Abstract
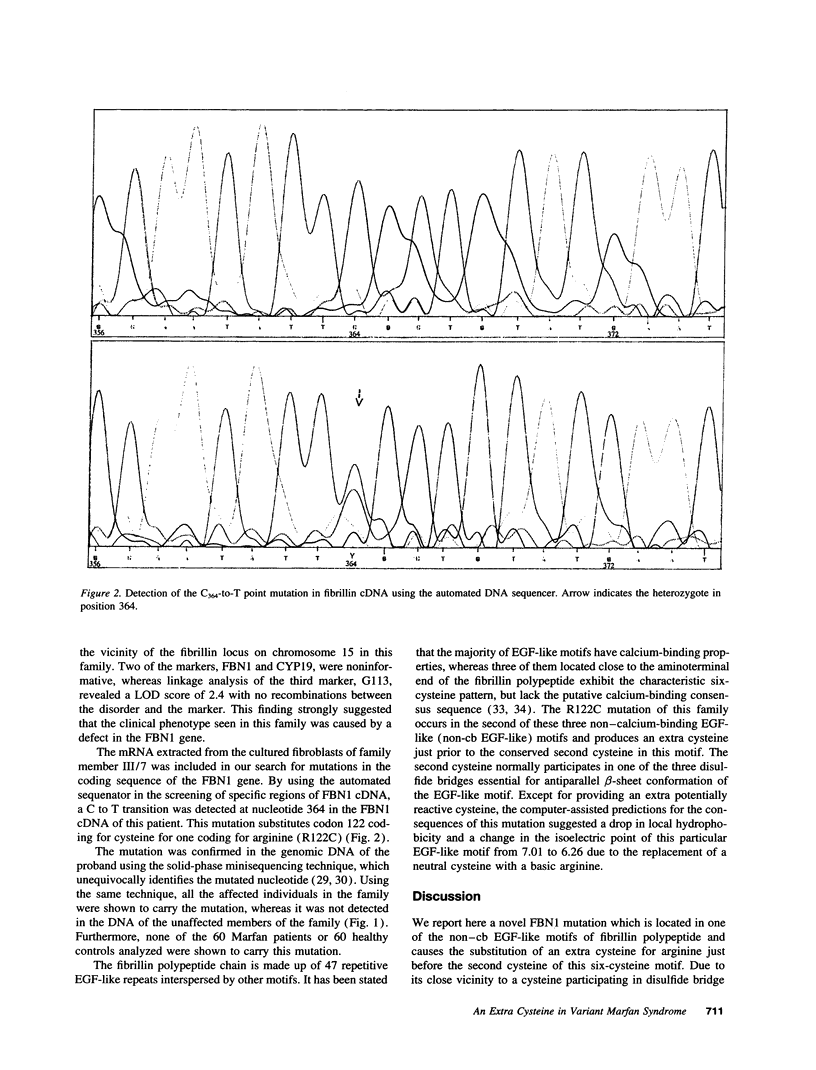
We present here a family with a clinical phenotype resembling Marfan syndrome (MFS), and displaying joint contracture and episodes of knee joint effusions, but lacking the cardiovascular features of the syndrome. The phenotype of this family represents a unique mixture of connective tissue symptoms, some of which are found in classical MFS and some of which are typical of dominant ectopia lentis. Linkage analyses suggested a linkage (LOD score 2.4; theta = 0) between the phenotype of the family and a polymorphic marker in the vicinity of the fibrillin locus on chromosome 15 (FBN1). Furthermore, a novel transition mutation was identified in the FBN1 gene in all the affected members of the family. In contrast to the majority of fibrillin mutations reported so far, this mutation substitutes a cysteine for arginine, producing an extra cysteine in one of the non-calcium-binding EGF-like motifs of the fibrillin polypeptide, most probably disturbing the formation of one of the three disulfide bridges known to be essential for the normal conformation of this motif.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansorge W., Sproat B., Stegemann J., Schwager C., Zenke M. Automated DNA sequencing: ultrasensitive detection of fluorescent bands during electrophoresis. Nucleic Acids Res. 1987 Jun 11;15(11):4593–4602. doi: 10.1093/nar/15.11.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appella E., Robinson E. A., Ullrich S. J., Stoppelli M. P., Corti A., Cassani G., Blasi F. The receptor-binding sequence of urokinase. A biological function for the growth-factor module of proteases. J Biol Chem. 1987 Apr 5;262(10):4437–4440. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of beta-turns. Biophys J. 1979 Jun;26(3):367–383. doi: 10.1016/S0006-3495(79)85259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H. H., Compton D. M. Fall accident patterns: characterization of most frequent work surface-related injuries. Prof Saf. 1982 Jun;27(6):16–22. [PubMed] [Google Scholar]
- Corson G. M., Chalberg S. C., Dietz H. C., Charbonneau N. L., Sakai L. Y. Fibrillin binds calcium and is coded by cDNAs that reveal a multidomain structure and alternatively spliced exons at the 5' end. Genomics. 1993 Aug;17(2):476–484. doi: 10.1006/geno.1993.1350. [DOI] [PubMed] [Google Scholar]
- Dietz H. C., Cutting G. R., Pyeritz R. E., Maslen C. L., Sakai L. Y., Corson G. M., Puffenberger E. G., Hamosh A., Nanthakumar E. J., Curristin S. M. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991 Jul 25;352(6333):337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- Dietz H. C., McIntosh I., Sakai L. Y., Corson G. M., Chalberg S. C., Pyeritz R. E., Francomano C. A. Four novel FBN1 mutations: significance for mutant transcript level and EGF-like domain calcium binding in the pathogenesis of Marfan syndrome. Genomics. 1993 Aug;17(2):468–475. doi: 10.1006/geno.1993.1349. [DOI] [PubMed] [Google Scholar]
- Dietz H. C., Pyeritz R. E., Puffenberger E. G., Kendzior R. J., Jr, Corson G. M., Maslen C. L., Sakai L. Y., Francomano C. A., Cutting G. R. Marfan phenotype variability in a family segregating a missense mutation in the epidermal growth factor-like motif of the fibrillin gene. J Clin Invest. 1992 May;89(5):1674–1680. doi: 10.1172/JCI115766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz H. C., Saraiva J. M., Pyeritz R. E., Cutting G. R., Francomano C. A. Clustering of fibrillin (FBN1) missense mutations in Marfan syndrome patients at cysteine residues in EGF-like domains. Hum Mutat. 1992;1(5):366–374. doi: 10.1002/humu.1380010504. [DOI] [PubMed] [Google Scholar]
