Abstract
The title compound, (1α,3α,6α,14α,15α,16β)-3,8,13,14,15-pentahydroxy-1,6,16-trimethoxy-4-methoxymethyl-20-methylaconitan-8,14-diyl 8-acetate 14-benzoate, C33H45NO11, a C19 diterpenoid alkaloid, obtained from the roots of Aconitum kusnezoffii, has been crystallographically characterized in this study. Rings A, B and E have chair conformations, rings C and F display envelope conformations, and ring D adopts a boat conformation. There are inter- and intramolecular O—H⋯O hydrogen bonds, the latter resulting in the formation of a non-planar seven-membered ring. The intermolecular interactions link the molecules into a two-dimensional network.
Related literature
For general background, see: Hikino et al. (1980 ▶); Li et al. (1997 ▶); Mitamura et al. (2002 ▶); Saito et al. (1982 ▶); For ring conformation details, see: Codding (1982 ▶); De Camp & Pelletier (1977 ▶); Parvez et al. (1999 ▶); Pelletier et al. (1982 ▶). For related literature, see: Pelletier & Djarmati (1976 ▶); Tsuda & Marion (1963 ▶); Zhapova et al. (1986 ▶).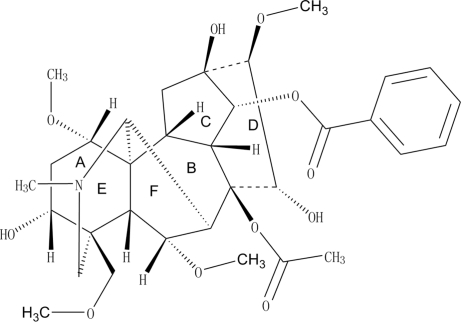
Experimental
Crystal data
C33H45NO11
M r = 631.70
Orthorhombic,
a = 12.6820 (6) Å
b = 15.3848 (7) Å
c = 15.6110 (7) Å
V = 3045.9 (2) Å3
Z = 4
Mo Kα radiation
μ = 0.10 mm−1
T = 173 (2) K
0.46 × 0.35 × 0.12 mm
Data collection
Bruker SMART 1000 CCD diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 2003 ▶) T min = 0.954, T max = 0.988
18319 measured reflections
3713 independent reflections
3026 reflections with I > 2σ(I)
R int = 0.042
Refinement
R[F 2 > 2σ(F 2)] = 0.038
wR(F 2) = 0.081
S = 1.07
3713 reflections
418 parameters
2 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.21 e Å−3
Δρmin = −0.22 e Å−3
Data collection: SMART (Bruker, 2001 ▶); cell refinement: SAINT-Plus (Bruker, 2003 ▶); data reduction: SAINT-Plus; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808013147/wn2256sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808013147/wn2256Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O10—H10A⋯O2 | 0.84 | 2.11 | 2.788 (3) | 138 |
| O7—H7⋯O8 | 0.84 | 2.04 | 2.560 (3) | 120 |
| O4—H4⋯O11i | 0.84 | 2.20 | 3.018 (3) | 163 |
Symmetry code: (i) 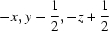 .
.
Acknowledgments
The authors thank Guangdong Provincial Natural Science Foundation of China (No. 04300531) for financial assistance.
supplementary crystallographic information
Comment
As an important Chinese herbal medicine belonging to the genus Aconitum, A. kusnzoffii has been therapeutically used to treat rheumatic pain, paralysis due to stroke, rheumatoid arthritis and some other inflammations. Mesaconitine, a C19 diterpenoid alkaloid, is pharmacologically one of the most active components obtained from the roots of A. kusnezoffii (Li et al., 1997). Mesaconitine has been reported to have an anti-inflammatory activity (Hikino et al.,1980; Saito et al., 1982), and the vasorelaxant effect of mesaconitine may contribute to the therapeutical effectiveness on persons with a weak constitution and poor metabolism, by improving peripheral blood circulation (Mitamura et al., 2002). The three-dimensional structure of most biologically active molecules plays a role in governing their interactions and activities. It is important to obtain information on the mode of action and selectivity of mesaconitine so that it can be used safely and efficiently. Many X-ray crystal structure determinations of C19 diterpenoid alkaloids have been reported, such as pseudaconitine, delphinine (Parvez et al., 1999; Pelletier et al., 1982.). However, the crystal structure of mesaconitine has not been reported. In view of this, the crystal structure determination of the title compound was carried out and the results are presented here.
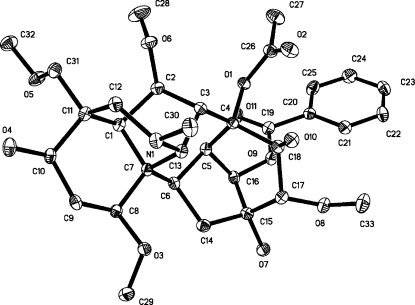
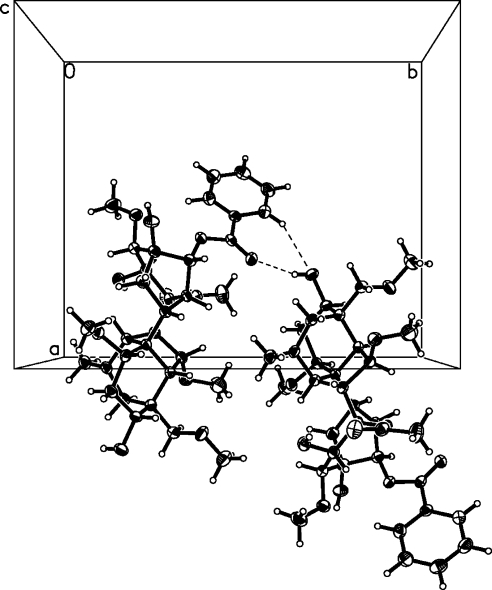
The structure of mesaconitine is similar to that of aconitine (Codding, 1982). The only difference between aconitine and mesaconitine is a methylene group at the tertiary nitrogen atom. The bond lengths and angles in the title compound are in good agreement with expected values. In the molecule of the title compound, (Fig. 1), rings A, B and E have a chair conformation, rings C and F display an envelope conformation, ring D adopts a boat conformation. The packing of the title compound is shown in Fig. 2. In the crystal structure, there are inter- and intramolecular O—H···O hydrogen bonds. The former link the molecules into a two-dimensional network, while the latter results in the formation of a non-planar seven-membered ring. These intramolecular hydrogen bonds may be effective in the stabilization of the structure.
