Abstract
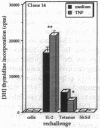
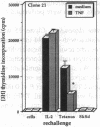
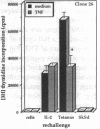
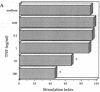
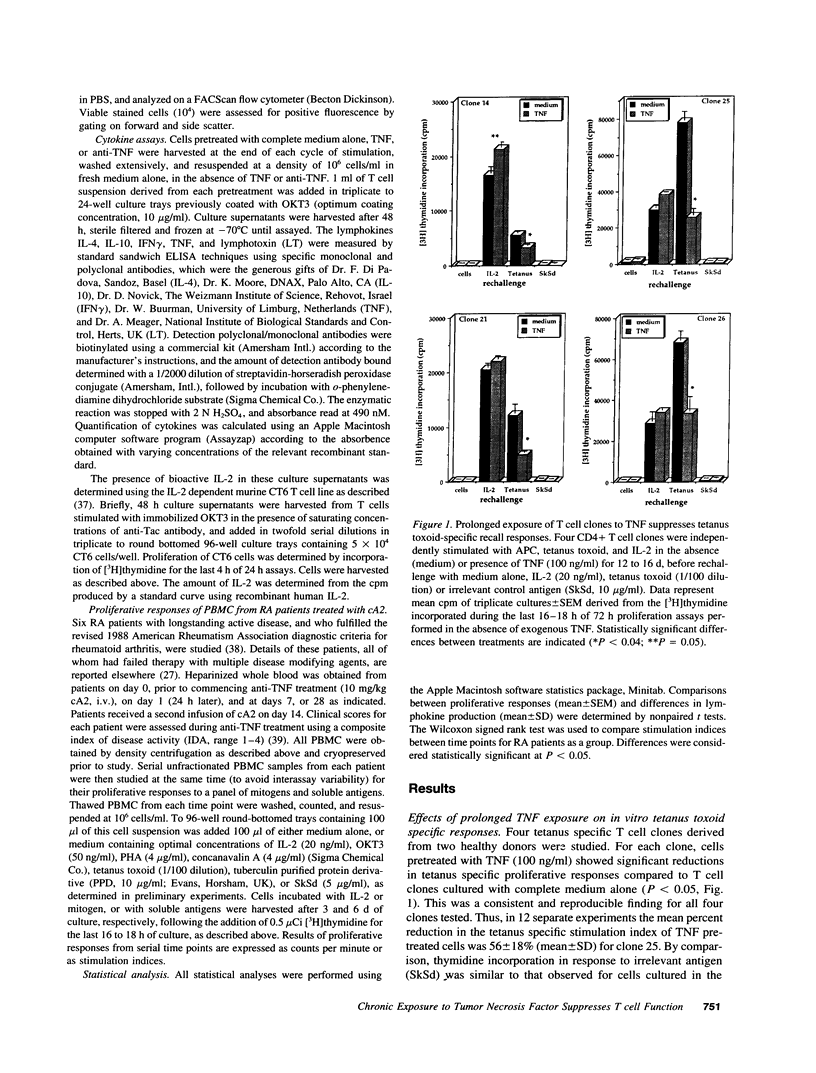
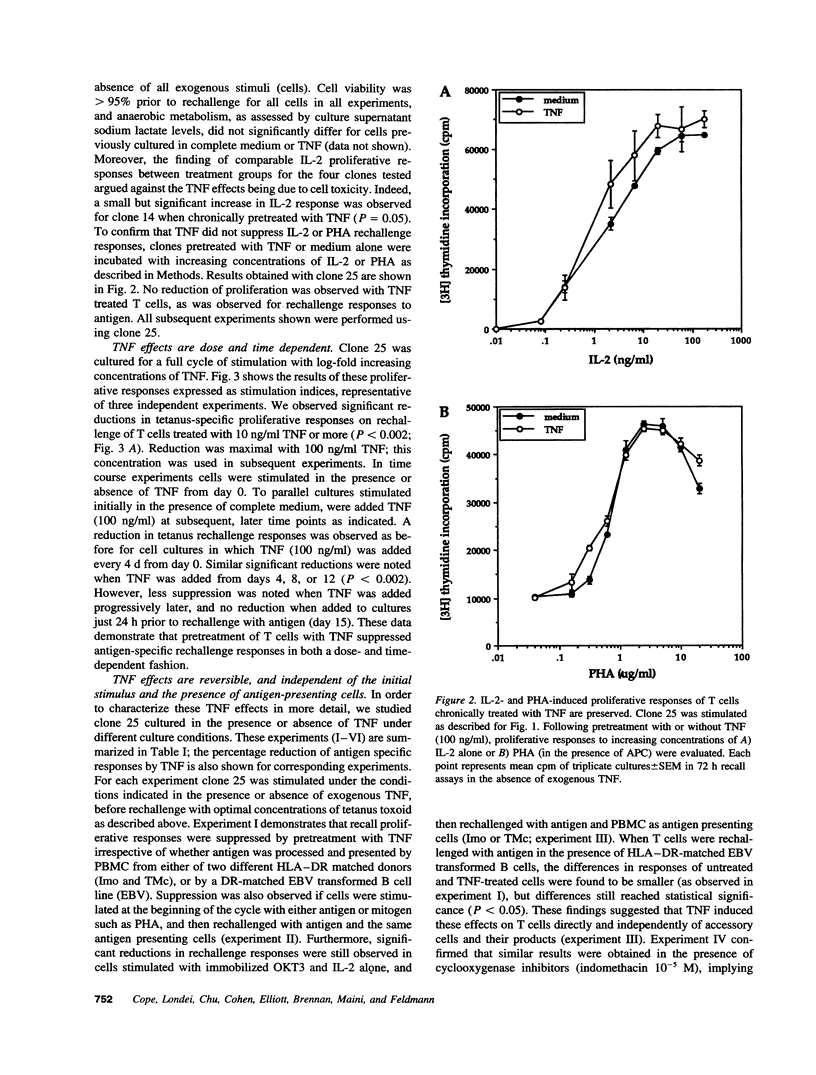
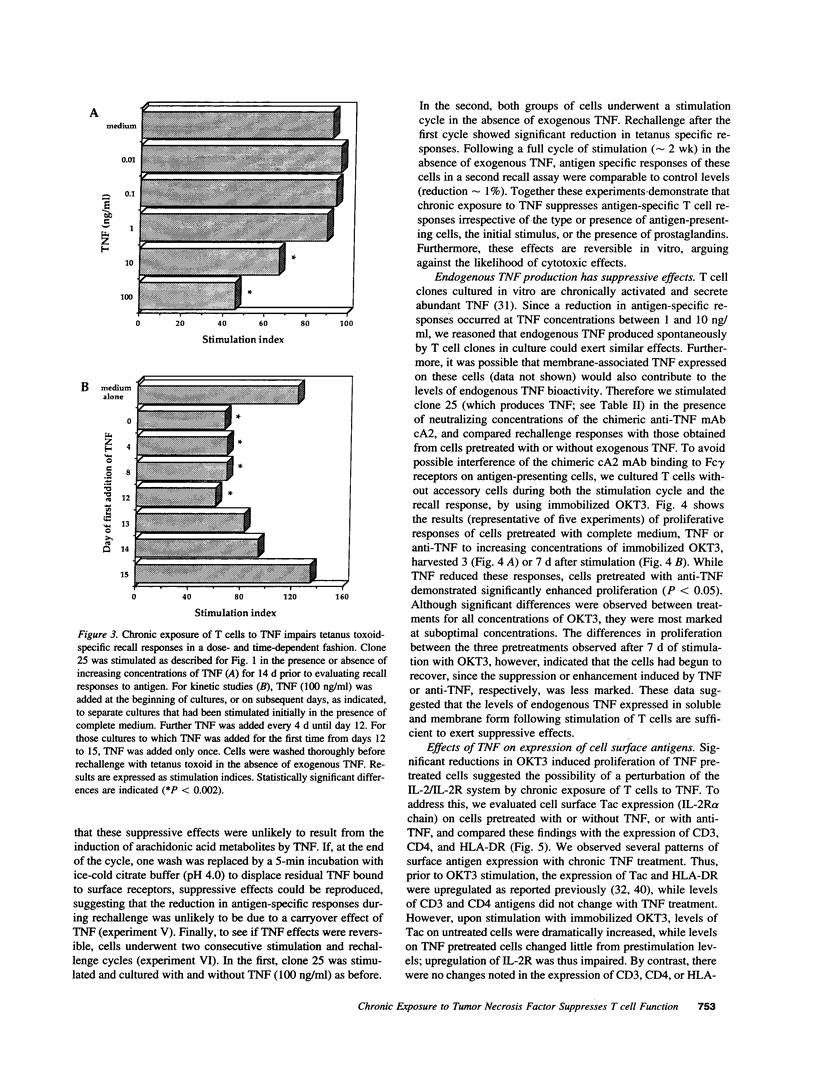
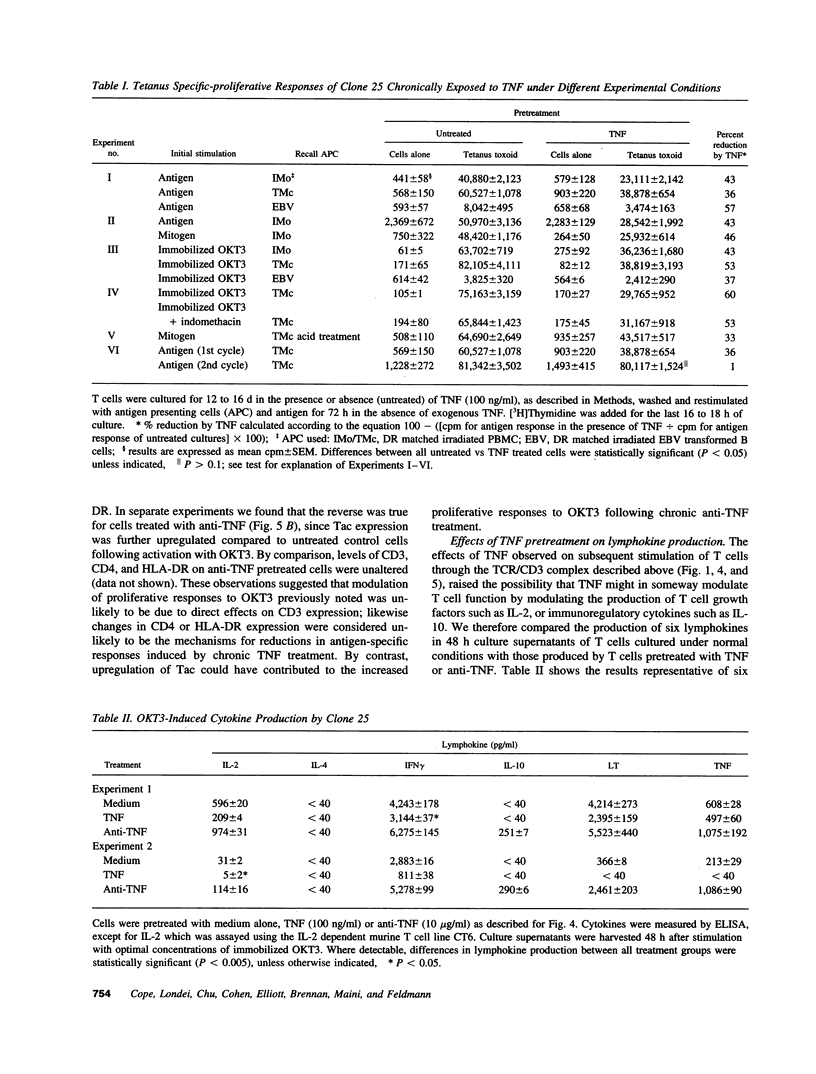
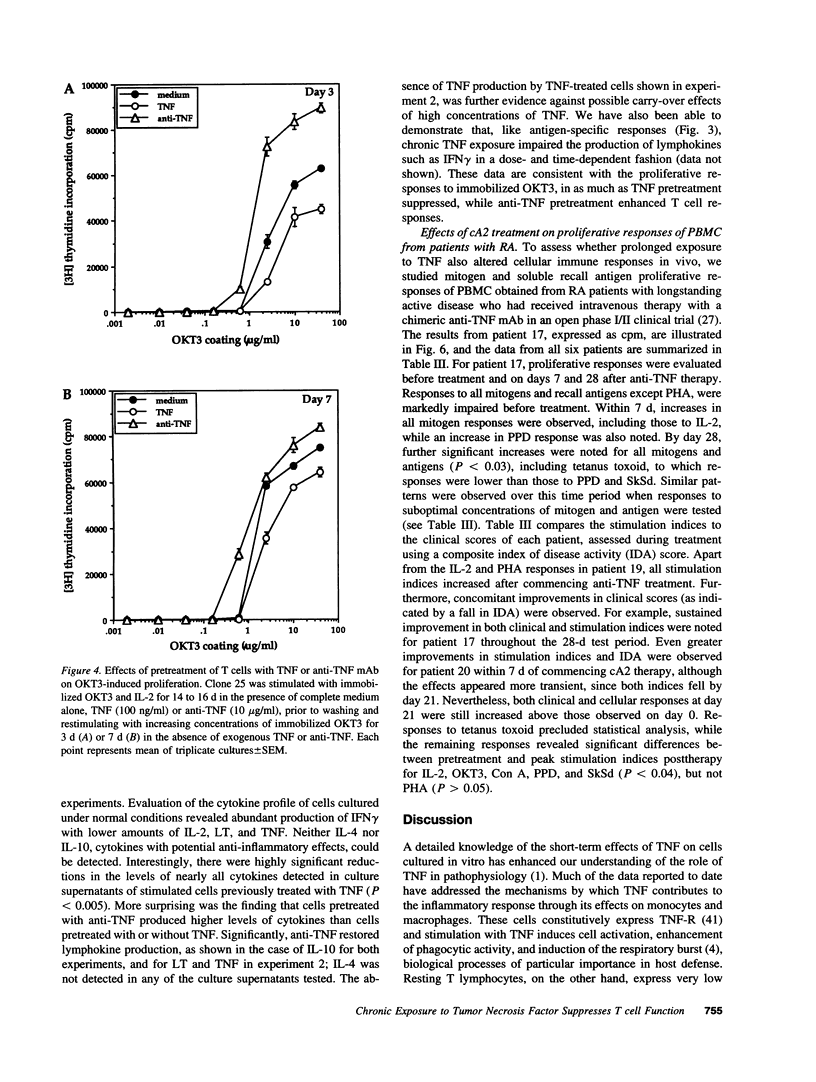
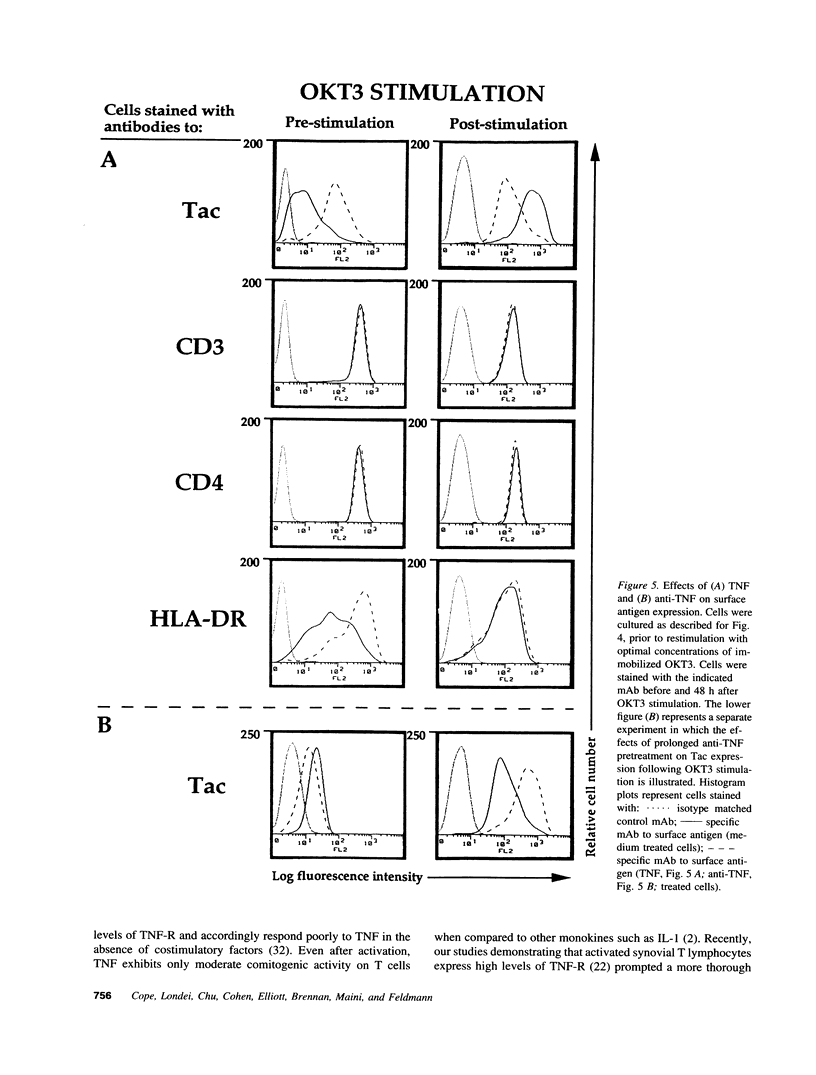
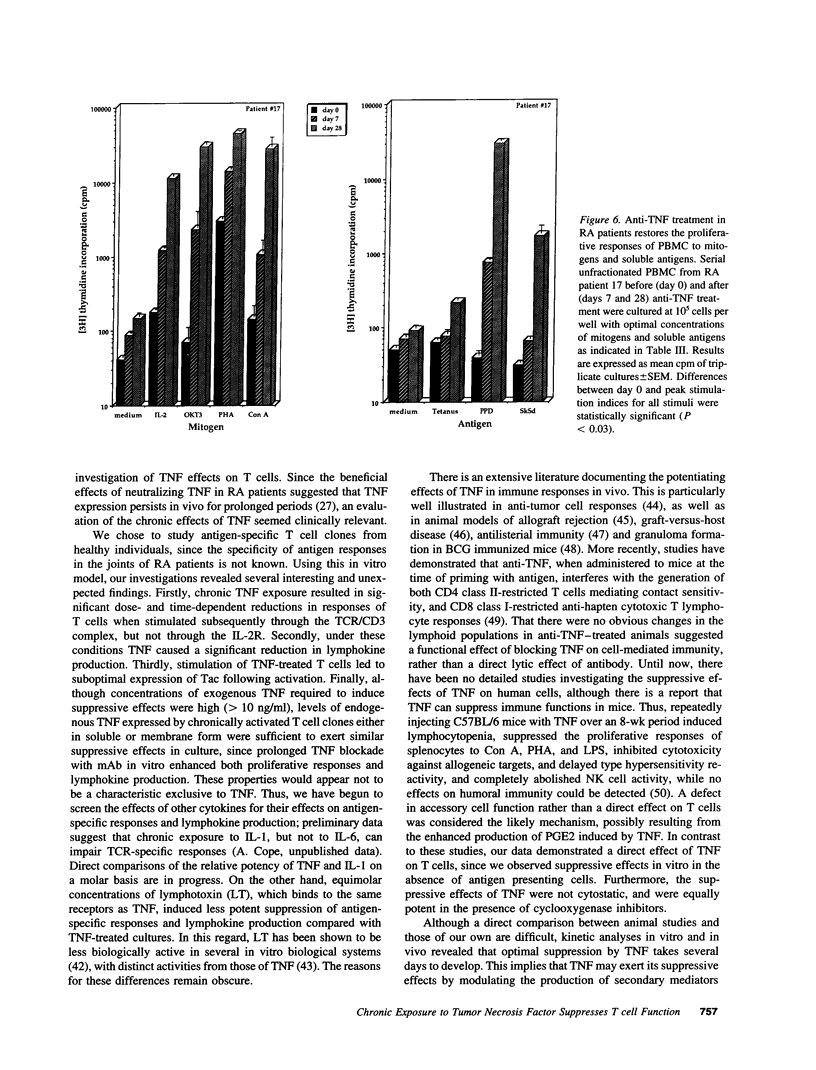
Experiments were designed to test the hypothesis that chronic exposure to tumor necrosis factor alpha (TNF) alters the function of activated T lymphocytes. Pretreatment of tetanus toxoid-specific T cell clones with TNF for up to 16 d impaired rechallenge proliferative responses to antigen in a dose- and time-dependent fashion. IL-2 and PHA responses were preserved. Prolonged treatment with TNF impaired production of IL-2, IL-10, IFN gamma, TNF, and lymphotoxin (LT) following stimulation with immobilized OKT3, and resulted in suboptimal expression of the IL-2R alpha chain (Tac) but not CD3, CD4, or HLA-DR antigens, when compared to untreated control cells. By contrast, pretreatment of T cells for prolonged periods in vitro with neutralizing anti-TNF monoclonal antibodies (mAb) enhanced proliferative responses, increased lymphokine production, and upregulated Tac expression following stimulation with OKT3. To determine whether TNF exerts immunosuppressive effects on T cells in vivo, we studied cell-mediated immunity in patients with active rheumatoid arthritis (RA), before and after treatment with a chimeric anti-TNF mAb. Treatment with anti-TNF restored the diminished proliferative responses of PBMC to mitogens and recall antigens towards normal in all patients tested. These data demonstrate that persistent expression of TNF in vitro and in vivo impairs cell-mediated immune responses.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bancroft G. J., Sheehan K. C., Schreiber R. D., Unanue E. R. Tumor necrosis factor is involved in the T cell-independent pathway of macrophage activation in scid mice. J Immunol. 1989 Jul 1;143(1):127–130. [PubMed] [Google Scholar]
