Abstract
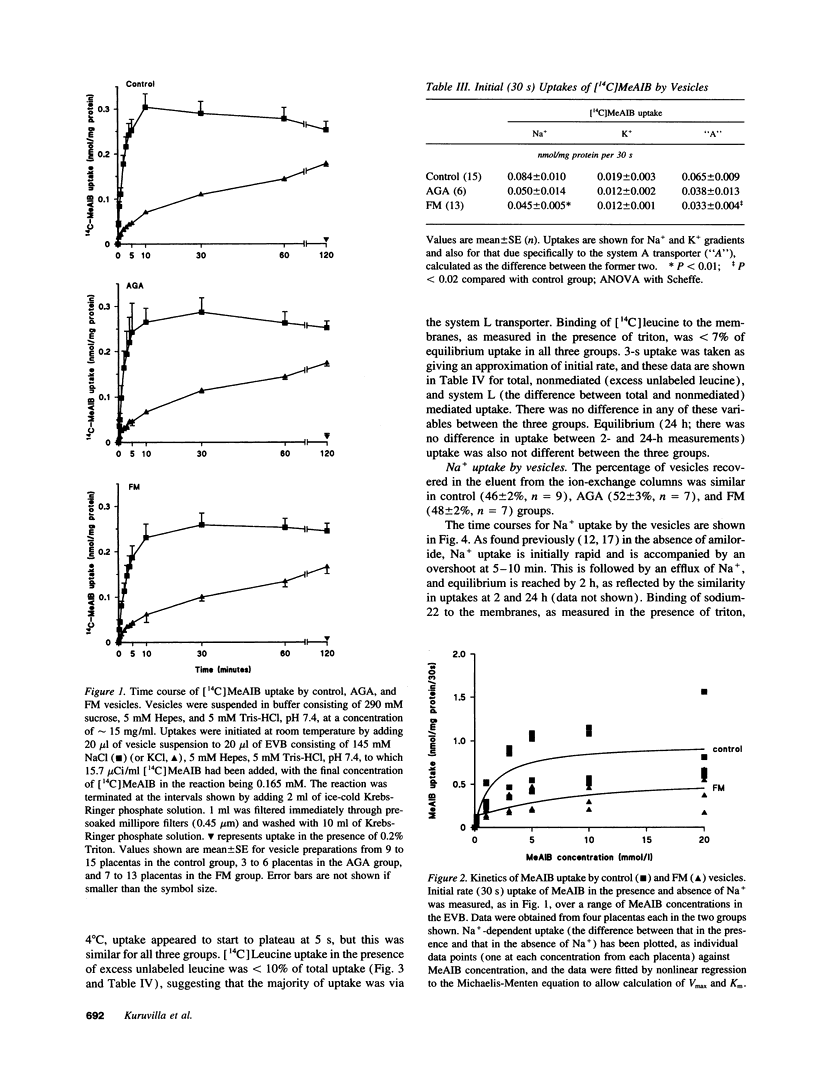
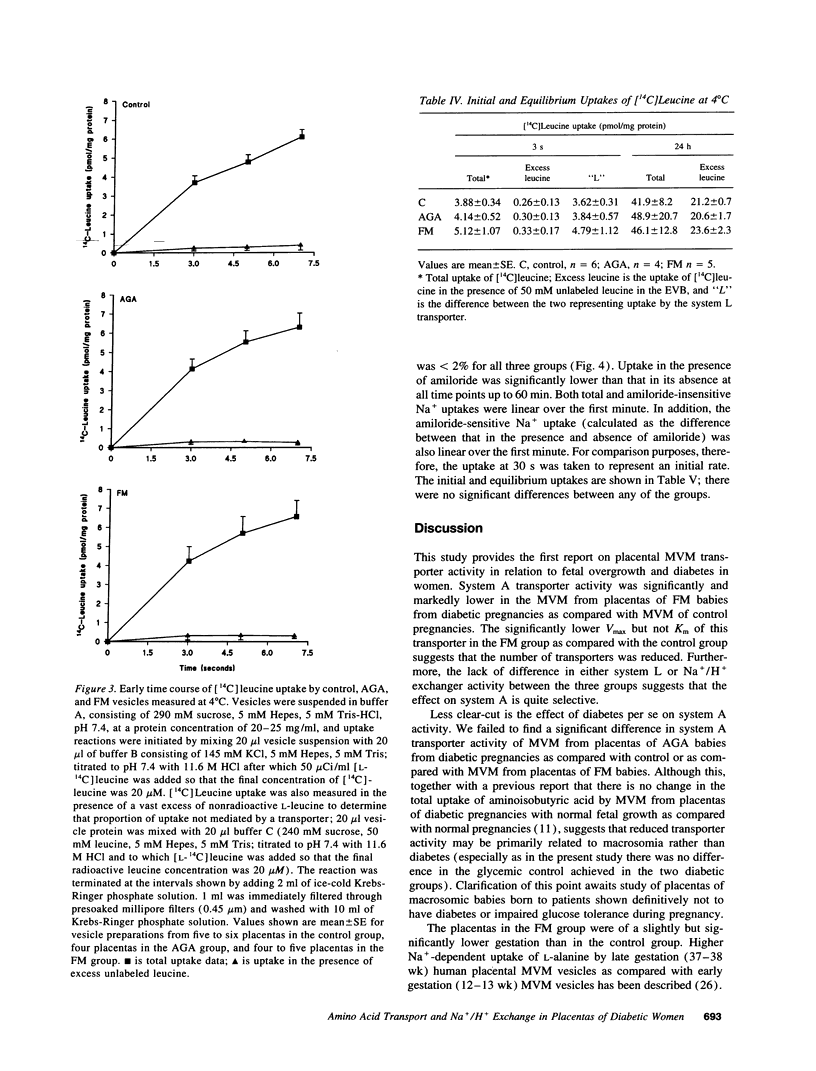
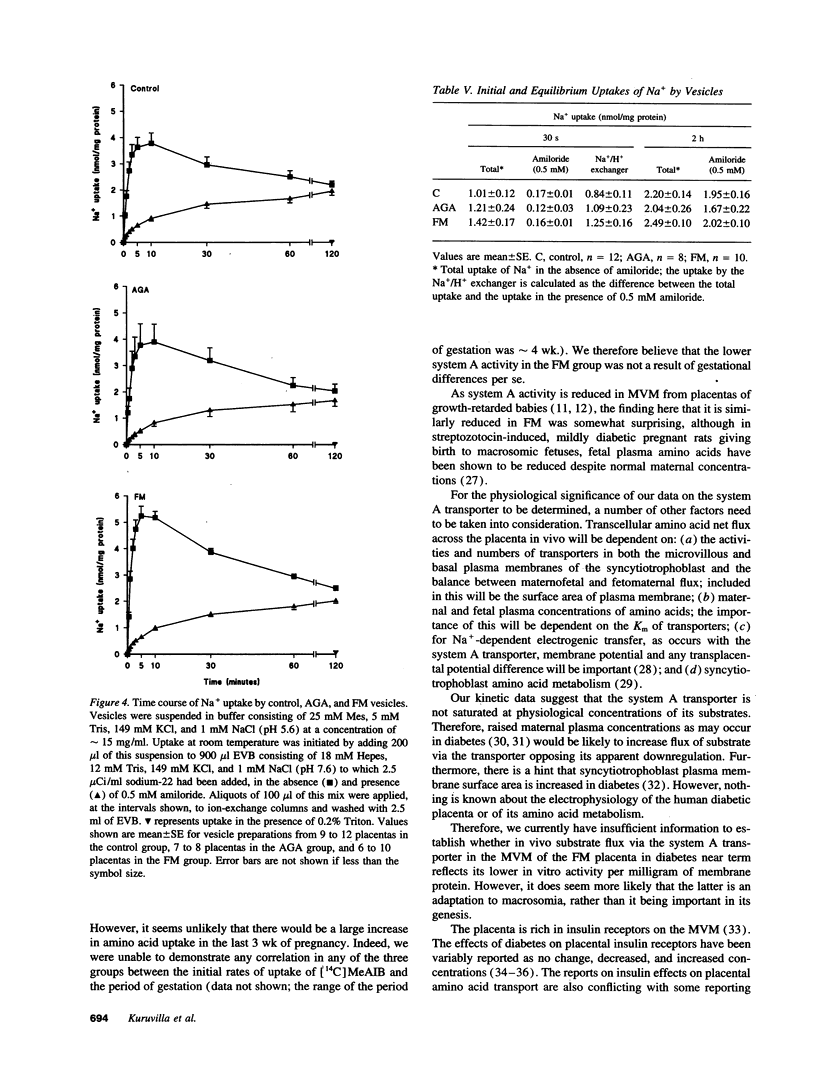
Fetal macrosomia (FM) is a well-recognized complication of diabetic pregnancy but it is not known whether placental transport mechanisms are altered. We therefore studied the activity of the system A amino acid transporter, the system L amino acid transporter, and the Na+/H+ exchanger in microvillous membrane vesicles from placentas of macrosomic babies born to diabetic women (FM group), from placentas of appropriately grown babies born to diabetic women (appropriate for gestational age group) and from placentas of appropriately grown babies of normal women (control group). Sodium-dependent uptake of [14C]-methylaminoisobutyric acid at 30 s (initial rate, a measure of system A activity) was 49% lower into FM vesicles than into control vesicles (P < 0.02); this effect was due to a decrease in Vmax of the transporter with no change in Km. There was no significant difference in system A activity between the appropriate for gestational age group and control or FM group. There was also no difference between system L transporter or Na+/H+ exchanger activity between the three groups. We conclude that the number of system A transporters per milligram of membrane protein in the placental microvillous membrane is selectively reduced in diabetic pregnancies associated with FM.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aerts L., Van Bree R., Feytons V., Rombauts W., Van Assche F. A. Plasma amino acids in diabetic pregnant rats and in their fetal and adult offspring. Biol Neonate. 1989;56(1):31–39. doi: 10.1159/000242984. [DOI] [PubMed] [Google Scholar]
- Berk M. A., Mimouni F., Miodovnik M., Hertzberg V., Valuck J. Macrosomia in infants of insulin-dependent diabetic mothers. Pediatrics. 1989 Jun;83(6):1029–1034. [PubMed] [Google Scholar]