Experimental
The title compound was isolated from the roots of A. kusnezoffii according to the literature procedure of Li et al. (1997) and crystals of X-ray quality were grown from methanol at room temperature by slow evaporation.
Refinement
The H atom attached to C2 was located and refined freely [C—H = 0.97 (2) Å]. Other H atoms were included in the refinement at idealized positions and refin ed as riding, with C—H = 0.95 (aromatic), 0.98 (CH2), 1.00 (CH), O—H = 0.84 Å. Uiso(H) = xUeq(carrier atom), where x = 1.5 for O and methyl, 1.2 for all other H atoms. In the absence of significant anomalous scattering effects, Friedel pairs were merged. The absolute configuration was assigned on the basis of the related literature (Pelletier & Djarmati, 1976; Tsuda & Marion, 1963; Zhapova et al., 1986).
Figures
Fig. 1.
A view of the structure of the title compound, showing the atom-numbering scheme. Displacement ellipsoids are drawn at the 30% probability level. H atoms have been omitted for clarity.
Fig. 2.
The packing of molecules of the title compound, viewed down the c axis. Dashed lines indicate hydrogen bonds.
Crystal data
| C33H45NO11 | F000 = 1352 |
| Mr = 631.70 | Dx = 1.378 Mg m−3 |
| Orthorhombic, P212121 | Mo Kα radiation λ = 0.71073 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 6314 reflections |
| a = 12.6820 (6) Å | θ = 2.5–26.8º |
| b = 15.3848 (7) Å | µ = 0.10 mm−1 |
| c = 15.6110 (7) Å | T = 173 (2) K |
| V = 3045.9 (2) Å3 | Block, colorless |
| Z = 4 | 0.46 × 0.35 × 0.12 mm |
Data collection
| Bruker SMART 1000 CCD diffractometer | 3713 independent reflections |
| Radiation source: fine-focus sealed tube | 3026 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.042 |
| T = 173(2) K | θmax = 27.0º |
| ω scans | θmin = 1.9º |
| Absorption correction: multi-scan(SADABS; Sheldrick, 2003) | h = −16→15 |
| Tmin = 0.954, Tmax = 0.988 | k = −16→19 |
| 18319 measured reflections | l = −16→19 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.038 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.081 | w = 1/[σ2(Fo2) + (0.0245P)2 + 1.2755P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.07 | (Δ/σ)max = 0.001 |
| 3713 reflections | Δρmax = 0.21 e Å−3 |
| 418 parameters | Δρmin = −0.22 e Å−3 |
| 2 restraints | Extinction correction: none |
| Primary atom site location: structure-invariant direct methods |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | −0.0391 (2) | 0.81198 (18) | 0.21029 (19) | 0.0220 (6) | |
| H1 | −0.0477 | 0.8647 | 0.1734 | 0.026* | |
| C2 | 0.0052 (2) | 0.8377 (2) | 0.3007 (2) | 0.0246 (7) | |
| C3 | 0.0859 (2) | 0.76493 (18) | 0.32284 (18) | 0.0218 (6) | |
| H3 | 0.0727 | 0.7412 | 0.3815 | 0.026* | |
| C4 | 0.1968 (2) | 0.80413 (18) | 0.31663 (18) | 0.0209 (6) | |
| C5 | 0.2045 (2) | 0.85444 (18) | 0.23165 (18) | 0.0219 (6) | |
| H5 | 0.1701 | 0.9126 | 0.2373 | 0.026* | |
| C6 | 0.1544 (2) | 0.80248 (19) | 0.15592 (18) | 0.0220 (6) | |
| H6 | 0.1380 | 0.8456 | 0.1099 | 0.026* | |
| C7 | 0.0505 (2) | 0.75172 (18) | 0.17449 (18) | 0.0207 (6) | |
| C8 | 0.0175 (2) | 0.70445 (19) | 0.09137 (19) | 0.0226 (6) | |
| H8 | 0.0235 | 0.7470 | 0.0432 | 0.027* | |
| C9 | −0.0936 (2) | 0.6672 (2) | 0.0894 (2) | 0.0256 (7) | |
| H9A | −0.1126 | 0.6534 | 0.0294 | 0.031* | |
| H9B | −0.0949 | 0.6123 | 0.1225 | 0.031* | |
| C10 | −0.1744 (2) | 0.72861 (19) | 0.12591 (19) | 0.0241 (6) | |
| H10 | −0.1762 | 0.7807 | 0.0877 | 0.029* | |
