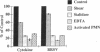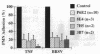Abstract
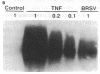
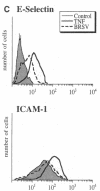
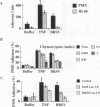
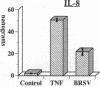
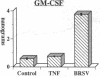
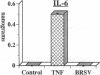
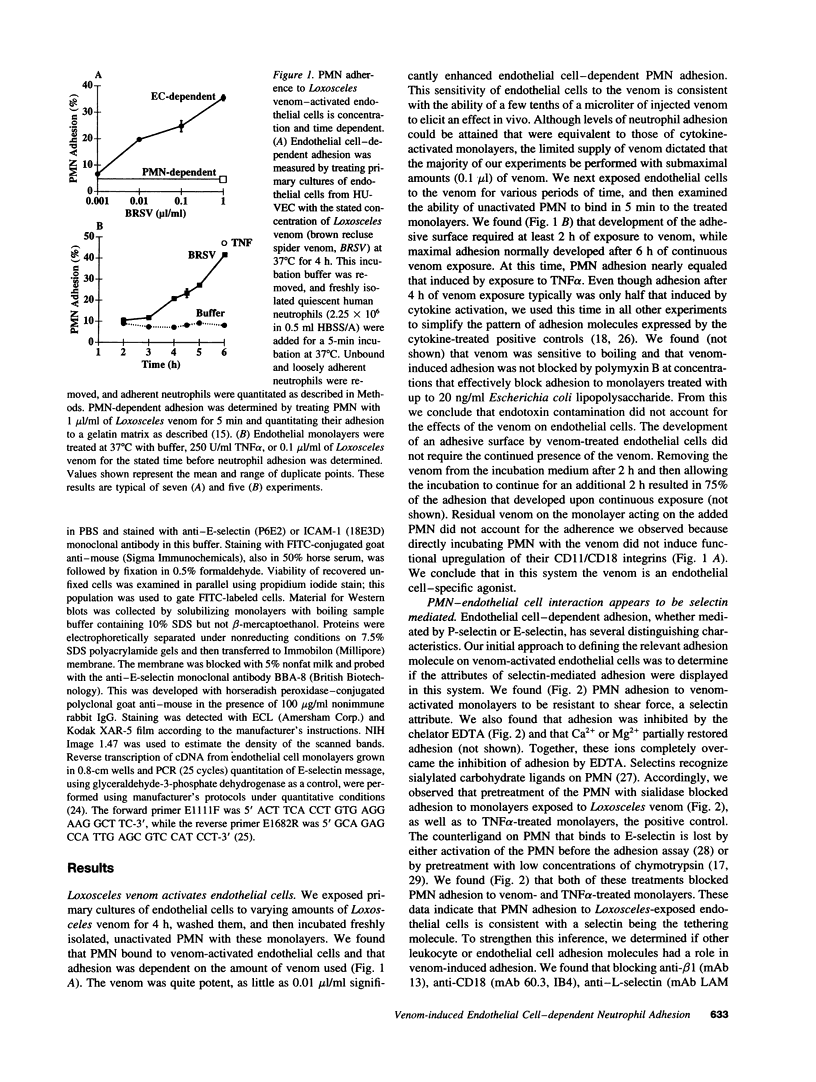
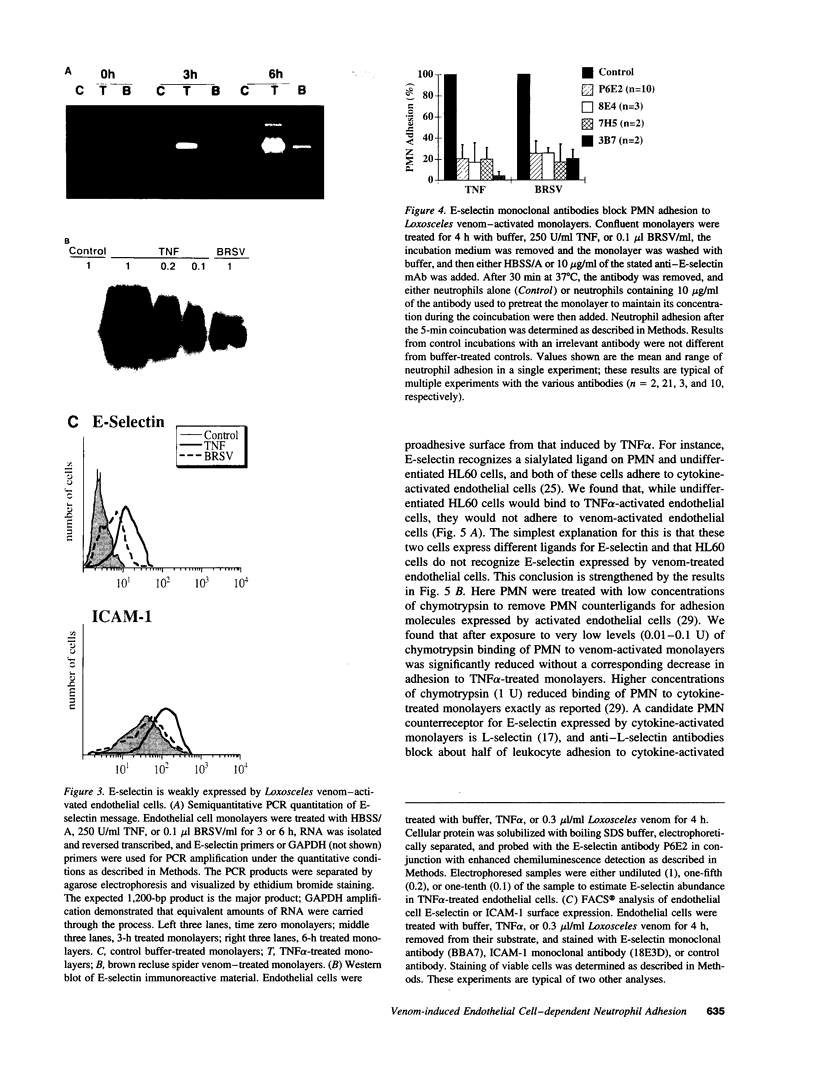
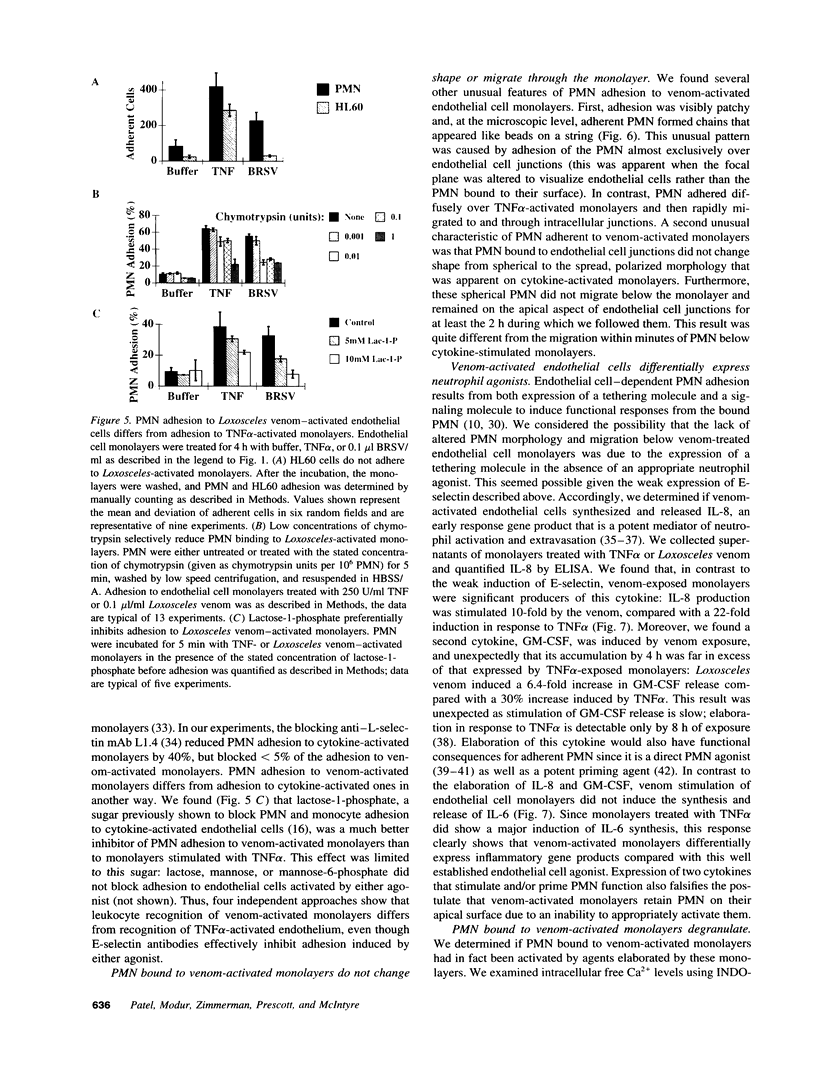
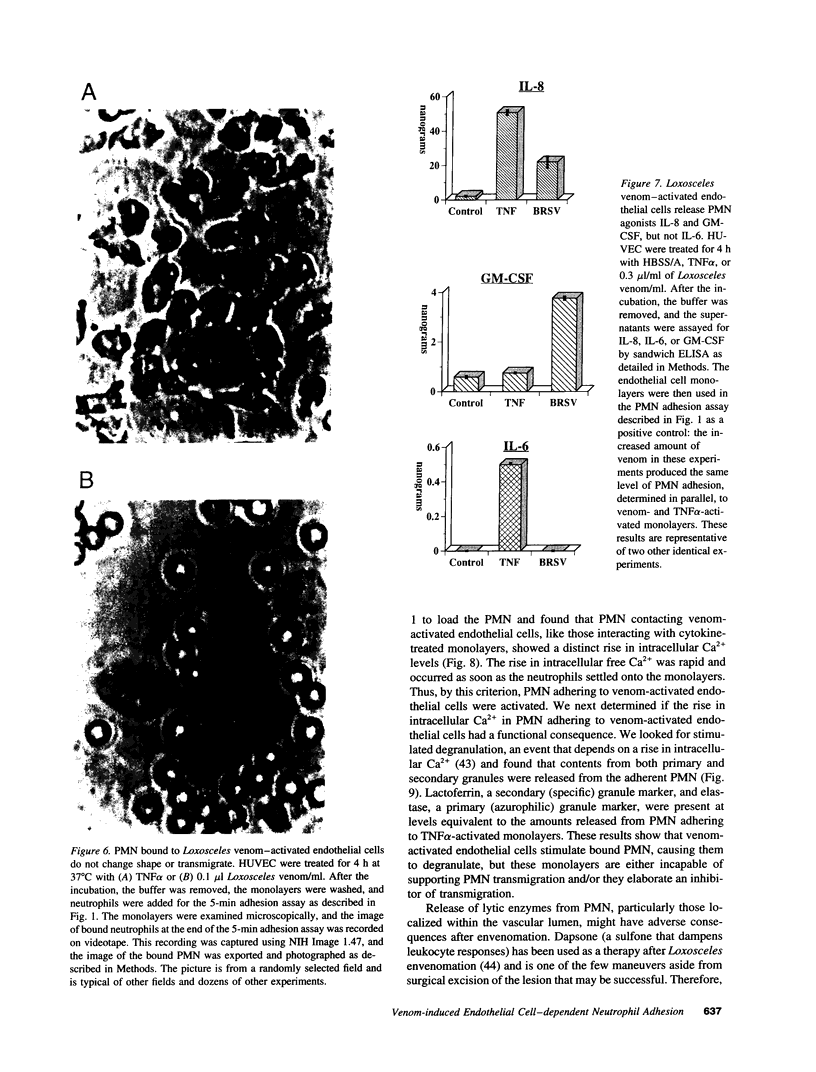
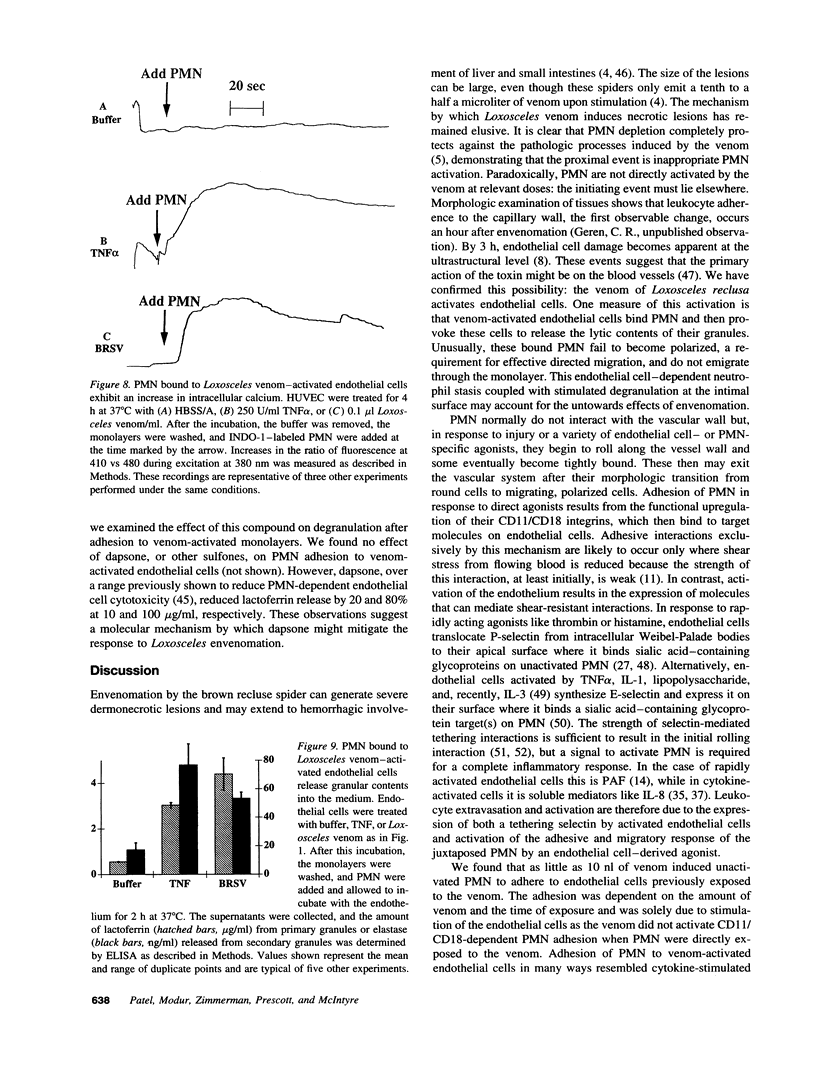
Brown recluse spider (Loxosceles reclusa) venom induces severe dermonecrotic lesions. The mechanism for this is unknown but presents an interesting paradox: necrosis is completely dependent on the victim's neutrophils, yet neutrophils are not activated by the venom. We show Loxosceles venom is a potent, but disjointed, endothelial cell agonist. It weakly induced E-selectin expression, but not intercellular adhesion molecule-1 or IL-6 expression, yet significantly stimulated release of IL-8 and large amounts of GM-CSF by 4 h. In contrast, TNF strongly induced all of these, except for GM-CSF. PMN bound to E-selectin on venom-activated endothelial cells, apparently via counterreceptors different from those that bind E-selectin on TNF alpha-activated monolayers. Notably, PMN bound venom-activated monolayers only at intercellular junctions, did not polarize, and completely failed to migrate beneath the monolayer. Despite this, bound PMN demonstrated increased intracellular Ca2+ levels and secreted primary and secondary granule markers. The latter event was suppressed by sulfones used to treat envenomation. We have defined a new endothelial cell agonist, Loxosceles venom, that differentially stimulates the inflammatory response of endothelial cells. This, in turn, leads to a dysregulated PMN response where adhesion and degranulation are completely dissociated from shape change and transmigration.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINS J. A., WINGO C. W., SODEMAN W. A., FLYNN J. E. Necrotic arachnidism. Am J Trop Med Hyg. 1958 Mar;7(2):165–184. doi: 10.4269/ajtmh.1958.7.165. [DOI] [PubMed] [Google Scholar]
- Abbassi O., Lane C. L., Krater S., Kishimoto T. K., Anderson D. C., McIntire L. V., Smith C. W. Canine neutrophil margination mediated by lectin adhesion molecule-1 in vitro. J Immunol. 1991 Oct 1;147(7):2107–2115. [PubMed] [Google Scholar]