- Dietz H. C., Valle D., Francomano C. A., Kendzior R. J., Jr, Pyeritz R. E., Cutting G. R. The skipping of constitutive exons in vivo induced by nonsense mutations. Science. 1993 Jan 29;259(5095):680–683. doi: 10.1126/science.8430317. [DOI] [PubMed] [Google Scholar]
- Godfrey M., Vandemark N., Wang M., Velinov M., Wargowski D., Tsipouras P., Han J., Becker J., Robertson W., Droste S. Prenatal diagnosis and a donor splice site mutation in fibrillin in a family with Marfan syndrome. Am J Hum Genet. 1993 Aug;53(2):472–480. [PMC free article] [PubMed] [Google Scholar]
- Hewett D. R., Lynch J. R., Smith R., Sykes B. C. A novel fibrillin mutation in the Marfan syndrome which could disrupt calcium binding of the epidermal growth factor-like module. Hum Mol Genet. 1993 Apr;2(4):475–477. doi: 10.1093/hmg/2.4.475. [DOI] [PubMed] [Google Scholar]
- Hudson T. J., Engelstein M., Lee M. K., Ho E. C., Rubenfield M. J., Adams C. P., Housman D. E., Dracopoli N. C. Isolation and chromosomal assignment of 100 highly informative human simple sequence repeat polymorphisms. Genomics. 1992 Jul;13(3):622–629. doi: 10.1016/0888-7543(92)90133-d. [DOI] [PubMed] [Google Scholar]
- Kainulainen K., Karttunen L., Puhakka L., Sakai L., Peltonen L. Mutations in the fibrillin gene responsible for dominant ectopia lentis and neonatal Marfan syndrome. Nat Genet. 1994 Jan;6(1):64–69. doi: 10.1038/ng0194-64. [DOI] [PubMed] [Google Scholar]
- Kainulainen K., Pulkkinen L., Savolainen A., Kaitila I., Peltonen L. Location on chromosome 15 of the gene defect causing Marfan syndrome. N Engl J Med. 1990 Oct 4;323(14):935–939. doi: 10.1056/NEJM199010043231402. [DOI] [PubMed] [Google Scholar]
- Kainulainen K., Sakai L. Y., Child A., Pope F. M., Puhakka L., Ryhänen L., Palotie A., Kaitila I., Peltonen L. Two mutations in Marfan syndrome resulting in truncated fibrillin polypeptides. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5917–5921. doi: 10.1073/pnas.89.13.5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komoriya A., Hortsch M., Meyers C., Smith M., Kanety H., Schlessinger J. Biologically active synthetic fragments of epidermal growth factor: localization of a major receptor-binding region. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1351–1355. doi: 10.1073/pnas.81.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Godfrey M., Vitale E., Hori H., Mattei M. G., Sarfarazi M., Tsipouras P., Ramirez F., Hollister D. W. Linkage of Marfan syndrome and a phenotypically related disorder to two different fibrillin genes. Nature. 1991 Jul 25;352(6333):330–334. doi: 10.1038/352330a0. [DOI] [PubMed] [Google Scholar]
- Lönnqvist L., Child A., Kainulainen K., Davidson R., Puhakka L., Peltonen L. A novel mutation of the fibrillin gene causing ectopia lentis. Genomics. 1994 Feb;19(3):573–576. doi: 10.1006/geno.1994.1110. [DOI] [PubMed] [Google Scholar]
- Magenis R. E., Maslen C. L., Smith L., Allen L., Sakai L. Y. Localization of the fibrillin (FBN) gene to chromosome 15, band q21.1. Genomics. 1991 Oct;11(2):346–351. doi: 10.1016/0888-7543(91)90142-2. [DOI] [PubMed] [Google Scholar]
