Abstract
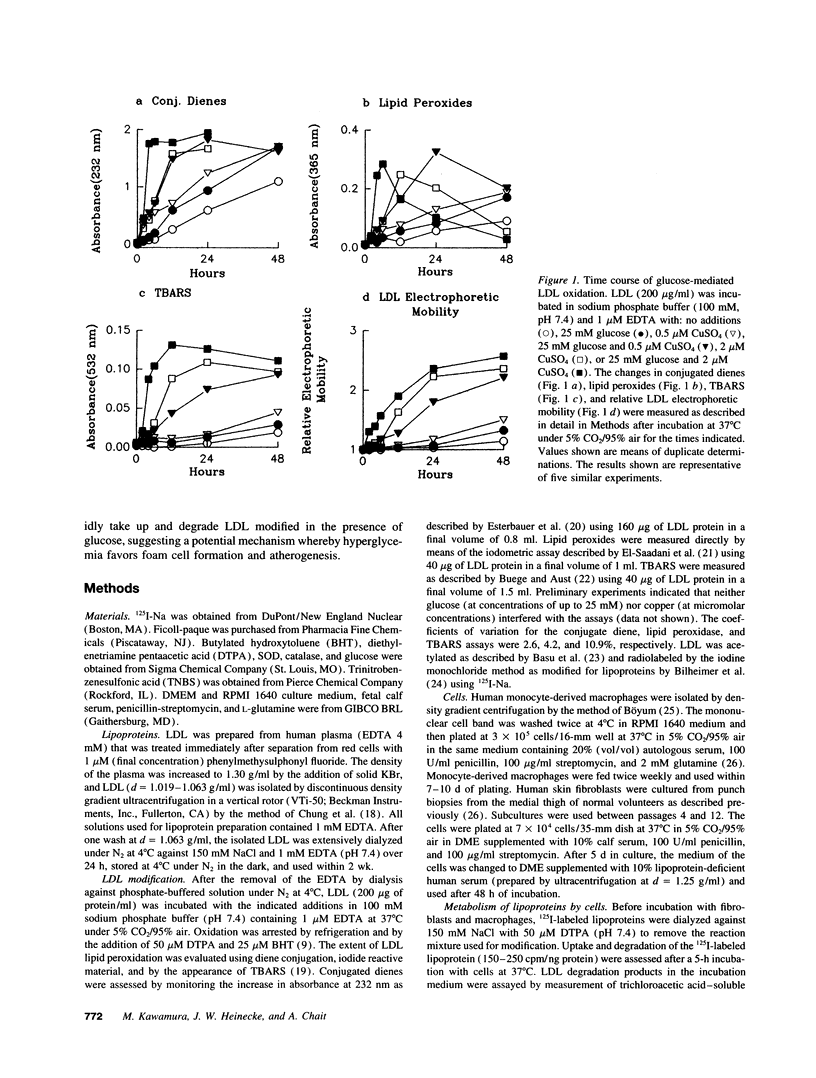
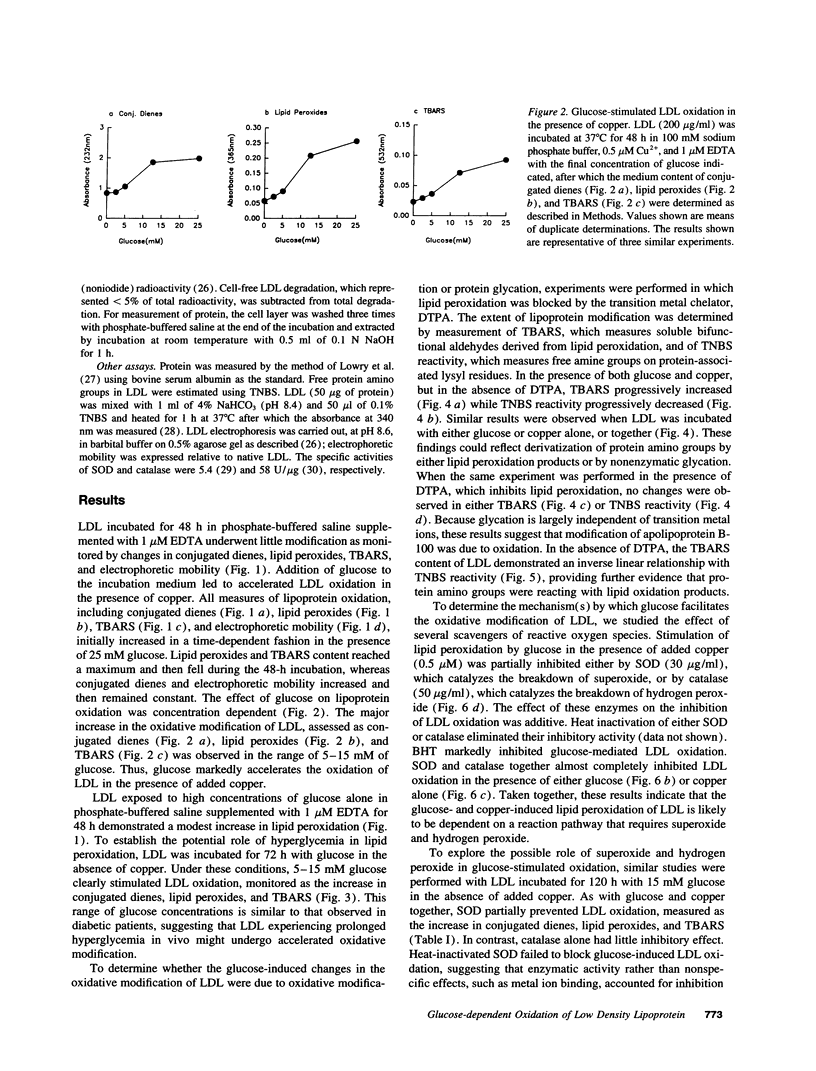
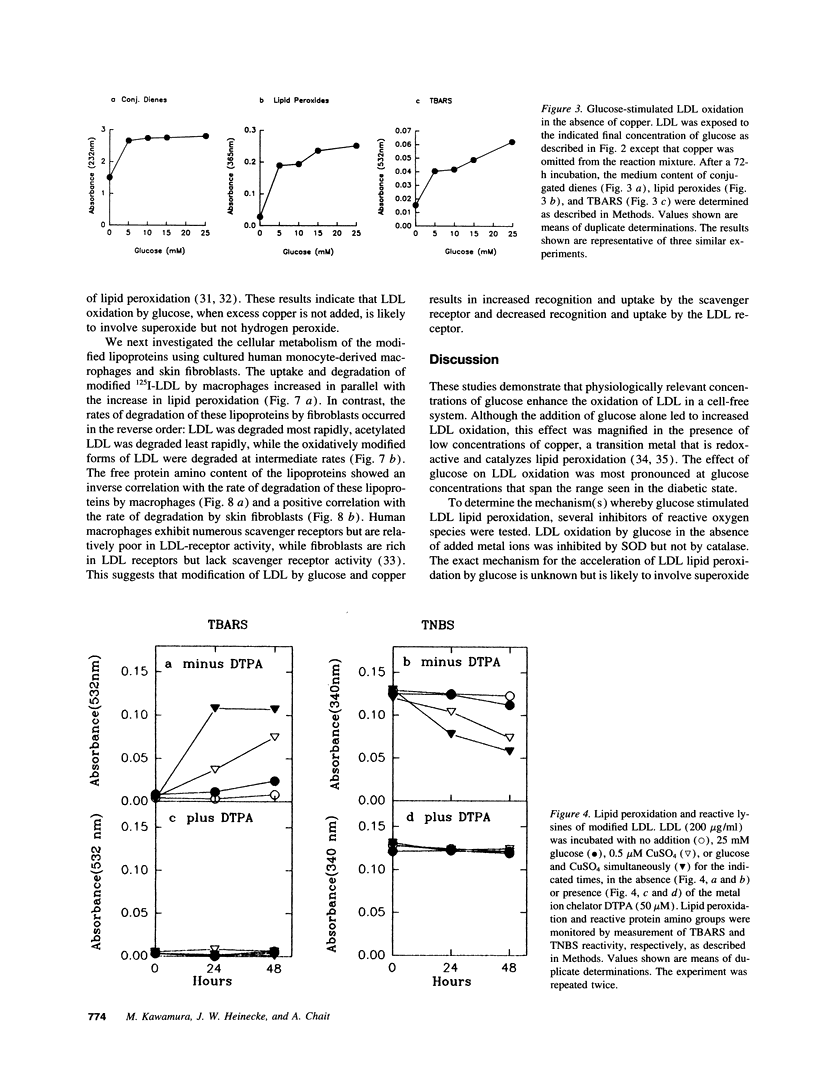
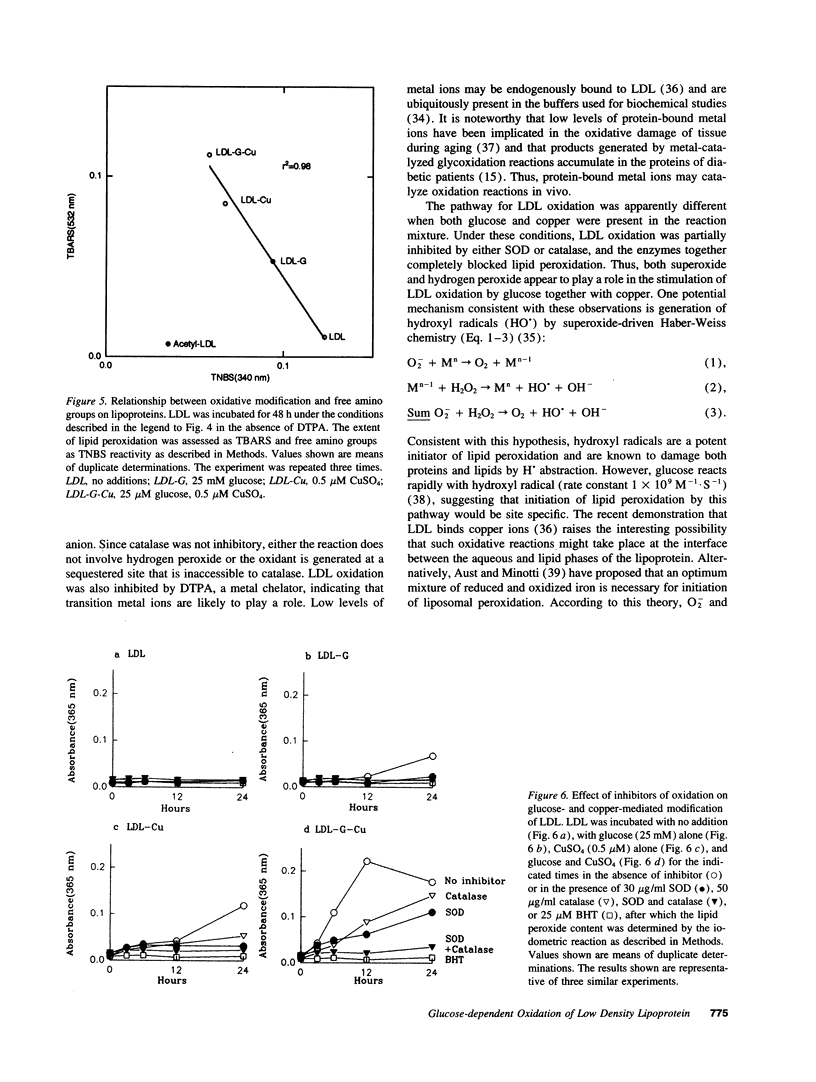
Oxidized lipoproteins may be important in the pathogenesis of atherosclerosis. Because diabetic subjects are particularly prone to vascular disease, and glucose autoxidation and protein glycation generate reactive oxygen species, we explored the role of glucose in lipoprotein oxidation. Glucose enhanced low density lipoprotein (LDL) oxidation at concentrations seen in the diabetic state. Conjugated dienes, thiobarbituric acid reactive substances, electrophoretic mobility, and degradation by macrophages were increased when LDL was modified in the presence of glucose. In contrast, free lysine groups and fibroblast degradation were reduced. Although loss of reactive lysine groups could be due to either oxidative modification or nonenzymatic glycation of apolipoprotein B-100, inhibition of lipid peroxidation by the metal chelator, diethylenetriamine pentaacetic acid, blocked the changes in free lysines. Thus, glycation of lysine residues is unlikely to account for the alterations in macrophage and fibroblast uptake of LDL modified in the presence of glucose. Glucose-mediated enhancement of LDL oxidation was partially blocked by superoxide dismutase and nearly completely inhibited by butylated hydroxytoluene. These findings indicate that glucose enhances LDL lipid peroxidation by an oxidative pathway involving superoxide and raise the possibility that the chronic hyperglycemia of diabetes accelerates lipoprotein oxidation, thereby promoting diabetic vascular disease.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azevedo M. S., Raposo J., Falcão J., Fontes G., Manso C. Oxygen radical generation by Maillard compounds. J Diabet Complications. 