- Akahane K., Pluznik D. H. Interferon-gamma destabilizes interleukin-1-induced granulocyte-macrophage colony-stimulating factor mRNA in murine vascular endothelial cells. Exp Hematol. 1993 Jul;21(7):878–884. [PubMed] [Google Scholar]
- Babcock J. L., Marmer D. J., Steele R. W. Immunotoxicology of brown recluse spider (Loxosceles reclusa) venom. Toxicon. 1986;24(8):783–790. doi: 10.1016/0041-0101(86)90103-0. [DOI] [PubMed] [Google Scholar]
- Babcock J. L., Suber R. L., Frith C. H., Geren C. R. Systemic effect in mice or venom apparatus extract and toxin from the brown recluse spider (Loxosceles reclusa). Toxicon. 1981;19(4):463–471. doi: 10.1016/0041-0101(81)90004-0. [DOI] [PubMed] [Google Scholar]
- Berger R. S., Adelstein E. H., Anderson P. C. Intravascular coagulation: the cause of necrotic arachnidism. J Invest Dermatol. 1973 Sep;61(3):142–150. doi: 10.1111/1523-1747.ep12676202. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Nelson R. M. Selectins. J Clin Invest. 1993 Feb;91(2):379–387. doi: 10.1172/JCI116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Bradley J. R., Johnson D. R., Pober J. S. Endothelial activation by hydrogen peroxide. Selective increases of intercellular adhesion molecule-1 and major histocompatibility complex class I. Am J Pathol. 1993 May;142(5):1598–1609. [PMC free article] [PubMed] [Google Scholar]
- Brizzi M. F., Garbarino G., Rossi P. R., Pagliardi G. L., Arduino C., Avanzi G. C., Pegoraro L. Interleukin 3 stimulates proliferation and triggers endothelial-leukocyte adhesion molecule 1 gene activation of human endothelial cells. J Clin Invest. 1993 Jun;91(6):2887–2892. doi: 10.1172/JCI116534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broudy V. C., Kaushansky K., Segal G. M., Harlan J. M., Adamson J. W. Tumor necrosis factor type alpha stimulates human endothelial cells to produce granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7467–7471. doi: 10.1073/pnas.83.19.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colditz I. G., Zwahlen R. D., Baggiolini M. Neutrophil accumulation and plasma leakage induced in vivo by neutrophil-activating peptide-1. J Leukoc Biol. 1990 Aug;48(2):129–137. doi: 10.1002/jlb.48.2.129. [DOI] [PubMed] [Google Scholar]
- DiCorleto P. E., de la Motte C. A. Role of cell surface carbohydrate moieties in monocytic cell adhesion to endothelium in vitro. J Immunol. 1989 Dec 1;143(11):3666–3672. [PubMed] [Google Scholar]