- Carroll M. J., Young M. The relationship between placental protein synthesis and transfer of amino acids. Biochem J. 1983 Jan 15;210(1):99–105. doi: 10.1042/bj2100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs C. A., Gunderson E., Kitzmiller J. L., Gavin L. A., Main E. K. Relationship of fetal macrosomia to maternal postprandial glucose control during pregnancy. Diabetes Care. 1992 Oct;15(10):1251–1257. doi: 10.2337/diacare.15.10.1251. [DOI] [PubMed] [Google Scholar]
- Dancis J., Money W. L., Springer D., Levitz M. Transport of amino acids by placenta. Am J Obstet Gynecol. 1968 Jul 15;101(6):820–829. doi: 10.1016/0002-9378(68)90038-0. [DOI] [PubMed] [Google Scholar]
- Dandona P., Besterman H. S., Freedman D. B., Boag F., Taylor A. M., Beckett A. G. Macrosomia despite well-controlled diabetic pregnancy. Lancet. 1984 Mar 31;1(8379):737–737. doi: 10.1016/s0140-6736(84)92248-7. [DOI] [PubMed] [Google Scholar]
- Desoye G., Hofmann H. H., Weiss P. A. Insulin binding to trophoblast plasma membranes and placental glycogen content in well-controlled gestational diabetic women treated with diet or insulin, in well-controlled overt diabetic patients and in healthy control subjects. Diabetologia. 1992 Jan;35(1):45–55. doi: 10.1007/BF00400851. [DOI] [PubMed] [Google Scholar]
- Dicke J. M., Henderson G. I. Placental amino acid uptake in normal and complicated pregnancies. Am J Med Sci. 1988 Mar;295(3):223–227. doi: 10.1097/00000441-198803000-00012. [DOI] [PubMed] [Google Scholar]
- Durán-García S., Nieto J. G., Cabello A. M. Effect of gestational diabetes on insulin receptors in human placenta. Diabetologia. 1979 Feb;16(2):87–91. doi: 10.1007/BF01225455. [DOI] [PubMed] [Google Scholar]
- FLEISCHER S., BRIERLEY G., KLOUWEN H., SLAUTTERBACK D. B. Studies of the electron transfer system. 47. The role of phospholipids in electron transfer. J Biol Chem. 1962 Oct;237:3264–3272. [PubMed] [Google Scholar]