1988 Jan-Mar;2(1):19–21. doi: 10.1016/0891-6632(88)90021-9. [DOI] [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Anderson G. W., Brown M. S. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3178–3182. doi: 10.1073/pnas.73.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baynes J. W. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991 Apr;40(4):405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Buege J. A., Aust S. D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Chung B. H., Wilkinson T., Geer J. C., Segrest J. P. Preparative and quantitative isolation of plasma lipoproteins: rapid, single discontinuous density gradient ultracentrifugation in a vertical rotor. J Lipid Res. 1980 Mar;21(3):284–291. [PubMed] [Google Scholar]
- Esterbauer H., Striegl G., Puhl H., Rotheneder M. Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic Res Commun. 1989;6(1):67–75. doi: 10.3109/10715768909073429. [DOI] [PubMed] [Google Scholar]
- Flohé L., Otting F. Superoxide dismutase assays. Methods Enzymol. 1984;105:93–104. doi: 10.1016/s0076-6879(84)05013-8. [DOI] [PubMed] [Google Scholar]
- Galle J., Mülsch A., Busse R., Bassenge E. Effects of native and oxidized low density lipoproteins on formation and inactivation of endothelium-derived relaxing factor. Arterioscler Thromb. 1991 Jan-Feb;11(1):198–203. doi: 10.1161/01.atv.11.1.198. [DOI] [PubMed] [Google Scholar]
- Gillery P., Monboisse J. C., Maquart F. X., Borel J. P. Glycation of proteins as a source of superoxide. Diabete Metab. 1988 Jan-Feb;14(1):25–30. [PubMed] [Google Scholar]
- Gutteridge J. M., Halliwell B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem Sci. 1990 Apr;15(4):129–135. doi: 10.1016/0968-0004(90)90206-q. [DOI] [PubMed] [Google Scholar]