- Beutler B., Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Brennan F. M., Chantry D., Jackson A., Maini R., Feldmann M. Inhibitory effect of TNF alpha antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet. 1989 Jul 29;2(8657):244–247. doi: 10.1016/s0140-6736(89)90430-3. [DOI] [PubMed] [Google Scholar]
- Brennan F. M., Gibbons D. L., Mitchell T., Cope A. P., Maini R. N., Feldmann M. Enhanced expression of tumor necrosis factor receptor mRNA and protein in mononuclear cells isolated from rheumatoid arthritis synovial joints. Eur J Immunol. 1992 Jul;22(7):1907–1912. doi: 10.1002/eji.1830220734. [DOI] [PubMed] [Google Scholar]
- Bromberg J. S., Chavin K. D., Kunkel S. L. Anti-tumor necrosis factor antibodies suppress cell-mediated immunity in vivo. J Immunol. 1992 Jun 1;148(11):3412–3417. [PubMed] [Google Scholar]
- Brouckaert P., Spriggs D. R., Demetri G., Kufe D. W., Fiers W. Circulating interleukin 6 during a continuous infusion of tumor necrosis factor and interferon gamma. J Exp Med. 1989 Jun 1;169(6):2257–2262. doi: 10.1084/jem.169.6.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broudy V. C., Kaushansky K., Segal G. M., Harlan J. M., Adamson J. W. Tumor necrosis factor type alpha stimulates human endothelial cells to produce granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7467–7471. doi: 10.1073/pnas.83.19.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan G., Barrett K., Turner M., Chantry D., Maini R. N., Feldmann M. Interleukin-1 and tumour necrosis factor mRNA expression in rheumatoid arthritis: prolonged production of IL-1 alpha. Clin Exp Immunol. 1988 Sep;73(3):449–455. [PMC free article] [PubMed] [Google Scholar]