| C11 | −0.1439 (2) | 0.76086 (19) | 0.21596 (19) | 0.0236 (6) | |
| C12 | −0.1281 (2) | 0.68527 (19) | 0.2794 (2) | 0.0254 (7) | |
| H12A | −0.1312 | 0.7082 | 0.3386 | 0.030* | |
| H12B | −0.1866 | 0.6432 | 0.2724 | 0.030* | |
| C13 | 0.0663 (2) | 0.69431 (18) | 0.25371 (18) | 0.0226 (6) | |
| H13 | 0.1306 | 0.6574 | 0.2466 | 0.027* | |
| C14 | 0.2486 (2) | 0.7463 (2) | 0.1230 (2) | 0.0275 (7) | |
| H14A | 0.2335 | 0.6837 | 0.1313 | 0.033* | |
| H14B | 0.2606 | 0.7570 | 0.0612 | 0.033* | |
| C15 | 0.3455 (2) | 0.7728 (2) | 0.17473 (18) | 0.0247 (6) | |
| C16 | 0.3180 (2) | 0.86471 (19) | 0.20170 (19) | 0.0242 (6) | |
| H16 | 0.3214 | 0.9048 | 0.1513 | 0.029* | |
| C17 | 0.3584 (2) | 0.71420 (19) | 0.25396 (19) | 0.0253 (7) | |
| H17 | 0.3397 | 0.6533 | 0.2376 | 0.030* | |
| C18 | 0.2910 (2) | 0.74092 (19) | 0.33247 (18) | 0.0240 (6) | |
| H18 | 0.3398 | 0.7717 | 0.3726 | 0.029* | |
| C19 | 0.3869 (2) | 0.97097 (19) | 0.3007 (2) | 0.0257 (7) | |
| C20 | 0.4690 (2) | 0.98015 (19) | 0.3682 (2) | 0.0250 (6) | |
| C21 | 0.5301 (3) | 0.9093 (2) | 0.3930 (2) | 0.0299 (7) | |
| H21 | 0.5187 | 0.8540 | 0.3677 | 0.036* | |
| C22 | 0.6072 (3) | 0.9198 (2) | 0.4544 (2) | 0.0356 (8) | |
| H22 | 0.6489 | 0.8715 | 0.4713 | 0.043* | |
| C23 | 0.6240 (3) | 0.9998 (2) | 0.4915 (2) | 0.0365 (8) | |
| H23 | 0.6767 | 1.0065 | 0.5342 | 0.044* | |
| C24 | 0.5643 (3) | 1.0702 (2) | 0.4664 (2) | 0.0401 (9) | |
| H24 | 0.5768 | 1.1255 | 0.4914 | 0.048* | |
| C25 | 0.4865 (3) | 1.0608 (2) | 0.4053 (2) | 0.0362 (8) | |
| H25 | 0.4451 | 1.1094 | 0.3887 | 0.043* | |
| C26 | 0.2065 (2) | 0.8593 (2) | 0.46489 (19) | 0.0284 (7) | |
| C27 | 0.2139 (3) | 0.9414 (2) | 0.5150 (2) | 0.0452 (9) | |
| H27A | 0.1483 | 0.9745 | 0.5086 | 0.068* | |
| H27B | 0.2731 | 0.9762 | 0.4937 | 0.068* | |
| H27C | 0.2251 | 0.9277 | 0.5756 | 0.068* | |
| C28 | −0.0892 (3) | 0.9341 (3) | 0.3905 (3) | 0.0584 (12) | |
| H28A | −0.0235 | 0.9626 | 0.4079 | 0.088* | |
| H28B | −0.1399 | 0.9362 | 0.4378 | 0.088* | |
| H28C | −0.1187 | 0.9641 | 0.3406 | 0.088* | |
| C29 | 0.1046 (3) | 0.6183 (2) | −0.0140 (2) | 0.0339 (8) | |
| H29A | 0.0383 | 0.5980 | −0.0393 | 0.051* | |
| H29B | 0.1589 | 0.5735 | −0.0212 | 0.051* | |
| H29C | 0.1272 | 0.6718 | −0.0427 | 0.051* | |
| C30 | −0.0119 (3) | 0.5751 (2) | 0.3337 (2) | 0.0311 (7) | |
| H30A | 0.0555 | 0.5451 | 0.3248 | 0.047* | |
| H30B | −0.0696 | 0.5328 | 0.3315 | 0.047* | |
| H30C | −0.0115 | 0.6038 | 0.3897 | 0.047* | |
| C31 | −0.2345 (2) | 0.81935 (19) | 0.2462 (2) | 0.0284 (7) | |
| H31A | −0.3027 | 0.7887 | 0.2400 | 0.034* | |
| H31B | −0.2249 | 0.8348 | 0.3073 | 0.034* | |
| C32 | −0.3153 (3) | 0.9539 (2) | 0.2150 (2) | 0.0399 (8) | |
| H32A | −0.3834 | 0.9252 | 0.2058 | 0.060* | |
| H32B | −0.3104 | 1.0054 | 0.1783 | 0.060* | |
| H32C | −0.3092 | 0.9714 | 0.2751 | 0.060* | |
| C33 | 0.5015 (3) | 0.6510 (2) | 0.3335 (2) | 0.0445 (9) | |
| H33A | 0.4696 | 0.6603 | 0.3899 | 0.067* | |
| H33B | 0.5785 | 0.6529 | 0.3386 | 0.067* | |
| H33C | 0.4800 | 0.5941 | 0.3113 | 0.067* | |
| N1 | −0.02662 (19) | 0.63950 (15) | 0.26699 (16) | 0.0239 (5) | |
| O1 | 0.20493 (15) | 0.87584 (12) | 0.38022 (12) | 0.0241 (4) | |
| O2 | 0.2048 (2) | 0.78785 (15) | 0.49642 (14) | 0.0402 (6) | |
| O3 | 0.08940 (16) | 0.63488 (13) | 0.07442 (13) | 0.0263 (5) | |
| O4 | −0.27732 (15) | 0.68993 (14) | 0.12299 (15) | 0.0316 (5) | |
| H4 | −0.2766 | 0.6427 | 0.1499 | 0.047* | |