- Erbe D. V., Wolitzky B. A., Presta L. G., Norton C. R., Ramos R. J., Burns D. K., Rumberger J. M., Rao B. N., Foxall C., Brandley B. K. Identification of an E-selectin region critical for carbohydrate recognition and cell adhesion. J Cell Biol. 1992 Oct;119(1):215–227. doi: 10.1083/jcb.119.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk C. D., FitzGerald G. A. Eicosanoid forming enzyme mRNA in human tissues. Analysis by quantitative polymerase chain reaction. J Biol Chem. 1991 Jul 5;266(19):12508–12513. [PubMed] [Google Scholar]
- Gamble J. R., Rand T. H., Lopez A. F., Clark-Lewis I., Vadas M. A. Heterogeneity of recombinant granulocyte-macrophage colony-stimulating factor-mediated enhancement of neutrophil adherence to endothelium. Exp Hematol. 1990 Sep;18(8):897–902. [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Obin M. S., Brock A. F., Luis E. A., Hass P. E., Hébert C. A., Yip Y. K., Leung D. W., Lowe D. G., Kohr W. J. Endothelial interleukin-8: a novel inhibitor of leukocyte-endothelial interactions. Science. 1989 Dec 22;246(4937):1601–1603. doi: 10.1126/science.2688092. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Spertini O., Ernst T. J., Belvin M. P., Levine H. B., Kanakura Y., Tedder T. F. Granulocyte-macrophage colony-stimulating factor and other cytokines regulate surface expression of the leukocyte adhesion molecule-1 on human neutrophils, monocytes, and their precursors. J Immunol. 1990 Jul 15;145(2):576–584. [PubMed] [Google Scholar]
- Hooft van Huijsduijnen R., Pescini R., DeLamarter J. F. Two distinct NF-kappa B complexes differing in their larger subunit bind the E-selectin promoter kappa B element. Nucleic Acids Res. 1993 Aug 11;21(16):3711–3717. doi: 10.1093/nar/21.16.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooft van Huijsduijnen R., Whelan J., Pescini R., Becker-André M., Schenk A. M., DeLamarter J. F. A T-cell enhancer cooperates with NF-kappa B to yield cytokine induction of E-selectin gene transcription in endothelial cells. J Biol Chem. 1992 Nov 5;267(31):22385–22391. [PubMed] [Google Scholar]