- Chantry D., Turner M., Abney E., Feldmann M. Modulation of cytokine production by transforming growth factor-beta. J Immunol. 1989 Jun 15;142(12):4295–4300. [PubMed] [Google Scholar]
- Cope A. P., Gibbons D. L., Aderka D., Foxwell B. M., Wallach D., Maini R. N., Feldmann M., Brennan F. M. Differential regulation of tumour necrosis factor receptors (TNF-R) by IL-4; upregulation of P55 and P75 TNF-R on synovial joint mononuclear cells. Cytokine. 1993 May;5(3):205–212. doi: 10.1016/1043-4666(93)90006-q. [DOI] [PubMed] [Google Scholar]
- Cush J. J., Lipsky P. E. Phenotypic analysis of synovial tissue and peripheral blood lymphocytes isolated from patients with rheumatoid arthritis. Arthritis Rheum. 1988 Oct;31(10):1230–1238. doi: 10.1002/art.1780311003. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Elliott M. J., Maini R. N., Feldmann M., Long-Fox A., Charles P., Katsikis P., Brennan F. M., Walker J., Bijl H., Ghrayeb J. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheum. 1993 Dec;36(12):1681–1690. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- Emery P., Panayi G. S., Nouri A. M. Interleukin-2 reverses deficient cell-mediated immune responses in rheumatoid arthritis. Clin Exp Immunol. 1984 Jul;57(1):123–129. [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Brennan F. M., Chantry D., Haworth C., Turner M., Abney E., Buchan G., Barrett K., Barkley D., Chu A. Cytokine production in the rheumatoid joint: implications for treatment. Ann Rheum Dis. 1990 Jun;49 (Suppl 1):480–486. [PubMed] [Google Scholar]
- Firestein G. S., Xu W. D., Townsend K., Broide D., Alvaro-Gracia J., Glasebrook A., Zvaifler N. J. Cytokines in chronic inflammatory arthritis. I. Failure to detect T cell lymphokines (interleukin 2 and interleukin 3) and presence of macrophage colony-stimulating factor (CSF-1) and a novel mast cell growth factor in rheumatoid synovitis. J Exp Med. 1988 Nov 1;168(5):1573–1586. doi: 10.1084/jem.168.