| O5 | −0.23341 (17) | 0.89599 (14) | 0.19455 (15) | 0.0349 (6) | |
| O6 | −0.06838 (16) | 0.84649 (14) | 0.36913 (14) | 0.0328 (5) | |
| O7 | 0.43716 (15) | 0.76827 (15) | 0.12268 (13) | 0.0309 (5) | |
| H7 | 0.4897 | 0.7566 | 0.1534 | 0.046* | |
| O8 | 0.46752 (16) | 0.71676 (14) | 0.27663 (14) | 0.0305 (5) | |
| O9 | 0.39220 (15) | 0.89139 (13) | 0.26615 (14) | 0.0257 (5) | |
| O10 | 0.25977 (17) | 0.66318 (13) | 0.37478 (14) | 0.0290 (5) | |
| H10A | 0.2344 | 0.6755 | 0.4230 | 0.044* | |
| O11 | 0.32507 (17) | 1.02599 (13) | 0.27820 (16) | 0.0349 (5) | |
| H2 | 0.037 (2) | 0.8949 (9) | 0.297 (2) | 0.042* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0223 (14) | 0.0175 (14) | 0.0261 (15) | −0.0007 (12) | 0.0000 (12) | 0.0027 (12) |
| C2 | 0.0242 (15) | 0.0225 (15) | 0.0272 (16) | −0.0032 (12) | 0.0023 (13) | −0.0019 (13) |
| C3 | 0.0241 (14) | 0.0210 (14) | 0.0204 (14) | −0.0024 (12) | 0.0003 (12) | 0.0007 (12) |
| C4 | 0.0231 (14) | 0.0190 (14) | 0.0207 (14) | −0.0019 (12) | −0.0004 (12) | −0.0012 (12) |
| C5 | 0.0216 (14) | 0.0180 (14) | 0.0261 (15) | −0.0037 (12) | −0.0013 (12) | 0.0012 (12) |
| C6 | 0.0224 (14) | 0.0220 (15) | 0.0215 (15) | −0.0024 (12) | −0.0015 (12) | 0.0018 (12) |
| C7 | 0.0203 (14) | 0.0180 (14) | 0.0239 (15) | −0.0014 (11) | −0.0029 (12) | −0.0007 (12) |
| C8 | 0.0251 (15) | 0.0201 (15) | 0.0227 (14) | 0.0031 (12) | −0.0018 (12) | 0.0023 (12) |
| C9 | 0.0263 (15) | 0.0254 (16) | 0.0251 (16) | −0.0045 (13) | −0.0068 (13) | −0.0003 (13) |
| C10 | 0.0192 (14) | 0.0249 (15) | 0.0283 (15) | −0.0032 (12) | −0.0032 (12) | 0.0052 (13) |
| C11 | 0.0217 (14) | 0.0211 (15) | 0.0281 (15) | −0.0004 (12) | −0.0001 (12) | 0.0029 (13) |
| C12 | 0.0231 (14) | 0.0248 (15) | 0.0282 (16) | −0.0053 (12) | −0.0001 (13) | 0.0023 (13) |
| C13 | 0.0218 (14) | 0.0198 (14) | 0.0263 (16) | −0.0016 (12) | −0.0013 (12) | 0.0010 (13) |
| C14 | 0.0273 (15) | 0.0290 (16) | 0.0263 (15) | −0.0029 (14) | 0.0031 (13) | −0.0012 (13) |
| C15 | 0.0242 (14) | 0.0255 (15) | 0.0243 (15) | −0.0033 (13) | 0.0022 (12) | 0.0001 (13) |
| C16 | 0.0244 (15) | 0.0248 (15) | 0.0235 (15) | −0.0030 (13) | −0.0028 (12) | 0.0058 (12) |
| C17 | 0.0230 (15) | 0.0217 (15) | 0.0311 (16) | −0.0013 (12) | 0.0002 (13) | −0.0003 (13) |
| C18 | 0.0259 (15) | 0.0225 (15) | 0.0237 (15) | −0.0018 (13) | 0.0000 (13) | 0.0021 (12) |
| C19 | 0.0233 (15) | 0.0203 (15) | 0.0337 (18) | −0.0055 (13) | 0.0016 (13) | 0.0034 (13) |
| C20 | 0.0217 (14) | 0.0266 (15) | 0.0267 (16) | −0.0045 (13) | 0.0035 (13) | 0.0004 (13) |
| C21 | 0.0336 (17) | 0.0256 (16) | 0.0307 (18) | −0.0019 (14) | −0.0003 (14) | 0.0026 (14) |
| C22 | 0.0373 (18) | 0.0350 (19) | 0.0343 (19) | 0.0011 (16) | −0.0072 (15) | 0.0090 (16) |
| C23 | 0.0339 (18) | 0.047 (2) | 0.0284 (18) | −0.0087 (16) | −0.0056 (15) | 0.0040 (16) |
| C24 | 0.0358 (19) | 0.036 (2) | 0.049 (2) | −0.0075 (16) | −0.0028 (17) | −0.0144 (17) |
| C25 | 0.0302 (17) | 0.0269 (17) | 0.051 (2) | 0.0008 (14) | −0.0034 (16) | −0.0027 (16) |
| C26 | 0.0288 (16) | 0.0304 (18) | 0.0260 (16) | −0.0039 (14) | 0.0002 (14) | −0.0031 (14) |
| C27 | 0.064 (3) | 0.035 (2) | 0.036 (2) | −0.0062 (19) | −0.0018 (19) | −0.0077 (16) |
| C28 | 0.043 (2) | 0.050 (2) | 0.082 (3) | −0.0042 (19) | 0.019 (2) | −0.034 (2) |
| C29 | 0.0347 (17) | 0.0335 (18) | 0.0335 (18) | 0.0068 (15) | 0.0028 (15) | −0.0042 (15) |
| C30 | 0.0338 (17) | 0.0234 (16) | 0.0362 (18) | −0.0041 (14) | −0.0038 (15) | 0.0080 (14) |