- Huber A. R., Kunkel S. L., Todd R. F., 3rd, Weiss S. J. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 1991 Oct 4;254(5028):99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- Isshiki H., Akira S., Tanabe O., Nakajima T., Shimamoto T., Hirano T., Kishimoto T. Constitutive and interleukin-1 (IL-1)-inducible factors interact with the IL-1-responsive element in the IL-6 gene. Mol Cell Biol. 1990 Jun;10(6):2757–2764. doi: 10.1128/mcb.10.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly R. D. The pathogenic action of the exotoxin of Corynebacterium ovis. J Comp Pathol. 1965 Oct;75(4):417–431. doi: 10.1016/0021-9975(65)90022-8. [DOI] [PubMed] [Google Scholar]
- Jutila M. A., Kishimoto T. K., Finken M. Low-dose chymotrypsin treatment inhibits neutrophil migration into sites of inflammation in vivo: effects on Mac-1 and MEL-14 adhesion protein expression and function. Cell Immunol. 1991 Jan;132(1):201–214. doi: 10.1016/0008-8749(91)90019-8. [DOI] [PubMed] [Google Scholar]
- Kaufman S. E., DiPersio J. F., Gasson J. C. Effects of human GM-CSF on neutrophil degranulation in vitro. Exp Hematol. 1989 Aug;17(7):800–804. [PubMed] [Google Scholar]
- Kaushansky K. Control of granulocyte-macrophage colony-stimulating factor production in normal endothelial cells by positive and negative regulatory elements. J Immunol. 1989 Oct 15;143(8):2525–2529. [PubMed] [Google Scholar]
- Kojima N., Handa K., Newman W., Hakomori S. Multi-recognition capability of E-selectin in a dynamic flow system, as evidenced by differential effects of sialidases and anti-carbohydrate antibodies on selectin-mediated cell adhesion at low vs. high wall shear stress: a preliminary note. Biochem Biophys Res Commun. 1992 Dec 30;189(3):1686–1694. doi: 10.1016/0006-291x(92)90272-m. [DOI] [PubMed] [Google Scholar]
- Larkin M., Ahern T. J., Stoll M. S., Shaffer M., Sako D., O'Brien J., Yuen C. T., Lawson A. M., Childs R. A., Barone K. M. Spectrum of sialylated and nonsialylated fuco-oligosaccharides bound by the endothelial-leukocyte adhesion molecule E-selectin. Dependence of the carbohydrate binding activity on E-selectin density. J Biol Chem. 1992 Jul 5;267(19):13661–13668. [PubMed] [Google Scholar]
- Lawrence M. B., Smith C. W., Eskin S. G., McIntire L. V. Effect of venous shear stress on CD18-mediated neutrophil adhesion to cultured endothelium. Blood. 1990 Jan 1;75(1):227–237. [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991 May 31;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Ley K., Gaehtgens P., Fennie C., Singer M. S., Lasky L. A., Rosen S. D. Lectin-like cell adhesion molecule 1 mediates leukocyte rolling in mesenteric venules in vivo. Blood. 1991 Jun 15;77(12):2553–2555. [PubMed] [Google Scholar]