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Zvaifler N. J. Peripheral blood and synovial fluid monocyte activation in inflammatory arthritis. II. Low levels of synovial fluid and synovial tissue interferon suggest that gamma-interferon is not the primary macrophage activating factor. Arthritis Rheum. 1987 Aug;30(8):864–871. doi: 10.1002/art.1780300804. [DOI] [PubMed] [Google Scholar]
- Franciotta D. M., Grimaldi L. M., Martino G. V., Piccolo G., Bergamaschi R., Citterio A., Melzi d'Eril G. V. Tumor necrosis factor in serum and cerebrospinal fluid of patients with multiple sclerosis. Ann Neurol. 1989 Dec;26(6):787–789. doi: 10.1002/ana.410260618. [DOI] [PubMed] [Google Scholar]
- Gordon C., Wofsy D. Effects of recombinant murine tumor necrosis factor-alpha on immune function. J Immunol. 1990 Mar 1;144(5):1753–1758. [PubMed] [Google Scholar]
- Haraoui B., Wilder R. L., Malone D. G., Allen J. B., Katona I. M., Wahl S. M. Immune function in severe, active rheumatoid arthritis: a relationship between peripheral blood mononuclear cell proliferation to soluble antigens and mononuclear cell subset profiles. J Immunol. 1984 Aug;133(2):697–701. [PubMed] [Google Scholar]
- Hart P. H., Vitti G. F., Burgess D. R., Whitty G. A., Piccoli D. S., Hamilton J. A. Potential antiinflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc Natl Acad Sci U S A. 1989 May;86(10):3803–3807. doi: 10.1073/pnas.86.10.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth C., Brennan F. M., Chantry D., Turner M., Maini R. N., Feldmann M. Expression of granulocyte-macrophage colony-stimulating factor in rheumatoid arthritis: regulation by tumor necrosis factor-alpha. Eur J Immunol. 1991 Oct;21(10):2575–2579. doi: 10.1002/eji.1830211039. [DOI] [PubMed] [Google Scholar]