| C31 | 0.0241 (15) | 0.0279 (16) | 0.0332 (17) | −0.0026 (13) | 0.0009 (13) | 0.0014 (14) |
| C32 | 0.041 (2) | 0.0364 (19) | 0.043 (2) | 0.0109 (16) | 0.0066 (17) | 0.0022 (17) |
| C33 | 0.0345 (19) | 0.045 (2) | 0.054 (2) | 0.0089 (17) | −0.0026 (17) | 0.0134 (19) |
| N1 | 0.0250 (13) | 0.0197 (12) | 0.0269 (13) | −0.0043 (11) | −0.0018 (11) | 0.0047 (11) |
| O1 | 0.0270 (10) | 0.0215 (10) | 0.0238 (11) | −0.0029 (9) | −0.0012 (9) | −0.0031 (9) |
| O2 | 0.0527 (15) | 0.0367 (14) | 0.0313 (12) | −0.0038 (12) | 0.0017 (11) | 0.0005 (11) |
| O3 | 0.0290 (11) | 0.0230 (11) | 0.0270 (11) | 0.0031 (9) | −0.0036 (9) | −0.0029 (9) |
| O4 | 0.0239 (11) | 0.0305 (12) | 0.0405 (13) | −0.0063 (9) | −0.0056 (10) | 0.0057 (11) |
| O5 | 0.0348 (12) | 0.0273 (12) | 0.0424 (14) | 0.0098 (10) | 0.0107 (11) | 0.0074 (10) |
| O6 | 0.0293 (11) | 0.0338 (12) | 0.0354 (12) | −0.0005 (10) | 0.0084 (10) | −0.0081 (11) |
| O7 | 0.0232 (10) | 0.0401 (13) | 0.0293 (11) | −0.0012 (10) | 0.0048 (9) | 0.0014 (11) |
| O8 | 0.0248 (11) | 0.0300 (12) | 0.0367 (12) | 0.0019 (9) | 0.0003 (10) | 0.0067 (10) |
| O9 | 0.0231 (10) | 0.0224 (10) | 0.0318 (12) | −0.0035 (9) | −0.0030 (9) | 0.0003 (9) |
| O10 | 0.0326 (11) | 0.0249 (11) | 0.0297 (12) | −0.0004 (9) | 0.0023 (10) | 0.0080 (10) |
| O11 | 0.0287 (12) | 0.0229 (11) | 0.0532 (15) | 0.0023 (10) | −0.0106 (11) | 0.0011 (11) |
Geometric parameters (Å, °)
| C1—C11 | 1.547 (4) | C18—O10 | 1.422 (3) |
| C1—C7 | 1.570 (4) | C18—H18 | 1.0000 |
| C1—C2 | 1.570 (4) | C19—O11 | 1.206 (3) |
| C1—H1 | 1.0000 | C19—O9 | 1.339 (3) |
| C2—O6 | 1.424 (4) | C19—C20 | 1.489 (4) |
| C2—C3 | 1.556 (4) | C20—C25 | 1.387 (4) |
| C2—H2 | 0.970 (16) | C20—C21 | 1.391 (4) |
| C3—C4 | 1.533 (4) | C21—C22 | 1.379 (4) |
| C3—C13 | 1.551 (4) | C21—H21 | 0.9500 |
| C3—H3 | 1.0000 | C22—C23 | 1.376 (5) |
| C4—O1 | 1.488 (3) | C22—H22 | 0.9500 |
| C4—C5 | 1.539 (4) | C23—C24 | 1.377 (5) |
| C4—C18 | 1.560 (4) | C23—H23 | 0.9500 |
| C5—C16 | 1.522 (4) | C24—C25 | 1.380 (5) |
| C5—C6 | 1.562 (4) | C24—H24 | 0.9500 |
| C5—H5 | 1.0000 | C25—H25 | 0.9500 |
| C6—C7 | 1.559 (4) | C26—O2 | 1.204 (4) |
| C6—C14 | 1.561 (4) | C26—O1 | 1.346 (4) |
| C6—H6 | 1.0000 | C26—C27 | 1.490 (4) |
| C7—C13 | 1.533 (4) | C27—H27A | 0.9800 |
| C7—C8 | 1.545 (4) | C27—H27B | 0.9800 |
| C8—O3 | 1.431 (3) | C27—H27C | 0.9800 |
| C8—C9 | 1.521 (4) | C28—O6 | 1.413 (4) |
| C8—H8 | 1.0000 | C28—H28A | 0.9800 |
| C9—C10 | 1.506 (4) | C28—H28B | 0.9800 |
| C9—H9A | 0.9900 | C28—H28C | 0.9800 |
| C9—H9B | 0.9900 | C29—O3 | 1.417 (4) |
| C10—O4 | 1.435 (3) | C29—H29A | 0.9800 |
| C10—C11 | 1.540 (4) | C29—H29B | 0.9800 |
| C10—H10 | 1.0000 | C29—H29C | 0.9800 |
| C11—C31 | 1.534 (4) | C30—N1 | 1.449 (4) |
| C11—C12 | 1.540 (4) | C30—H30A | 0.9800 |
| C12—N1 | 1.480 (4) | C30—H30B | 0.9800 |
| C12—H12A | 0.9900 | C30—H30C | 0.9800 |
| C12—H12B | 0.9900 | C31—O5 | 1.429 (4) |
| C13—N1 | 1.464 (4) | C31—H31A | 0.9900 |
| C13—H13 | 1.0000 | C31—H31B | 0.9900 |
| C14—C15 | 1.526 (4) | C32—O5 | 1.405 (4) |
| C14—H14A | 0.9900 | C32—H32A | 0.9800 |
| C14—H14B | 0.9900 | C32—H32B | 0.9800 |
| C15—O7 | 1.420 (3) | C32—H32C | 0.9800 |
| C15—C16 | 1.516 (4) | C33—O8 | 1.413 (4) |
| C15—C17 | 1.539 (4) | C33—H33A | 0.9800 |
| C16—O9 | 1.437 (3) | C33—H33B | 0.9800 |
| C16—H16 | 1.0000 | C33—H33C | 0.9800 |
| C17—O8 | 1.429 (3) | O4—H4 | 0.8400 |
| C17—C18 | 1.550 (4) | O7—H7 | 0.8400 |