- Freinkel N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes. 1980 Dec;29(12):1023–1035. doi: 10.2337/diab.29.12.1023. [DOI] [PubMed] [Google Scholar]
- Gairdner D., Pearson J. A growth chart for premature and other infants. Arch Dis Child. 1971 Dec;46(250):783–787. doi: 10.1136/adc.46.250.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasko O. D., Knowles A. F., Shertzer H. G., Suolinna E. M., Racker E. The use of ion-exchange resins for studying ion transport in biological systems. Anal Biochem. 1976 May 7;72:57–65. doi: 10.1016/0003-2697(76)90506-6. [DOI] [PubMed] [Google Scholar]
- Glazier J. D., Jones C. J., Sibley C. P. Purification and Na+ uptake by human placental microvillus membrane vesicles prepared by three different methods. Biochim Biophys Acta. 1988 Nov 22;945(2):127–134. doi: 10.1016/0005-2736(88)90475-0. [DOI] [PubMed] [Google Scholar]
- Greenwood S. L., Boyd R. D., Sibley C. P. Transtrophoblast and microvillus membrane potential difference in mature intermediate human placental villi. Am J Physiol. 1993 Aug;265(2 Pt 1):C460–C466. doi: 10.1152/ajpcell.1993.265.2.C460. [DOI] [PubMed] [Google Scholar]
- Harrison L. C., Billington T., Clark S., Nichols R., East I., Martin F. I. Decreased binding of insulin by receptors on placental membranes from diabetic mothers. J Clin Endocrinol Metab. 1977 Jan;44(1):206–209. doi: 10.1210/jcem-44-1-206. [DOI] [PubMed] [Google Scholar]
- Iioka H., Hisanaga H., Moriyama I. S., Akada S., Shimamoto T., Yamada Y., Ichijo M. Characterization of human placental activity for transport of L-alanine, using brush border (microvillous) membrane vesicles. Placenta. 1992 Mar-Apr;13(2):179–190. doi: 10.1016/0143-4004(92)90032-o. [DOI] [PubMed] [Google Scholar]
- Johnson L. W., Smith C. H. Neutral amino acid transport systems of microvillous membrane of human placenta. Am J Physiol. 1988 Jun;254(6 Pt 1):C773–C780. doi: 10.1152/ajpcell.1988.254.6.C773. [DOI] [PubMed] [Google Scholar]