- Ho S. N., Abraham R. T., Gillis S., McKean D. J. Differential bioassay of interleukin 2 and interleukin 4. J Immunol Methods. 1987 Apr 2;98(1):99–104. doi: 10.1016/0022-1759(87)90441-8. [DOI] [PubMed] [Google Scholar]
- Imagawa D. K., Millis J. M., Olthoff K. M., Seu P., Dempsey R. A., Hart J., Terasaki P. I., Wasef E. M., Busuttil R. W. The role of tumor necrosis factor in allograft rejection. II. Evidence that antibody therapy against tumor necrosis factor-alpha and lymphotoxin enhances cardiac allograft survival in rats. Transplantation. 1990 Aug;50(2):189–193. doi: 10.1097/00007890-199008000-00003. [DOI] [PubMed] [Google Scholar]
- Imamura K., Spriggs D., Kufe D. Expression of tumor necrosis factor receptors on human monocytes and internalization of receptor bound ligand. J Immunol. 1987 Nov 1;139(9):2989–2992. [PubMed] [Google Scholar]
- Keffer J., Probert L., Cazlaris H., Georgopoulos S., Kaslaris E., Kioussis D., Kollias G. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991 Dec;10(13):4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrl J. H., Miller A., Fauci A. S. Effect of tumor necrosis factor alpha on mitogen-activated human B cells. J Exp Med. 1987 Sep 1;166(3):786–791. doi: 10.1084/jem.166.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Kinkhabwala M., Sehajpal P., Skolnik E., Smith D., Sharma V. K., Vlassara H., Cerami A., Suthanthiran M. A novel addition to the T cell repertory. Cell surface expression of tumor necrosis factor/cachectin by activated normal human T cells. J Exp Med. 1990 Mar 1;171(3):941–946. doi: 10.1084/jem.171.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircheis R., Milleck J., Korobko V. G., Shingarova L. N., Schmidt H. E. Differences in the biological activity of TNF alpha and TNF beta correlate with their different abilities for binding to the target cells. Eur Cytokine Netw. 1992 Jul-Aug;3(4):381–390. [PubMed] [Google Scholar]