| C17—H17 | 1.0000 | O10—H10A | 0.8400 |
| C11—C1—C7 | 110.0 (2) | O9—C16—H16 | 110.3 |
| C11—C1—C2 | 112.6 (2) | C15—C16—H16 | 110.3 |
| C7—C1—C2 | 102.1 (2) | C5—C16—H16 | 110.3 |
| C11—C1—H1 | 110.6 | O8—C17—C15 | 106.6 (2) |
| C7—C1—H1 | 110.6 | O8—C17—C18 | 109.3 (2) |
| C2—C1—H1 | 110.6 | C15—C17—C18 | 114.9 (2) |
| O6—C2—C3 | 109.5 (2) | O8—C17—H17 | 108.6 |
| O6—C2—C1 | 117.6 (2) | C15—C17—H17 | 108.6 |
| C3—C2—C1 | 104.7 (2) | C18—C17—H17 | 108.6 |
| O6—C2—H2 | 104 (2) | O10—C18—C17 | 107.3 (2) |
| C3—C2—H2 | 113 (2) | O10—C18—C4 | 112.6 (2) |
| C1—C2—H2 | 109 (2) | C17—C18—C4 | 117.6 (2) |
| C4—C3—C13 | 112.2 (2) | O10—C18—H18 | 106.2 |
| C4—C3—C2 | 107.8 (2) | C17—C18—H18 | 106.2 |
| C13—C3—C2 | 104.1 (2) | C4—C18—H18 | 106.2 |
| C4—C3—H3 | 110.8 | O11—C19—O9 | 123.9 (3) |
| C13—C3—H3 | 110.8 | O11—C19—C20 | 126.5 (3) |
| C2—C3—H3 | 110.8 | O9—C19—C20 | 109.7 (2) |
| O1—C4—C3 | 108.3 (2) | C25—C20—C21 | 119.7 (3) |
| O1—C4—C5 | 101.4 (2) | C25—C20—C19 | 119.5 (3) |
| C3—C4—C5 | 108.1 (2) | C21—C20—C19 | 120.8 (3) |
| O1—C4—C18 | 107.7 (2) | C22—C21—C20 | 119.7 (3) |
| C3—C4—C18 | 116.6 (2) | C22—C21—H21 | 120.1 |
| C5—C4—C18 | 113.7 (2) | C20—C21—H21 | 120.1 |
| C16—C5—C4 | 112.2 (2) | C23—C22—C21 | 120.5 (3) |
| C16—C5—C6 | 101.9 (2) | C23—C22—H22 | 119.8 |
| C4—C5—C6 | 111.7 (2) | C21—C22—H22 | 119.8 |
| C16—C5—H5 | 110.3 | C22—C23—C24 | 119.9 (3) |
| C4—C5—H5 | 110.3 | C22—C23—H23 | 120.1 |
| C6—C5—H5 | 110.3 | C24—C23—H23 | 120.1 |
| C7—C6—C14 | 115.5 (2) | C23—C24—C25 | 120.4 (3) |
| C7—C6—C5 | 117.3 (2) | C23—C24—H24 | 119.8 |
| C14—C6—C5 | 102.8 (2) | C25—C24—H24 | 119.8 |
| C7—C6—H6 | 106.8 | C24—C25—C20 | 119.8 (3) |
| C14—C6—H6 | 106.8 | C24—C25—H25 | 120.1 |
| C5—C6—H6 | 106.8 | C20—C25—H25 | 120.1 |
| C13—C7—C8 | 116.2 (2) | O2—C26—O1 | 125.0 (3) |
| C13—C7—C6 | 109.1 (2) | O2—C26—C27 | 124.1 (3) |
| C8—C7—C6 | 108.0 (2) | O1—C26—C27 | 110.8 (3) |
| C13—C7—C1 | 98.5 (2) | C26—C27—H27A | 109.5 |
| C8—C7—C1 | 112.4 (2) | C26—C27—H27B | 109.5 |
| C6—C7—C1 | 112.5 (2) | H27A—C27—H27B | 109.5 |
| O3—C8—C9 | 107.7 (2) | C26—C27—H27C | 109.5 |
| O3—C8—C7 | 109.5 (2) | H27A—C27—H27C | 109.5 |
| C9—C8—C7 | 116.4 (2) | H27B—C27—H27C | 109.5 |
| O3—C8—H8 | 107.6 | O6—C28—H28A | 109.5 |
| C9—C8—H8 | 107.6 | O6—C28—H28B | 109.5 |
| C7—C8—H8 | 107.6 | H28A—C28—H28B | 109.5 |
| C10—C9—C8 | 112.7 (2) | O6—C28—H28C | 109.5 |
| C10—C9—H9A | 109.0 | H28A—C28—H28C | 109.5 |
| C8—C9—H9A | 109.0 | H28B—C28—H28C | 109.5 |
| C10—C9—H9B | 109.0 | O3—C29—H29A | 109.5 |
| C8—C9—H9B | 109.0 | O3—C29—H29B | 109.5 |
| H9A—C9—H9B | 107.8 | H29A—C29—H29B | 109.5 |
| O4—C10—C9 | 110.3 (2) | O3—C29—H29C | 109.5 |
| O4—C10—C11 | 113.0 (2) | H29A—C29—H29C | 109.5 |
| C9—C10—C11 | 112.1 (2) | H29B—C29—H29C | 109.5 |
| O4—C10—H10 | 107.0 | N1—C30—H30A | 109.5 |
| C9—C10—H10 | 107.0 | N1—C30—H30B | 109.5 |
| C11—C10—H10 | 107.0 | H30A—C30—H30B | 109.5 |
| C31—C11—C10 | 106.4 (2) | N1—C30—H30C | 109.5 |
| C31—C11—C12 | 110.0 (2) | H30A—C30—H30C | 109.5 |
| C10—C11—C12 | 112.1 (2) | H30B—C30—H30C | 109.5 |
| C31—C11—C1 | 111.3 (2) | O5—C31—C11 | 107.6 (2) |
| C10—C11—C1 | 109.1 (2) | O5—C31—H31A | 110.2 |
| C12—C11—C1 | 108.0 (2) | C11—C31—H31A | 110.2 |
| N1—C12—C11 | 112.9 (2) | O5—C31—H31B | 110.2 |
| N1—C12—H12A | 109.0 | C11—C31—H31B | 110.2 |
| C11—C12—H12A | 109.0 | H31A—C31—H31B | 108.5 |
| N1—C12—H12B | 109.0 | O5—C32—H32A | 109.5 |
| C11—C12—H12B | 109.0 | O5—C32—H32B | 109.5 |