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Haberland M. E., Olch C. L., Folgelman A. M. Role of lysines in mediating interaction of modified low density lipoproteins with the scavenger receptor of human monocyte macrophages. J Biol Chem. 1984 Sep 25;259(18):11305–11311. [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Hamilton T. A., Ma G. P., Chisolm G. M. Oxidized low density lipoprotein suppresses the expression of tumor necrosis factor-alpha mRNA in stimulated murine peritoneal macrophages. J Immunol. 1990 Mar 15;144(6):2343–2350. [PubMed] [Google Scholar]
- Heinecke J. W., Baker L., Rosen H., Chait A. Superoxide-mediated modification of low density lipoprotein by arterial smooth muscle cells. J Clin Invest. 1986 Mar;77(3):757–761. doi: 10.1172/JCI112371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinecke J. W., Rosen H., Chait A. Iron and copper promote modification of low density lipoprotein by human arterial smooth muscle cells in culture. J Clin Invest. 1984 Nov;74(5):1890–1894. doi: 10.1172/JCI111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinecke J. W., Rosen H., Suzuki L. A., Chait A. The role of sulfur-containing amino acids in superoxide production and modification of low density lipoprotein by arterial smooth muscle cells. J Biol Chem. 1987 Jul 25;262(21):10098–10103. [PubMed] [Google Scholar]
- Hiramatsu K., Rosen H., Heinecke J. W., Wolfbauer G., Chait A. Superoxide initiates oxidation of low density lipoprotein by human monocytes. Arteriosclerosis. 1987 Jan-Feb;7(1):55–60. doi: 10.1161/01.atv.7.1.55. [DOI] [PubMed] [Google Scholar]