- Libermann T. A., Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990 May;10(5):2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb R. R., Chi-Rosso G., Leone D. R., Rosa M. D., Bixler S., Newman B. M., Luhowskyj S., Benjamin C. D., Dougas I. G., Goelz S. E. Expression and functional characterization of a soluble form of endothelial-leukocyte adhesion molecule 1. J Immunol. 1991 Jul 1;147(1):124–129. [PubMed] [Google Scholar]
- Lorant D. E., Patel K. D., McIntyre T. M., McEver R. P., Prescott S. M., Zimmerman G. A. Coexpression of GMP-140 and PAF by endothelium stimulated by histamine or thrombin: a juxtacrine system for adhesion and activation of neutrophils. J Cell Biol. 1991 Oct;115(1):223–234. doi: 10.1083/jcb.115.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorant D. E., Topham M. K., Whatley R. E., McEver R. P., McIntyre T. M., Prescott S. M., Zimmerman G. A. Inflammatory roles of P-selectin. J Clin Invest. 1993 Aug;92(2):559–570. doi: 10.1172/JCI116623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscinskas F. W., Cybulsky M. I., Kiely J. M., Peckins C. S., Davis V. M., Gimbrone M. A., Jr Cytokine-activated human endothelial monolayers support enhanced neutrophil transmigration via a mechanism involving both endothelial-leukocyte adhesion molecule-1 and intercellular adhesion molecule-1. J Immunol. 1991 Mar 1;146(5):1617–1625. [PubMed] [Google Scholar]
- Mahé Y., Mukaida N., Kuno K., Akiyama M., Ikeda N., Matsushima K., Murakami S. Hepatitis B virus X protein transactivates human interleukin-8 gene through acting on nuclear factor kB and CCAAT/enhancer-binding protein-like cis-elements. J Biol Chem. 1991 Jul 25;266(21):13759–13763. [PubMed] [Google Scholar]
- Majeski J. A., Stinnett J. D., Alexander J. W., Durst G. G., Sr Action of venom from the brown recluse spider (Loxosceles reclusa) on human neutrophils. Toxicon. 1977;15(5):423–427. doi: 10.1016/0041-0101(77)90120-9. [DOI] [PubMed] [Google Scholar]
- Martin W. J., 2nd, Kachel D. L. Reduction of neutrophil-mediated injury to pulmonary endothelial cells by dapsone. Am Rev Respir Dis. 1985 Apr;131(4):544–547. doi: 10.1164/arrd.1985.131.4.544. [DOI] [PubMed] [Google Scholar]