- Jones C. J., Fox H. Ultrastructure of the normal human placenta. Electron Microsc Rev. 1991;4(1):129–178. doi: 10.1016/0892-0354(91)90019-9. [DOI] [PubMed] [Google Scholar]
- Kalkhoff R. K., Kandaraki E., Morrow P. G., Mitchell T. H., Kelber S., Borkowf H. I. Relationship between neonatal birth weight and maternal plasma amino acid profiles in lean and obese nondiabetic women and in type I diabetic pregnant women. Metabolism. 1988 Mar;37(3):234–239. doi: 10.1016/0026-0495(88)90101-1. [DOI] [PubMed] [Google Scholar]
- Karl P. I., Alpy K. L., Fisher S. E. Amino acid transport by the cultured human placental trophoblast: effect of insulin on AIB transport. Am J Physiol. 1992 Apr;262(4 Pt 1):C834–C839. doi: 10.1152/ajpcell.1992.262.4.C834. [DOI] [PubMed] [Google Scholar]
- Kudo Y., Boyd C. A. Characterization of amino acid transport systems in human placental basal membrane vesicles. Biochim Biophys Acta. 1990 Jan 29;1021(2):169–174. doi: 10.1016/0005-2736(90)90030-r. [DOI] [PubMed] [Google Scholar]
- Kulanthaivel P., Furesz T. C., Moe A. J., Smith C. H., Mahesh V. B., Leibach F. H., Ganapathy V. Human placental syncytiotrophoblast expresses two pharmacologically distinguishable types of Na(+)-H+ exchangers, NHE-1 in the maternal-facing (brush border) membrane and NHE-2 in the fetal-facing (basal) membrane. Biochem J. 1992 May 15;284(Pt 1):33–38. doi: 10.1042/bj2840033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahendran D., Donnai P., Glazier J. D., D'Souza S. W., Boyd R. D., Sibley C. P. Amino acid (system A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr Res. 1993 Nov;34(5):661–665. doi: 10.1203/00006450-199311000-00019. [DOI] [PubMed] [Google Scholar]
- McComb R. B., Bowers G. N., Jr Study of optimum buffer conditions for measuring alkaline phosphatase activity in human serum. Clin Chem. 1972 Feb;18(2):97–104. [PubMed] [Google Scholar]