- Hunt J. V., Dean R. T., Wolff S. P. Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochem J. 1988 Nov 15;256(1):205–212. doi: 10.1042/bj2560205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J. V., Smith C. C., Wolff S. P. Autoxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucose. Diabetes. 1990 Nov;39(11):1420–1424. doi: 10.2337/diab.39.11.1420. [DOI] [PubMed] [Google Scholar]
- Jessup W., Simpson J. A., Dean R. T. Does superoxide radical have a role in macrophage-mediated oxidative modification of LDL? Atherosclerosis. 1993 Feb;99(1):107–120. doi: 10.1016/0021-9150(93)90056-z. [DOI] [PubMed] [Google Scholar]
- Kannel W. B., McGee D. L. Diabetes and cardiovascular risk factors: the Framingham study. Circulation. 1979 Jan;59(1):8–13. doi: 10.1161/01.cir.59.1.8. [DOI] [PubMed] [Google Scholar]
- Kosugi K., Morel D. W., DiCorleto P. E., Chisolm G. M. Toxicity of oxidized low-density lipoprotein to cultured fibroblasts is selective for S phase of the cell cycle. J Cell Physiol. 1987 Mar;130(3):311–320. doi: 10.1002/jcp.1041300302. [DOI] [PubMed] [Google Scholar]
- Kuzuya M., Yamada K., Hayashi T., Funaki C., Naito M., Asai K., Kuzuya F. Role of lipoprotein-copper complex in copper catalyzed-peroxidation of low-density lipoprotein. Biochim Biophys Acta. 1992 Feb 12;1123(3):334–341. doi: 10.1016/0005-2760(92)90015-n. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lopes-Virella M. F., Griffith R. L., Shunk K. A., Virella G. T. Enhanced uptake and impaired intracellular metabolism of low density lipoprotein complexed with anti-low density lipoprotein antibodies. Arterioscler Thromb. 1991 Sep-Oct;11(5):1356–1367. doi: 10.1161/01.atv.11.5.1356. [DOI] [PubMed] [Google Scholar]
- Lopes-Virella M. F., Klein R. L., Lyons T. J., Stevenson H. C., Witztum J. L. Glycosylation of low-density lipoprotein enhances cholesteryl ester synthesis in human monocyte-derived macrophages. Diabetes. 1988 May;37(5):550–557. doi: 10.2337/diab.37.5.550. [DOI] [PubMed] [Google Scholar]
