Abstract
The title compound, C15H11FO2, is an important intermediate in the synthesis of side-chain ligands for polymeric liquid crystals. The vinyl group is almost coplanar with both the aromatic rings. The crystal structure is stabilized by intermolecular O—H⋯O hydrogen bonding.
Related literature
For related literature, see: Ahmad et al. (2003 ▶); Collings & Hird (1997 ▶); Frazee & Foraker (2008 ▶); Hameed & Rama (2004 ▶); Hussain et al. (2005 ▶); Nazir et al. (2008 ▶); Ribeiro et al. (2008 ▶); Wang et al. (2008 ▶); Higashi (1999 ▶); Yasuda et al. (2000 ▶).
Experimental
Crystal data
C15H11FO2
M r = 242.24
Monoclinic,
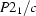
a = 6.261 (4) Å
b = 23.096 (15) Å
c = 8.269 (5) Å
β = 107.072 (8)°
V = 1143.1 (13) Å3
Z = 4
Mo Kα radiation
μ = 0.10 mm−1
T = 123 (2) K
0.45 × 0.30 × 0.18 mm
Data collection
Rigaku/MSC Mercury CCD diffractometer
Absorption correction: none
9111 measured reflections
2589 independent reflections
2399 reflections with I > 2σ(I)
R int = 0.036
Refinement
R[F 2 > 2σ(F 2)] = 0.065
wR(F 2) = 0.155
S = 1.16
2589 reflections
167 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.55 e Å−3
Δρmin = −0.28 e Å−3
Data collection: CrystalClear (MSC/Rigaku, 2001 ▶); cell refinement: CrystalClear; data reduction: TEXSAN (MSC/Rigaku, 2004 ▶); program(s) used to solve structure: SIR97 (Altomare et al., 1999 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEPII (Johnson, 1976 ▶); software used to prepare material for publication: SHELXL97 and TEXSAN.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808012920/hg2398sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808012920/hg2398Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O2—H2⋯O1i | 1.04 (4) | 1.57 (4) | 2.610 (2) | 174 (3) |
Symmetry code: (i) 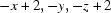 .
.
Acknowledgments
MKR is grateful to the Higher Education Commission of Pakistan for financial support under the International Support Initiative for Doctoral Fellowships at Gifu University, Japan.
supplementary crystallographic information
Comment
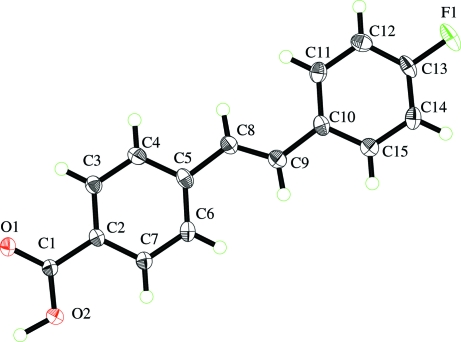
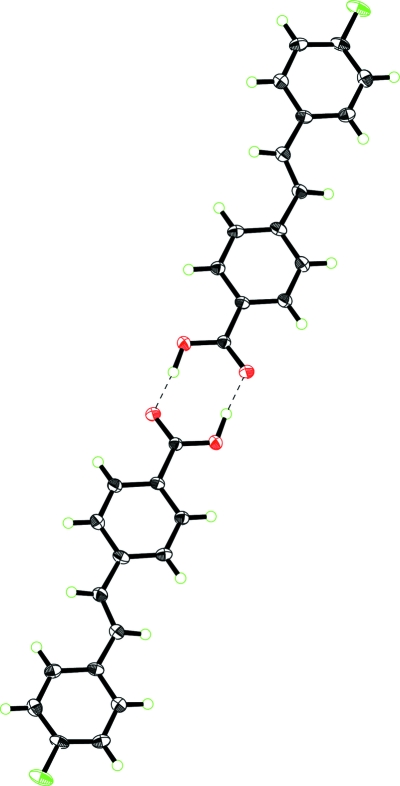
Carboxylic acids constitute an important class of organic compounds and have been used by the medicinal industry as important drugs (Ribeiro et al., 2008; Frazee & Foraker 2008; Hameed & Rama 2004). The carboxylic acids have also found applications as intermediates in the synthesis of an enormous number of organic compounds, in general (Hussain et al., 2005; Ahmad et al., 2003), and in the synthesis of side chain ligands for polymeric liquid crystals, in particular (Wang et al., 2008; Nazir et al., 