| H12A—C12—H12B | 107.8 | H32A—C32—H32B | 109.5 |
| N1—C13—C7 | 109.9 (2) | O5—C32—H32C | 109.5 |
| N1—C13—C3 | 115.7 (2) | H32A—C32—H32C | 109.5 |
| C7—C13—C3 | 100.3 (2) | H32B—C32—H32C | 109.5 |
| N1—C13—H13 | 110.2 | O8—C33—H33A | 109.5 |
| C7—C13—H13 | 110.2 | O8—C33—H33B | 109.5 |
| C3—C13—H13 | 110.2 | H33A—C33—H33B | 109.5 |
| C15—C14—C6 | 107.1 (2) | O8—C33—H33C | 109.5 |
| C15—C14—H14A | 110.3 | H33A—C33—H33C | 109.5 |
| C6—C14—H14A | 110.3 | H33B—C33—H33C | 109.5 |
| C15—C14—H14B | 110.3 | C30—N1—C13 | 113.1 (2) |
| C6—C14—H14B | 110.3 | C30—N1—C12 | 110.1 (2) |
| H14A—C14—H14B | 108.5 | C13—N1—C12 | 116.4 (2) |
| O7—C15—C16 | 113.1 (2) | C26—O1—C4 | 121.0 (2) |
| O7—C15—C14 | 110.1 (2) | C29—O3—C8 | 113.7 (2) |
| C16—C15—C14 | 102.2 (2) | C10—O4—H4 | 109.5 |
| O7—C15—C17 | 110.1 (2) | C32—O5—C31 | 112.8 (2) |
| C16—C15—C17 | 110.4 (2) | C28—O6—C2 | 112.9 (3) |
| C14—C15—C17 | 110.8 (2) | C15—O7—H7 | 109.5 |
| O9—C16—C15 | 108.1 (2) | C33—O8—C17 | 115.5 (2) |
| O9—C16—C5 | 115.7 (2) | C19—O9—C16 | 120.7 (2) |
| C15—C16—C5 | 101.9 (2) | C18—O10—H10A | 109.5 |
| C11—C1—C2—O6 | −26.0 (4) | C2—C3—C13—C7 | 40.7 (3) |
| C7—C1—C2—O6 | −143.9 (2) | C7—C6—C14—C15 | −133.1 (2) |
| C11—C1—C2—C3 | 95.8 (3) | C5—C6—C14—C15 | −4.0 (3) |
| C7—C1—C2—C3 | −22.2 (3) | C6—C14—C15—O7 | −145.6 (2) |
| O6—C2—C3—C4 | −124.5 (2) | C6—C14—C15—C16 | −25.2 (3) |
| C1—C2—C3—C4 | 108.5 (2) | C6—C14—C15—C17 | 92.4 (3) |
| O6—C2—C3—C13 | 116.1 (2) | O7—C15—C16—O9 | −73.8 (3) |
| C1—C2—C3—C13 | −10.9 (3) | C14—C15—C16—O9 | 167.8 (2) |
| C13—C3—C4—O1 | 175.3 (2) | C17—C15—C16—O9 | 50.0 (3) |
| C2—C3—C4—O1 | 61.2 (3) | O7—C15—C16—C5 | 163.8 (2) |
| C13—C3—C4—C5 | 66.2 (3) | C14—C15—C16—C5 | 45.5 (3) |
| C2—C3—C4—C5 | −47.9 (3) | C17—C15—C16—C5 | −72.4 (3) |
| C13—C3—C4—C18 | −63.2 (3) | C4—C5—C16—O9 | −45.8 (3) |
| C2—C3—C4—C18 | −177.4 (2) | C6—C5—C16—O9 | −165.3 (2) |
| O1—C4—C5—C16 | 89.2 (3) | C4—C5—C16—C15 | 71.2 (3) |
| C3—C4—C5—C16 | −157.1 (2) | C6—C5—C16—C15 | −48.4 (3) |
| C18—C4—C5—C16 | −26.0 (3) | O7—C15—C17—O8 | 33.8 (3) |
| O1—C4—C5—C6 | −157.2 (2) | C16—C15—C17—O8 | −91.8 (3) |
| C3—C4—C5—C6 | −43.5 (3) | C14—C15—C17—O8 | 155.8 (2) |
| C18—C4—C5—C6 | 87.6 (3) | O7—C15—C17—C18 | 155.1 (2) |
| C16—C5—C6—C7 | 159.5 (2) | C16—C15—C17—C18 | 29.5 (3) |
| C4—C5—C6—C7 | 39.7 (3) | C14—C15—C17—C18 | −82.9 (3) |
| C16—C5—C6—C14 | 31.6 (3) | O8—C17—C18—O10 | −95.0 (3) |
| C4—C5—C6—C14 | −88.3 (3) | C15—C17—C18—O10 | 145.2 (2) |
| C14—C6—C7—C13 | 69.8 (3) | O8—C17—C18—C4 | 136.9 (3) |
| C5—C6—C7—C13 | −51.7 (3) | C15—C17—C18—C4 | 17.0 (4) |
| C14—C6—C7—C8 | −57.4 (3) | O1—C4—C18—O10 | 104.2 (3) |
| C5—C6—C7—C8 | −178.9 (2) | C3—C4—C18—O10 | −17.5 (3) |
| C14—C6—C7—C1 | 178.0 (2) | C5—C4—C18—O10 | −144.3 (2) |
| C5—C6—C7—C1 | 56.5 (3) | O1—C4—C18—C17 | −130.2 (2) |
| C11—C1—C7—C13 | −72.6 (3) | C3—C4—C18—C17 | 108.0 (3) |
| C2—C1—C7—C13 | 47.1 (2) | C5—C4—C18—C17 | −18.7 (3) |
| C11—C1—C7—C8 | 50.4 (3) | O11—C19—C20—C25 | −6.9 (5) |
| C2—C1—C7—C8 | 170.1 (2) | O9—C19—C20—C25 | 172.2 (3) |
| C11—C1—C7—C6 | 172.5 (2) | O11—C19—C20—C21 | 174.7 (3) |
| C2—C1—C7—C6 | −67.8 (3) | O9—C19—C20—C21 | −6.2 (4) |
| C13—C7—C8—O3 | −52.6 (3) | C25—C20—C21—C22 | 0.2 (5) |
| C6—C7—C8—O3 | 70.3 (3) | C19—C20—C21—C22 | 178.6 (3) |
| C1—C7—C8—O3 | −165.0 (2) | C20—C21—C22—C23 | 0.1 (5) |
| C13—C7—C8—C9 | 69.9 (3) | C21—C22—C23—C24 | −0.7 (5) |
| C6—C7—C8—C9 | −167.2 (2) | C22—C23—C24—C25 | 0.9 (5) |