- Kriegler M., Perez C., DeFay K., Albert I., Lu S. D. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988 Apr 8;53(1):45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- Londei M., Bottazzo G. F., Feldmann M. Human T-cell clones from autoimmune thyroid glands: specific recognition of autologous thyroid cells. Science. 1985 Apr 5;228(4695):85–89. doi: 10.1126/science.3871967. [DOI] [PubMed] [Google Scholar]
- Mallya R. K., Mace B. E. The assessment of disease activity in rheumatoid arthritis using a multivariate analysis. Rheumatol Rehabil. 1981 Feb 1;20(1):14–17. doi: 10.1093/rheumatology/20.1.14. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Romain P. L., Fox D. A., Anderson P., DiMaggio M., Levine H., Schlossman S. F. Abnormalities in CD4+ T-lymphocyte subsets in inflammatory rheumatic diseases. Am J Med. 1988 May;84(5):817–825. doi: 10.1016/0002-9343(88)90058-7. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Munker R., Gasson J., Ogawa M., Koeffler H. P. Recombinant human TNF induces production of granulocyte-monocyte colony-stimulating factor. Nature. 1986 Sep 4;323(6083):79–82. doi: 10.1038/323079a0. [DOI] [PubMed] [Google Scholar]
- Murch S. H., Lamkin V. A., Savage M. O., Walker-Smith J. A., MacDonald T. T. Serum concentrations of tumour necrosis factor alpha in childhood chronic inflammatory bowel disease. Gut. 1991 Aug;32(8):913–917. doi: 10.1136/gut.32.8.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff A., Defeo-Jones D., Boyer M., Martinez D., Kiefer D., Vuocolo G., Wolfe A., Socher S. H. Tumors secreting human TNF/cachectin induce cachexia in mice. Cell. 1987 Aug 14;50(4):555–563. doi: 10.1016/0092-8674(87)90028-6. [DOI] [PubMed] [Google Scholar]
- Pfizenmaier K., Scheurich P., Schlüter C., Krönke M. Tumor necrosis factor enhances HLA-A,B,C and HLA-DR gene expression in human tumor cells. J Immunol. 1987 Feb 1;138(3):975–980. [PubMed] [Google Scholar]