2008). The liquid crystalline molecules containing substituents at 4-position behave as well ordered calamitic ligands as a side chain group (Collings & Hird, 1997) in side chain polymeric liquid crysrtals (SCPLCs). The derivatives of 4-(4-substituted styryl)benzoic acids have found applications as side chain groups in SCPLCs (Wang et al., 2008). As a part of a project to synthesize ligands for SCPLCs, the title compound, (E)-4-(4-fluorostyryl)benzoic acid (I), was synthesized by reacting 4-fluorobenzaldehyde with methyl [4-(methoxycarbonyl)benzyl]triphenylphosphonium bromide (Nazir et al., 2008) followed by hydrolysis. In the present article, the crystal structure of (I) is being reported. Bond lengths and angles are within the normal ranges as given for vinylbenzoic acid (Yasuda et al., 2000). The C(1)—O(1) and C(1)—O(2) bond lengths are 1.252 (2) and 1.291 (2) respectively,clearly indicating the partial double bond character of the carboxylate group. The carboxylic acid group subtends a dihedral angle[13.72 (16)°] with the phenyl ring C(2)/C(3)/C(4)/C(5)/C(6)/C(7).The vinyl group is almost coplanar with both the phenyl rings. The torsion angles between the phenyl rings and vinyl group fulfill the condition of coplanarity[near to 0° or 180 °]. Two molecules related by an inversion center form a dimer via two hydrogen bonds composed of two carboxyl groups as shown in Fig. 2.
Experimental
Methyl 4-(4-fluorostyryl)benzoate 0.8g (0.0031moles) and sodium hydroxide 0.126g (0.0031 moles) were dissolved in a mixture of 10 ml of methanol and 30 ml of water, and the mixture refluxed for 3 hours. The reaction mixture was cooled to room temperature and acidified with 6M HCl. The precipitated solid was filtered and recrystallized from hot ethanol. Yield: 76%, m.p: 240-252°C, Rf = 0.22 (n-hexane : ethyl acetate 7 : 3). IR (νmax, KBr, cm-1): 3300-2500, 1715, 1620, 1600, 1580, 1188, 1119, 965,834. 1H-NMR (300 MHz,DMSO-d6): δ 7.25 (2H, d, J = 9.0 Hz),7.28 (1H, d, J= 16.2 Hz), 7.42 (1H, d, J = 16.2 Hz), 7.71-7.67 (4H, m),7.95 (2H, d, J = 8.1 Hz), 12.93 (1H, s). 13C-NMR (75 MHz, DMSO-d6): δ 116.16 (d, J= 23 Hz), 126.90, 127.77, 129.23, 130.08 (d, J = 8 Hz), 131.95, 132.49, 133.71 (d, J = 3 Hz), 141.83, 162.42 (d, J = 246 Hz), 167.54.
Refinement
The O-bound H atom was refined isotropically. All the other H atoms were placed in idealized positions and treated as riding atoms with C—H distance in the range 0.95–0.99 Å and Uiso(H) = 1.2Ueq(C) or 1.5Ueq(C).
Figures
Fig. 1.
Molecular structure of (I) showing atom-labelling scheme and displacement ellipsoids at the 30% probability level.
Fig. 2.
Showing hydrogen bonded molecules through N—H···O.