- Montgomery K. F., Osborn L., Hession C., Tizard R., Goff D., Vassallo C., Tarr P. I., Bomsztyk K., Lobb R., Harlan J. M. Activation of endothelial-leukocyte adhesion molecule 1 (ELAM-1) gene transcription. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6523–6527. doi: 10.1073/pnas.88.15.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. L., Stults N. L., Diaz S., Smith D. F., Cummings R. D., Varki A., McEver R. P. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J Cell Biol. 1992 Jul;118(2):445–456. doi: 10.1083/jcb.118.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W. A., Weigl S. A., Deng X., Phillips D. M. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993 Aug 1;178(2):449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parhami F., Fang Z. T., Fogelman A. M., Andalibi A., Territo M. C., Berliner J. A. Minimally modified low density lipoprotein-induced inflammatory responses in endothelial cells are mediated by cyclic adenosine monophosphate. J Clin Invest. 1993 Jul;92(1):471–478. doi: 10.1172/JCI116590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K. D., Zimmerman G. A., Prescott S. M., McEver R. P., McIntyre T. M. Oxygen radicals induce human endothelial cells to express GMP-140 and bind neutrophils. J Cell Biol. 1991 Feb;112(4):749–759. doi: 10.1083/jcb.112.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K. D., Zimmerman G. A., Prescott S. M., McIntyre T. M. Novel leukocyte agonists are released by endothelial cells exposed to peroxide. J Biol Chem. 1992 Jul 25;267(21):15168–15175. [PubMed] [Google Scholar]
- Picker L. J., Warnock R. A., Burns A. R., Doerschuk C. M., Berg E. L., Butcher E. C. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell. 1991 Sep 6;66(5):921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Slowik M. R., De Luca L. G., Ritchie A. J. Elevated cyclic AMP inhibits endothelial cell synthesis and expression of TNF-induced endothelial leukocyte adhesion molecule-1, and vascular cell adhesion molecule-1, but not intercellular adhesion molecule-1. J Immunol. 1993 Jun 1;150(11):5114–5123. [PubMed] [Google Scholar]
- Rees R. S., Altenbern D. P., Lynch J. B., King L. E., Jr Brown recluse spider bites. A comparison of early surgical excision versus dapsone and delayed surgical excision. Ann Surg. 1985 Nov;202(5):659–663. doi: 10.1097/00000658-198511000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J., Andersson T., Olsson I. Effect of tumor necrosis factor and granulocyte/macrophage colony-stimulating factor on neutrophil degranulation. J Immunol. 1989 May 1;142(9):3199–3205. [PubMed] [Google Scholar]