- Maslen C. L., Corson G. M., Maddox B. K., Glanville R. W., Sakai L. Y. Partial sequence of a candidate gene for the Marfan syndrome. Nature. 1991 Jul 25;352(6333):334–337. doi: 10.1038/352334a0. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Novotný J., Auffray C. A program for prediction of protein secondary structure from nucleotide sequence data: application to histocompatibility antigens. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):243–255. doi: 10.1093/nar/12.1part1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., D'Alessio M., Ramirez F., Lynch J. R., Sykes B., Pangilinan T., Bonadio J. Genomic organization of the sequence coding for fibrillin, the defective gene product in Marfan syndrome. Hum Mol Genet. 1993 Jul;2(7):961–968. doi: 10.1093/hmg/2.7.961. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos M. H., Xiao H., Rath D. S., Merril C. R. Tetranucleotide repeat polymorphism at the human aromatase cytochrome P-450 gene (CYP19). Nucleic Acids Res. 1991 Jan 11;19(1):195–195. doi: 10.1093/nar/19.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantamäki T., Lönnqvist L., Karttunen L., Kainulainen K., Peltonen L. DNA diagnostics of the Marfan syndrome: application of amplifiable polymorphic markers. Eur J Hum Genet. 1994;2(1):66–75. doi: 10.1159/000472343. [DOI] [PubMed] [Google Scholar]
- Sakai L. Y., Keene D. R., Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol. 1986 Dec;103(6 Pt 1):2499–2509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai L. Y., Keene D. R., Glanville R. W., Bächinger H. P. Purification and partial characterization of fibrillin, a cysteine-rich structural component of connective tissue microfibrils. J Biol Chem. 1991 Aug 5;266(22):14763–14770. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syvänen A. C., Aalto-Setälä K., Harju L., Kontula K., Söderlund H. A primer-guided nucleotide incorporation assay in the genotyping of apolipoprotein E. Genomics. 1990 Dec;8(4):684–692. doi: 10.1016/0888-7543(90)90255-s. [DOI] [PubMed] [Google Scholar]
- Syvänen A. C., Aalto-Setälä K., Kontula K., Söderlund H. Direct sequencing of affinity-captured amplified human DNA application to the detection of apolipoprotein E polymorphism. FEBS Lett. 1989 Nov 20;258(1):71–74. doi: 10.1016/0014-5793(89)81618-7. [DOI] [PubMed] [Google Scholar]
- Syvänen A. C., Ikonen E., Manninen T., Bengtström M., Söderlund H., Aula P., Peltonen L. Convenient and quantitative determination of the frequency of a mutant allele using solid-phase minisequencing: application to aspartylglucosaminuria in Finland. Genomics. 1992 Mar;12(3):590–595. doi: 10.1016/0888-7543(92)90452-x. [DOI] [PubMed] [Google Scholar]
- Tsipouras P., Del Mastro R., Sarfarazi M., Lee B., Vitale E., Child A. H., Godfrey M., Devereux R. B., Hewett D., Steinmann B. Genetic linkage of the Marfan syndrome, ectopia lentis, and congenital contractural arachnodactyly to the fibrillin genes on chromosomes 15 and 5. The International Marfan Syndrome Collaborative Study. N Engl J Med. 1992 Apr 2;326(14):905–909. doi: 10.1056/NEJM199204023261401. [DOI] [PubMed] [Google Scholar]
- Vandenplas S., Wiid I., Grobler-Rabie A., Brebner K., Ricketts M., Wållis G., Bester A., Boyd C., Måthew C. Blot hybridisation analysis of genomic DNA. J Med Genet. 1984 Jun;21(3):164–172. doi: 10.1136/jmg.21.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton K. A., Johansen K. M., Xu T., Artavanis-Tsakonas S. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell. 1985 Dec;43(3 Pt 2):567–581. doi: 10.1016/0092-8674(85)90229-6. [DOI] [PubMed] [Google Scholar]