- Modanlou H. D., Dorchester W. L., Thorosian A., Freeman R. K. Macrosomia--maternal, fetal, and neonatal implications. Obstet Gynecol. 1980 Apr;55(4):420–424. [PubMed] [Google Scholar]
- PEDERSEN J. Weight and length at birth of infants of diabetic mothers. Acta Endocrinol (Copenh) 1954 Aug;16(4):330–342. doi: 10.1530/acta.0.0160330. [DOI] [PubMed] [Google Scholar]
- Potter J. M., Green A., Cullen D. R., Milner R. D. Amino acid profiles in early diabetic and non-diabetic pregnancy. Diabetes Res Clin Pract. 1986 Jun;2(3):123–126. doi: 10.1016/s0168-8227(86)80012-2. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel R. B., Mosley J. D., Smith C. H. Insulin and placenta: degradation and stabilization, binding to microvillous membrane receptors, and amino acid uptake. Am J Obstet Gynecol. 1979 Oct 15;135(4):522–529. doi: 10.1016/0002-9378(79)90444-7. [DOI] [PubMed] [Google Scholar]
- Stenninger E., Schollin J., Aman J. Neonatal macrosomia and hypoglycaemia in children of mothers with insulin-treated gestational diabetes mellitus. Acta Paediatr Scand. 1991 Nov;80(11):1014–1018. doi: 10.1111/j.1651-2227.1991.tb11776.x. [DOI] [PubMed] [Google Scholar]
- Teasdale F. Histomorphometry of the human placenta in Class C diabetes mellitus. Placenta. 1985 Jan-Feb;6(1):69–81. doi: 10.1016/s0143-4004(85)80034-5. [DOI] [PubMed] [Google Scholar]
- Whitsett J. A., Johnson C. L., Hawkins K. Differences in localization of insulin receptors and adenylate cyclase in the human placenta. Am J Obstet Gynecol. 1979 Jan 15;133(2):204–207. doi: 10.1016/0002-9378(79)90477-0. [DOI] [PubMed] [Google Scholar]
- Yudilevich D. L., Sweiry J. H. Transport of amino acids in the placenta. Biochim Biophys Acta. 1985 Sep 9;822(2):169–201. doi: 10.1016/0304-4157(85)90007-3. [DOI] [PubMed] [Google Scholar]