- Rot A. Endothelial cell binding of NAP-1/IL-8: role in neutrophil emigration. Immunol Today. 1992 Aug;13(8):291–294. doi: 10.1016/0167-5699(92)90039-A. [DOI] [PubMed] [Google Scholar]
- Segal G. M., Smith T. D., Heinrich M. C., Ey F. S., Bagby G. C., Jr Specific repression of granulocyte-macrophage and granulocyte colony-stimulating factor gene expression in interleukin-1-stimulated endothelial cells with antisense oligodeoxynucleotides. Blood. 1992 Aug 1;80(3):609–616. [PubMed] [Google Scholar]
- Sengeløv H., Kjeldsen L., Borregaard N. Control of exocytosis in early neutrophil activation. J Immunol. 1993 Feb 15;150(4):1535–1543. [PubMed] [Google Scholar]
- Smiley P. L., Stremler K. E., Prescott S. M., Zimmerman G. A., McIntyre T. M. Oxidatively fragmented phosphatidylcholines activate human neutrophils through the receptor for platelet-activating factor. J Biol Chem. 1991 Jun 15;266(17):11104–11110. [PubMed] [Google Scholar]
- Smith C. W., Kishimoto T. K., Abbassi O., Hughes B., Rothlein R., McIntire L. V., Butcher E., Anderson D. C., Abbass O. Chemotactic factors regulate lectin adhesion molecule 1 (LECAM-1)-dependent neutrophil adhesion to cytokine-stimulated endothelial cells in vitro. J Clin Invest. 1991 Feb;87(2):609–618. doi: 10.1172/JCI115037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Micks D. W. A comparative study of the venom and other components of three species of Loxosceles. Am J Trop Med Hyg. 1968 Jul;17(4):651–656. doi: 10.4269/ajtmh.1968.17.651. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Micks D. W. The role of polymorphonuclear leukocytes in the lesion caused by the venom of the brown spider, Loxosceles reclusa. Lab Invest. 1970 Jan;22(1):90–93. [PubMed] [Google Scholar]
- Spertini O., Kansas G. S., Reimann K. A., Mackay C. R., Tedder T. F. Function and evolutionary conservation of distinct epitopes on the leukocyte adhesion molecule-1 (TQ-1, Leu-8) that regulate leukocyte migration. J Immunol. 1991 Aug 1;147(3):942–949. [PubMed] [Google Scholar]
- Spertini O., Luscinskas F. W., Kansas G. S., Munro J. M., Griffin J. D., Gimbrone M. A., Jr, Tedder T. F. Leukocyte adhesion molecule-1 (LAM-1, L-selectin) interacts with an inducible endothelial cell ligand to support leukocyte adhesion. J Immunol. 1991 Oct 15;147(8):2565–2573. [PubMed] [Google Scholar]
- Varki A. Selectins and other mammalian sialic acid-binding lectins. Curr Opin Cell Biol. 1992 Apr;4(2):257–266. doi: 10.1016/0955-0674(92)90041-a. [DOI] [PubMed] [Google Scholar]