- Lyons T. J. Oxidized low density lipoproteins: a role in the pathogenesis of atherosclerosis in diabetes? Diabet Med. 1991 Jun;8(5):411–419. doi: 10.1111/j.1464-5491.1991.tb01624.x. [DOI] [PubMed] [Google Scholar]
- Malden L. T., Chait A., Raines E. W., Ross R. The influence of oxidatively modified low density lipoproteins on expression of platelet-derived growth factor by human monocyte-derived macrophages. J Biol Chem. 1991 Jul 25;266(21):13901–13907. [PubMed] [Google Scholar]
- McNally A. K., Chisolm G. M., 3rd, Morel D. W., Cathcart M. K. Activated human monocytes oxidize low-density lipoprotein by a lipoxygenase-dependent pathway. J Immunol. 1990 Jul 1;145(1):254–259. [PubMed] [Google Scholar]
- Minotti G., Aust S. D. Redox cycling of iron and lipid peroxidation. Lipids. 1992 Mar;27(3):219–226. doi: 10.1007/BF02536182. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Morel D. W., Chisolm G. M. Antioxidant treatment of diabetic rats inhibits lipoprotein oxidation and cytotoxicity. J Lipid Res. 1989 Dec;30(12):1827–1834. [PubMed] [Google Scholar]
- Nishigaki I., Hagihara M., Tsunekawa H., Maseki M., Yagi K. Lipid peroxide levels of serum lipoprotein fractions of diabetic patients. Biochem Med. 1981 Jun;25(3):373–378. doi: 10.1016/0006-2944(81)90096-x. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. E. Oxidized LDL affects multiple atherogenic cellular responses. Circulation. 1991 Jun;83(6):2137–2140. doi: 10.1161/01.cir.83.6.2137. [DOI] [PubMed] [Google Scholar]
- Schleicher E., Olgemöller B., Schön J., Dürst T., Wieland O. H. Limited nonenzymatic glucosylation of low-density lipoprotein does not alter its catabolism in tissue culture. Biochim Biophys Acta. 1985 Aug 30;846(2):226–233. doi: 10.1016/0167-4889(85)90069-2. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R. Protein oxidation and aging. Science. 1992 Aug 28;257(5074):1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P., Lougheed M., Kwan W. C., Dirks M. Recognition of oxidized low density lipoprotein by the scavenger receptor of macrophages results from derivatization of apolipoprotein B by products of fatty acid peroxidation. J Biol Chem. 1989 Sep 15;264(26):15216–15223. [PubMed] [Google Scholar]
- Thomas C. E. The influence of medium components on Cu(2+)-dependent oxidation of low-density lipoproteins and its sensitivity to superoxide dismutase. Biochim Biophys Acta. 1992 Sep 22;1128(1):50–57. doi: 10.1016/0005-2760(92)90256-u. [DOI] [PubMed] [Google Scholar]
- Thornalley P. J. Monosaccharide autoxidation in health and disease. Environ Health Perspect. 1985 Dec;64:297–307. doi: 10.1289/ehp.8564297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornalley P. J., Stern A. The production of free radicals during the autoxidation of monosaccharides by buffer ions. Carbohydr Res. 1984 Dec 1;134(2):191–204. doi: 10.1016/0008-6215(84)85037-5. [DOI] [PubMed] [Google Scholar]
- Witztum J. L., Koschinsky T. Metabolic and immunological consequences of glycation of low density lipoproteins. Prog Clin Biol Res. 1989;304:219–234. [PubMed] [Google Scholar]
- Wolff S. P., Dean R. T. Glucose autoxidation and protein modification. The potential role of 'autoxidative glycosylation' in diabetes. Biochem J. 1987 Jul 1;245(1):243–250. doi: 10.1042/bj2450243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Saadani M., Esterbauer H., el-Sayed M., Goher M., Nassar A. Y., Jürgens G. A spectrophotometric assay for lipid peroxides in serum lipoproteins using a commercially available reagent. J Lipid Res. 1989 Apr;30(4):627–630. [PubMed] [Google Scholar]