Crystal data
| C15H11FO2 | F(000) = 504 |
| Mr = 242.24 | Dx = 1.408 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71070 Å |
| Hall symbol: -P 2ybc | Cell parameters from 3252 reflections |
| a = 6.261 (4) Å | θ = 3.1–27.5° |
| b = 23.096 (15) Å | µ = 0.10 mm−1 |
| c = 8.269 (5) Å | T = 123 K |
| β = 107.072 (8)° | Needle, colourless |
| V = 1143.1 (13) Å3 | 0.45 × 0.30 × 0.18 mm |
| Z = 4 |
Data collection
| Rigaku/MSC Mercury CCD diffractometer | 2399 reflections with I > 2σ(I) |
| Radiation source: Rotating anode | Rint = 0.036 |
| graphite | θmax = 27.5°, θmin = 3.1° |
| ω scans | h = −8→6 |
| 9111 measured reflections | k = −25→29 |
| 2589 independent reflections | l = −10→9 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.065 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.155 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.16 | w = 1/[σ2(Fo2) + (0.0616P)2 + 0.7669P] where P = (Fo2 + 2Fc2)/3 |
| 2589 reflections | (Δ/σ)max < 0.001 |
| 167 parameters | Δρmax = 0.55 e Å−3 |
| 0 restraints | Δρmin = −0.28 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.7742 (3) | 0.03235 (7) | 0.8139 (2) | 0.0188 (4) | |
| O1 | 0.9748 (2) | 0.04569 (6) | 0.83715 (16) | 0.0268 (3) | |
| O2 | 0.7095 (2) | −0.00358 (6) | 0.90952 (16) | 0.0258 (3) | |
| H2 | 0.843 (6) | −0.0202 (16) | 1.006 (5) | 0.089 (12)* | |
| C2 | 0.5982 (3) | 0.05820 (7) | 0.6720 (2) | 0.0188 (4) | |
| C3 | 0.6547 (3) | 0.10173 (8) | 0.5755 (2) | 0.0221 (4) | |
| H3 | 0.8054 | 0.1143 | 0.6002 | 0.027* | |
| C4 | 0.4916 (3) | 0.12671 (8) | 0.4437 (2) | 0.0246 (4) | |
| H4 | 0.5313 | 0.1568 | 0.3796 | 0.030* | |
| C5 | 0.2699 (3) | 0.10851 (8) | 0.4031 (2) | 0.0231 (4) | |
| C6 | 0.2149 (3) | 0.06476 (8) | 0.5012 (2) | 0.0232 (4) | |
| H6 | 0.0645 | 0.0519 | 0.4756 | 0.028* | |
| C7 | 0.3771 (3) | 0.03999 (8) | 0.6354 (2) | 0.0213 (4) | |
| H7 | 0.3374 | 0.0107 | 0.7020 | 0.026* | |
| C8 | 0.1067 (3) | 0.13727 (8) | 0.2605 (2) | 0.0250 (4) | |
| H8 | 0.1586 | 0.1694 | 0.2111 | 0.030* | |
| C9 | −0.1064 (3) | 0.12250 (8) | 0.1943 (2) | 0.0246 (4) | |
| H9 | −0.1587 | 0.0904 | 0.2436 | 0.030* | |
| C10 | −0.2687 (3) | 0.15134 (8) | 0.0517 (2) | 0.0224 (4) | |
| C11 | −0.2127 (3) | 0.19609 (8) | −0.0431 (2) | 0.0250 (4) | |
| H11 | −0.0628 | 0.2094 | −0.0148 | 0.030* | |
| C12 | −0.3734 (3) | 0.22120 (8) | −0.1778 (2) | 0.0273 (4) | |
| H12 | −0.3353 | 0.2514 | −0.2424 | 0.033* | |
| C13 | −0.5900 (3) | 0.20104 (8) | −0.2150 (2) | 0.0262 (4) | |
| C14 | −0.6531 (3) | 0.15774 (8) | −0.1256 (2) | 0.0257 (4) | |
| H14 | −0.8040 | 0.1451 | −0.1539 | 0.031* | |
| C15 | −0.4898 (3) | 0.13277 (8) | 0.0075 (2) | 0.0241 (4) | |
| H15 | −0.5300 | 0.1023 | 0.0700 | 0.029* | |
| F1 | −0.7470 (2) | 0.22525 (6) | −0.34838 (15) | 0.0419 (4) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0185 (8) | 0.0204 (8) | 0.0175 (8) | 0.0011 (6) | 0.0052 (6) | −0.0007 (6) |
| O1 | 0.0173 (6) | 0.0346 (8) | 0.0269 (7) | 0.0001 (5) | 0.0040 (5) | 0.0061 (5) |