- Piguet P. F., Grau G. E., Allet B., Vassalli P. Tumor necrosis factor/cachectin is an effector of skin and gut lesions of the acute phase of graft-vs.-host disease. J Exp Med. 1987 Nov 1;166(5):1280–1289. doi: 10.1084/jem.166.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisa P., Gennene M., Söder O., Ottenhoff T., Hansson M., Kiessling R. Serum tumor necrosis factor levels and disease dissemination in leprosy and leishmaniasis. J Infect Dis. 1990 May;161(5):988–991. doi: 10.1093/infdis/161.5.988. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Porter A. G. Human tumour necrosis factors-alpha and -beta: differences in their structure, expression and biological properties. FEMS Microbiol Immunol. 1990 Nov;2(4):193–199. doi: 10.1111/j.1574-6968.1990.tb03519.x. [DOI] [PubMed] [Google Scholar]
- Scheurich P., Thoma B., Ucer U., Pfizenmaier K. Immunoregulatory activity of recombinant human tumor necrosis factor (TNF)-alpha: induction of TNF receptors on human T cells and TNF-alpha-mediated enhancement of T cell responses. J Immunol. 1987 Mar 15;138(6):1786–1790. [PubMed] [Google Scholar]
- Silver R. M., Redelman D., Zvaifler N. J. Studies of rheumatoid synovial fluid lymphocytes. II. A comparison of their behavior with blood mononuclear cells in the autologous mixed lymphocyte reaction and response to TCGF. Clin Immunol Immunopathol. 1983 Apr;27(1):15–27. doi: 10.1016/0090-1229(83)90052-1. [DOI] [PubMed] [Google Scholar]
- Silverman H. A., Johnson J. S., Vaughan J. H., McGlamory J. C. Altered lymphocyte reactivity in rheumatoid arthritis. Arthritis Rheum. 1976 May-Jun;19(3):509–515. doi: 10.1002/art.1780190301. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Kunkel S. L., Showell H. J., Remick D. G., Phan S. H., Ward P. A., Marks R. M. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science. 1989 Mar 17;243(4897):1467–1469. doi: 10.1126/science.2648570. [DOI] [PubMed] [Google Scholar]