- Voraberger G., Schäfer R., Stratowa C. Cloning of the human gene for intercellular adhesion molecule 1 and analysis of its 5'-regulatory region. Induction by cytokines and phorbol ester. J Immunol. 1991 Oct 15;147(8):2777–2786. [PubMed] [Google Scholar]
- Walcheck B., Watts G., Jutila M. A. Bovine gamma/delta T cells bind E-selectin via a novel glycoprotein receptor: first characterization of a lymphocyte/E-selectin interaction in an animal model. J Exp Med. 1993 Sep 1;178(3):853–863. doi: 10.1084/jem.178.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman G. S., Anderson P. C. Loxoscelism and necrotic arachnidism. J Toxicol Clin Toxicol. 1983;21(4-5):451–472. doi: 10.3109/15563658308990434. [DOI] [PubMed] [Google Scholar]
- Wertheimer S. J., Myers C. L., Wallace R. W., Parks T. P. Intercellular adhesion molecule-1 gene expression in human endothelial cells. Differential regulation by tumor necrosis factor-alpha and phorbol myristate acetate. J Biol Chem. 1992 Jun 15;267(17):12030–12035. [PubMed] [Google Scholar]
- Whatley R. E., Fennell D. F., Kurrus J. A., Zimmerman G. A., McIntyre T. M., Prescott S. M. Synthesis of platelet-activating factor by endothelial cells. The role of G proteins. J Biol Chem. 1990 Sep 15;265(26):15550–15559. [PubMed] [Google Scholar]
- Whatley R. E., Nelson P., Zimmerman G. A., Stevens D. L., Parker C. J., McIntyre T. M., Prescott S. M. The regulation of platelet-activating factor production in endothelial cells. The role of calcium and protein kinase C. J Biol Chem. 1989 Apr 15;264(11):6325–6333. [PubMed] [Google Scholar]
- Zimmerman G. A., McIntyre T. M., Mehra M., Prescott S. M. Endothelial cell-associated platelet-activating factor: a novel mechanism for signaling intercellular adhesion. J Cell Biol. 1990 Feb;110(2):529–540. doi: 10.1083/jcb.110.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman G. A., McIntyre T. M., Prescott S. M. Thrombin stimulates the adherence of neutrophils to human endothelial cells in vitro. J Clin Invest. 1985 Dec;76(6):2235–2246. doi: 10.1172/JCI112232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman G. A., Prescott S. M., McIntyre T. M. Endothelial cell interactions with granulocytes: tethering and signaling molecules. Immunol Today. 1992 Mar;13(3):93–100. doi: 10.1016/0167-5699(92)90149-2. [DOI] [PubMed] [Google Scholar]