| O2 | 0.0227 (6) | 0.0285 (7) | 0.0251 (6) | 0.0005 (5) | 0.0053 (5) | 0.0084 (5) |
| C2 | 0.0190 (8) | 0.0200 (8) | 0.0165 (8) | 0.0030 (6) | 0.0038 (6) | −0.0018 (6) |
| C3 | 0.0213 (8) | 0.0251 (9) | 0.0195 (8) | 0.0016 (7) | 0.0054 (6) | −0.0001 (6) |
| C4 | 0.0267 (9) | 0.0253 (9) | 0.0219 (8) | 0.0031 (7) | 0.0073 (7) | 0.0034 (7) |
| C5 | 0.0246 (9) | 0.0249 (9) | 0.0175 (8) | 0.0057 (7) | 0.0029 (7) | −0.0022 (6) |
| C6 | 0.0175 (8) | 0.0265 (9) | 0.0237 (8) | 0.0019 (7) | 0.0032 (7) | −0.0034 (7) |
| C7 | 0.0196 (8) | 0.0226 (9) | 0.0211 (8) | 0.0012 (7) | 0.0052 (6) | −0.0003 (6) |
| C8 | 0.0238 (9) | 0.0262 (9) | 0.0234 (8) | 0.0025 (7) | 0.0045 (7) | 0.0031 (7) |
| C9 | 0.0264 (9) | 0.0248 (9) | 0.0221 (8) | 0.0011 (7) | 0.0062 (7) | 0.0004 (7) |
| C10 | 0.0220 (9) | 0.0243 (9) | 0.0187 (8) | 0.0044 (7) | 0.0028 (6) | −0.0041 (6) |
| C11 | 0.0216 (9) | 0.0259 (10) | 0.0265 (9) | −0.0002 (7) | 0.0055 (7) | −0.0057 (7) |
| C12 | 0.0347 (10) | 0.0217 (9) | 0.0265 (9) | 0.0025 (8) | 0.0104 (8) | 0.0006 (7) |
| C13 | 0.0280 (9) | 0.0233 (9) | 0.0215 (8) | 0.0116 (7) | −0.0018 (7) | −0.0012 (7) |
| C14 | 0.0188 (8) | 0.0273 (9) | 0.0290 (9) | 0.0019 (7) | 0.0038 (7) | −0.0072 (7) |
| C15 | 0.0260 (9) | 0.0229 (9) | 0.0232 (8) | 0.0013 (7) | 0.0067 (7) | −0.0006 (7) |
| F1 | 0.0413 (7) | 0.0402 (8) | 0.0330 (7) | 0.0202 (6) | −0.0063 (5) | 0.0036 (5) |
Geometric parameters (Å, °)
| C1—O1 | 1.252 (2) | C8—C9 | 1.330 (3) |
| C1—O2 | 1.291 (2) | C8—H8 | 0.9500 |
| C1—C2 | 1.479 (2) | C9—C10 | 1.471 (2) |
| O2—H2 | 1.04 (4) | C9—H9 | 0.9500 |
| C2—C3 | 1.392 (3) | C10—C15 | 1.392 (3) |
| C2—C7 | 1.393 (3) | C10—C11 | 1.402 (3) |
| C3—C4 | 1.382 (2) | C11—C12 | 1.390 (3) |
| C3—H3 | 0.9500 | C11—H11 | 0.9500 |
| C4—C5 | 1.394 (3) | C12—C13 | 1.380 (3) |
| C4—H4 | 0.9500 | C12—H12 | 0.9500 |
| C5—C6 | 1.401 (3) | C13—F1 | 1.363 (2) |
| C5—C8 | 1.473 (2) | C13—C14 | 1.369 (3) |
| C6—C7 | 1.389 (2) | C14—C15 | 1.389 (3) |
| C6—H6 | 0.9500 | C14—H14 | 0.9500 |
| C7—H7 | 0.9500 | C15—H15 | 0.9500 |
| O1—C1—O2 | 123.07 (15) | C9—C8—H8 | 116.9 |
| O1—C1—C2 | 120.16 (15) | C5—C8—H8 | 116.9 |
| O2—C1—C2 | 116.78 (15) | C8—C9—C10 | 125.99 (18) |
| C1—O2—H2 | 112 (2) | C8—C9—H9 | 117.0 |
| C3—C2—C7 | 119.79 (15) | C10—C9—H9 | 117.0 |
| C3—C2—C1 | 119.41 (16) | C15—C10—C11 | 118.25 (16) |
| C7—C2—C1 | 120.80 (16) | C15—C10—C9 | 118.07 (17) |
| C4—C3—C2 | 120.05 (17) | C11—C10—C9 | 123.68 (17) |
| C4—C3—H3 | 120.0 | C12—C11—C10 | 120.96 (18) |
| C2—C3—H3 | 120.0 | C12—C11—H11 | 119.5 |
| C3—C4—C5 | 121.11 (18) | C10—C11—H11 | 119.5 |
| C3—C4—H4 | 119.4 | C13—C12—C11 | 118.08 (18) |
| C5—C4—H4 | 119.4 | C13—C12—H12 | 121.0 |
| C4—C5—C6 | 118.38 (16) | C11—C12—H12 | 121.0 |
| C4—C5—C8 | 117.69 (17) | F1—C13—C14 | 118.85 (18) |
| C6—C5—C8 | 123.92 (17) | F1—C13—C12 | 118.02 (18) |
| C7—C6—C5 | 120.88 (17) | C14—C13—C12 | 123.12 (17) |
