2; (viii) −x+1, −y+2, −z+2; (ix) −x+1, −y+1, −z+1; (x) −x+2, −y, −z+1; (xi) −x+2, y+1/2, −z+1/2; (xii) −x+2, y−1/2, −z+1/2; (xiii) x, −y+1/2, z−1/2; (xiv) x, y−1, z.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C2—H2···O2iii | 0.93 | 2.47 | 3.3470 (19) | 157 |
| C18—H18B···O1 | 0.97 | 2.56 | 3.0755 (18) | 113 |
| C20—H20B···O2 | 0.97 | 2.35 | 2.759 (2) | 105 |
Symmetry codes: (iii) x, −y+3/2, z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: TK2267).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst.32, 115–119.
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc.97, 1354–1358.
- Doğruer, D. S., Ünlü, S., Küpeli, E., Banoğlu, E. & Şahin, M. F. (2007). Turk. J. Pharm. Sci.4, 57–70.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Stoe & Cie (2002). X-AREA and X-RED32 Stoe & Cie, Darmstadt, Germany.
- Swenson, D. C., Yamamoto, M. & Burton, D. J. (1997). Acta Cryst. C53, 1445–1447.
- Yüksektepe, Ç., Saraçoğlu, H., Koca, M., Çukurovali, A. & Çalışkan, N. (2004). Acta Cryst. C60, o509–o510. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808013159/tk2267sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808013159/tk2267Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