- Talmadge J. E., Phillips H., Schneider M., Rowe T., Pennington R., Bowersox O., Lenz B. Immunomodulatory properties of recombinant murine and human tumor necrosis factor. Cancer Res. 1988 Feb 1;48(3):544–550. [PubMed] [Google Scholar]
- Tracey K. J., Wei H., Manogue K. R., Fong Y., Hesse D. G., Nguyen H. T., Kuo G. C., Beutler B., Cotran R. S., Cerami A. Cachectin/tumor necrosis factor induces cachexia, anemia, and inflammation. J Exp Med. 1988 Mar 1;167(3):1211–1227. doi: 10.1084/jem.167.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M., Chantry D., Buchan G., Barrett K., Feldmann M. Regulation of expression of human IL-1 alpha and IL-1 beta genes. J Immunol. 1989 Dec 1;143(11):3556–3561. [PubMed] [Google Scholar]
- Turner M., Londei M., Feldmann M. Human T cells from autoimmune and normal individuals can produce tumor necrosis factor. Eur J Immunol. 1987 Dec;17(12):1807–1814. doi: 10.1002/eji.1830171220. [DOI] [PubMed] [Google Scholar]
- Van Boxel J. A., Paget S. A. Predominantly T-cell infiltrate in rheumatoid synovial membranes. N Engl J Med. 1975 Sep 11;293(11):517–520. doi: 10.1056/NEJM197509112931101. [DOI] [PubMed] [Google Scholar]
- Whittle H. C., Brown J., Marsh K., Blackman M., Jobe O., Shenton F. The effects of Plasmodium falciparum malaria on immune control of B lymphocytes in Gambian children. Clin Exp Immunol. 1990 May;80(2):213–218. doi: 10.1111/j.1365-2249.1990.tb05236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. O., Feldmann M., Maini R. N. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S., Geppert T. D., Lipsky P. E. Enhancement of antigen- and mitogen-induced human T lymphocyte proliferation by tumor necrosis factor-alpha. J Immunol. 1988 Jan 15;140(2):531–536. [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]