| C7—C6—H6 | 119.6 | C13—C14—C15 | 118.00 (17) |
| C5—C6—H6 | 119.6 | C13—C14—H14 | 121.0 |
| C6—C7—C2 | 119.77 (17) | C15—C14—H14 | 121.0 |
| C6—C7—H7 | 120.1 | C14—C15—C10 | 121.58 (18) |
| C2—C7—H7 | 120.1 | C14—C15—H15 | 119.2 |
| C9—C8—C5 | 126.22 (18) | C10—C15—H15 | 119.2 |
| O1—C1—C2—C3 | −6.5 (2) | C6—C5—C8—C9 | −7.2 (3) |
| O2—C1—C2—C3 | 173.26 (15) | C5—C8—C9—C10 | −179.93 (17) |
| O1—C1—C2—C7 | 174.23 (16) | C8—C9—C10—C15 | −175.06 (18) |
| O2—C1—C2—C7 | −6.0 (2) | C8—C9—C10—C11 | 5.5 (3) |
| C7—C2—C3—C4 | −0.1 (3) | C15—C10—C11—C12 | −0.1 (3) |
| C1—C2—C3—C4 | −179.31 (16) | C9—C10—C11—C12 | 179.26 (17) |
| C2—C3—C4—C5 | −1.0 (3) | C10—C11—C12—C13 | 0.4 (3) |
| C3—C4—C5—C6 | 1.1 (3) | C11—C12—C13—F1 | −179.25 (16) |
| C3—C4—C5—C8 | −179.83 (16) | C11—C12—C13—C14 | 0.1 (3) |
| C4—C5—C6—C7 | −0.2 (3) | F1—C13—C14—C15 | 178.64 (16) |
| C8—C5—C6—C7 | −179.19 (17) | C12—C13—C14—C15 | −0.7 (3) |
| C5—C6—C7—C2 | −0.9 (3) | C13—C14—C15—C10 | 0.9 (3) |
| C3—C2—C7—C6 | 1.0 (3) | C11—C10—C15—C14 | −0.5 (3) |
| C1—C2—C7—C6 | −179.80 (15) | C9—C10—C15—C14 | −179.93 (16) |
| C4—C5—C8—C9 | 173.72 (19) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O2—H2···O1i | 1.04 (4) | 1.57 (4) | 2.610 (2) | 174 (3) |
Symmetry codes: (i) −x+2, −y, −z+2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HG2398).
References
- Ahmad, H. B., Rama, N. H., Hussain, M., Hussain, M. T., Qasim, M. M., Hameed, S., Malana, M. A. & Malik, A. (2003). Indian J. Chem. B42, 611–615.
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst.32, 115–119.
- Collings, P. J. & Hird, M. (1997). Introduction to Liquid Crystals, Chemistry and Physics, edited by G. W. Gray, J. W. Goodby & A. Fukuda, pp. 43–78. Bristol: Taylor and Francis.
- Frazee, L. A. & Foraker, K. C. (2008). Annals Pharmacother.42, 403–407. [DOI] [PubMed]
- Hameed, S. & Rama, N. H. (2004). J. Chem. Soc. Pak.26, 157–162.
- Higashi, T. (1999). NUMABS Rigaku Corporation, 3-9-12 Akishima, Tokyo 196-8666, Japan.
- Hussain, M. T., Rama, N. H., Hameed, S., Malik, A. & Khan, K. M. (2005). Nat. Prod. Res.19, 41–51. [DOI] [PubMed]
- Johnson, C. K. (1976). ORTEPII Report ORNL-5138. Oak Ridge National Laboratory, Tennessee, USA.
- MSC/Rigaku (2001). CrystalClear MSC/Rigaku, The Woodlands, Texas, USA.
- MSC/Rigaku (2004). TEXSAN MSC/Rigaku, The Woodlands, Texas, USA.
- Nazir, S., Khawar Rauf, M., Ebihara, M. & Hameed, S. (2008). Acta Cryst. E64, o423. [DOI] [PMC free article] [PubMed]
- Ribeiro, G., Benadiba, M., Colquhoun, A. & Silva, D. D. (2008). Polyhedron, 27, 1131–1137.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Wang, M. L., Liu, B. L. & Lin, S. J. (2008). Chem. Eng. Commun.195, 770–786.
- Yasuda, N., Uekusa, H. & Ohashi, Y. (2000). Acta Cryst. C56, 1364–1366. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808012920/hg2398sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808012920/hg2398Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report