| C1—C7—C8—C9 | −42.5 (3) | C23—C24—C25—C20 | −0.6 (5) |
| O3—C8—C9—C10 | 166.8 (2) | C21—C20—C25—C24 | 0.0 (5) |
| C7—C8—C9—C10 | 43.4 (3) | C19—C20—C25—C24 | −178.4 (3) |
| C8—C9—C10—O4 | −179.6 (2) | C10—C11—C31—O5 | −68.7 (3) |
| C8—C9—C10—C11 | −52.7 (3) | C12—C11—C31—O5 | 169.7 (2) |
| O4—C10—C11—C31 | −52.4 (3) | C1—C11—C31—O5 | 50.1 (3) |
| C9—C10—C11—C31 | −177.8 (2) | C7—C13—N1—C30 | 172.1 (2) |
| O4—C10—C11—C12 | 67.9 (3) | C3—C13—N1—C30 | −75.2 (3) |
| C9—C10—C11—C12 | −57.5 (3) | C7—C13—N1—C12 | −59.0 (3) |
| O4—C10—C11—C1 | −172.6 (2) | C3—C13—N1—C12 | 53.7 (3) |
| C9—C10—C11—C1 | 62.0 (3) | C11—C12—N1—C30 | 174.3 (2) |
| C7—C1—C11—C31 | −177.0 (2) | C11—C12—N1—C13 | 44.0 (3) |
| C2—C1—C11—C31 | 69.9 (3) | O2—C26—O1—C4 | 2.3 (5) |
| C7—C1—C11—C10 | −59.9 (3) | C27—C26—O1—C4 | −179.4 (3) |
| C2—C1—C11—C10 | −173.0 (2) | C3—C4—O1—C26 | 69.5 (3) |
| C7—C1—C11—C12 | 62.2 (3) | C5—C4—O1—C26 | −176.9 (2) |
| C2—C1—C11—C12 | −51.0 (3) | C18—C4—O1—C26 | −57.3 (3) |
| C31—C11—C12—N1 | −165.4 (2) | C9—C8—O3—C29 | 84.5 (3) |
| C10—C11—C12—N1 | 76.4 (3) | C7—C8—O3—C29 | −148.0 (2) |
| C1—C11—C12—N1 | −43.8 (3) | C11—C31—O5—C32 | 178.3 (3) |
| C8—C7—C13—N1 | −51.9 (3) | C3—C2—O6—C28 | 136.2 (3) |
| C6—C7—C13—N1 | −174.2 (2) | C1—C2—O6—C28 | −104.5 (3) |
| C1—C7—C13—N1 | 68.3 (3) | C15—C17—O8—C33 | −164.5 (3) |
| C8—C7—C13—C3 | −174.2 (2) | C18—C17—O8—C33 | 70.7 (3) |
| C6—C7—C13—C3 | 63.4 (3) | O11—C19—O9—C16 | −3.7 (4) |
| C1—C7—C13—C3 | −54.0 (2) | C20—C19—O9—C16 | 177.3 (2) |
| C4—C3—C13—N1 | 166.2 (2) | C15—C16—O9—C19 | 179.1 (2) |
| C2—C3—C13—N1 | −77.4 (3) | C5—C16—O9—C19 | −67.5 (3) |
| C4—C3—C13—C7 | −75.7 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O10—H10A···O2 | 0.84 | 2.11 | 2.788 (3) | 138 |
| O7—H7···O8 | 0.84 | 2.04 | 2.560 (3) | 120 |
| O4—H4···O11i | 0.84 | 2.20 | 3.018 (3) | 163 |
Symmetry codes: (i) −x, y−1/2, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: WN2256).
References
- Bruker (2001). SMART. Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2003). SAINT-Plus. Bruker AXS Inc., Madison, Wisconsin, USA.
- Codding, P. W. (1982). Acta Cryst. B38, 2519–2522.
- De Camp, W. H. & Pelletier, S. W. (1977). Acta Cryst. B33, 722–727.
- Hikino, H., Konno, C., Takata, H., Yamada, Y., Yamada, C., Ohizumi, Y., Sugio, K. & Fujimura, H. (1980). J. Pharm. Dyn.3, 514–525. [DOI] [PubMed]
- Li, Z. B., Lu, G. H., Chen, D. L. & Wang, F. P. (1997). Nat. Prod. Res. Dev.9, 9-14.
- Mitamura, M., Horie, S., Sakaguchi, M., Someya, A., Tsuchiya, S. V., Murayama, T. & Watanabe, K. (2002). Eur. J. Pharmacol.436, 217–225. [DOI] [PubMed]
- Parvez, M., Gul, W., Atta-ur-Rahman, Choudhary, M. I., Nasreen, A. & Fatima, N. (1999). Acta Cryst. C55, 72–74.
- Pelletier, S. W. & Djarmati, Z. (1976). J Am Chem Soc 98, 2626–2636.
- Pelletier, S. W., Finer-Moore, J., Desai, R. C., Mody, N. V. & Desai, H. K. (1982). J. Org. Chem.47, 5290–5297.
- Saito, H., Ueyama, T., Naka, N., Yagi, J. & Okamoto, T. (1982). Chem. Pharm. Bull.30, 1844–1850. [DOI] [PubMed]
- Sheldrick, G. M. (2003). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Tsuda, Y. & Marion, L. (1963). Can. J. Chem 41, 1485-1489.
- Zhapova, T., Modonova, L. D. & Semenov, A. A. (1986). Chem Nat Compd, 21, 7678–679.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808013147/wn2256sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808013147/wn2256Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report