Abstract
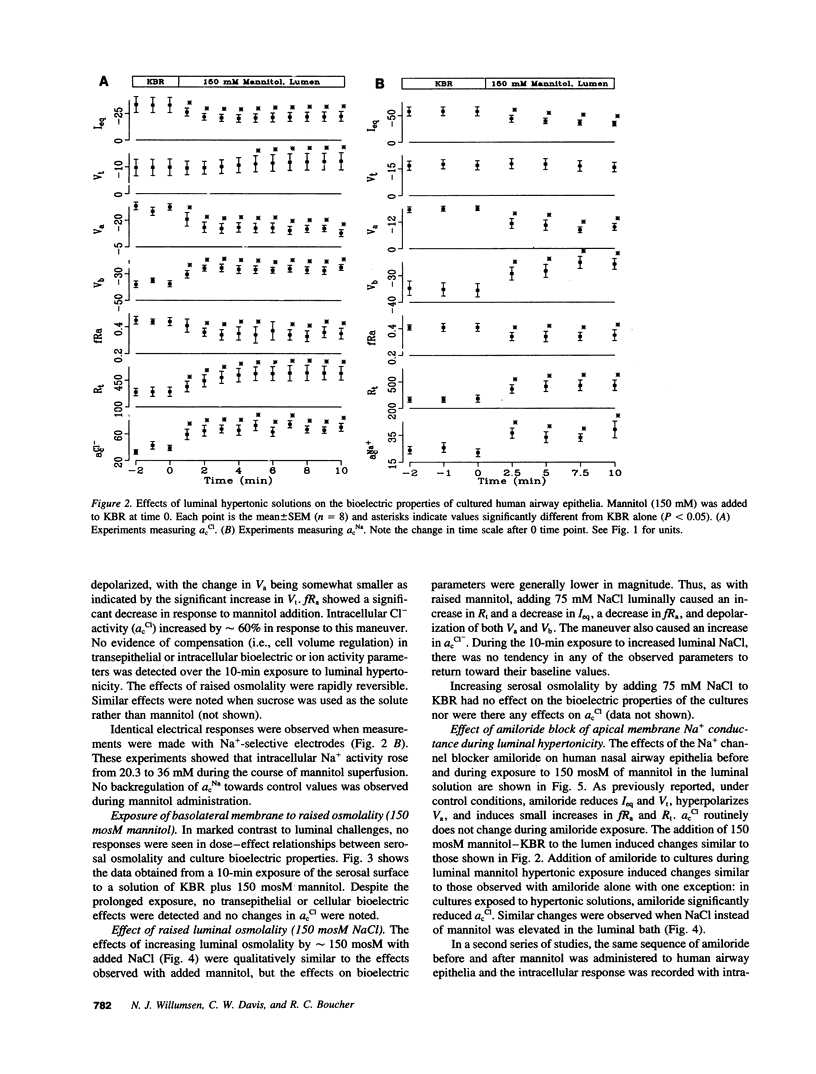
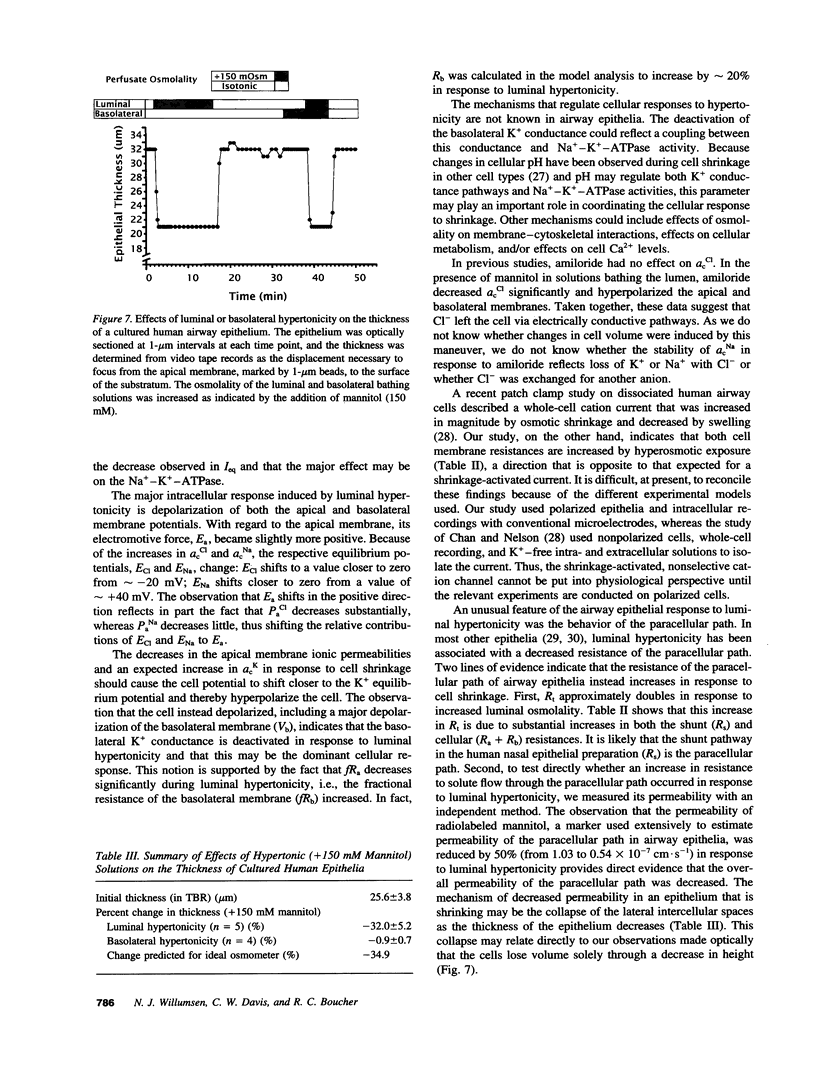
The response of cultured human nasal epithelia to hypertonic bathing solutions was tested using ion-selective microelectrode and quantitative microscopy. Raised luminal, but not serosal, osmolality (+/- 150 mM mannitol) decreased Na+ absorption but did not induce Cl- secretion. Raised luminal osmolality increased cell Cl- activity, Na+ activity, and transepithelial resistance and decreased both apical and basolateral membrane potentials and the fractional resistance of the apical membrane; equivalent circuit analysis revealed increases in apical, basolateral, and shunt resistances. Prolonged exposure (10 min) to 430 mosM luminal solution elicited no regulation of any parameter. Optical measurements revealed a reduction in the thickness of preparations only in response to luminal hypertonic solutions. We conclude that (a) airway epithelial cells exhibit asymmetric water transport properties, with the apical membrane water permeability exceeding that of the basolateral membrane; (b) the cellular response to volume loss is a deactivation of the basolateral membrane K+ conductance and the apical membrane Cl- conductance; (c) luminal hypertonicity slows the rate of Na+ absorption but does not induce Cl- secretion; and (d) cell volume loss increases the resistance of the paracellular path. We speculate that these properties configure human nasal epithelium to behave as an osmotic sensor, transducing information about luminal solutions to the airway wall.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agard D. A. Optical sectioning microscopy: cellular architecture in three dimensions. Annu Rev Biophys Bioeng. 1984;13:191–219. doi: 10.1146/annurev.bb.13.060184.001203. [DOI] [PubMed] [Google Scholar]
- Anderson S. D., Daviskas E., Smith C. M. Exercise-induced asthma: a difference in opinion regarding the stimulus. Allergy Proc. 1989 May-Jun;10(3):215–226. doi: 10.2500/108854189778960054. [DOI] [PubMed] [Google Scholar]
- Baile E. M., Guillemi S., Paré P. D. Tracheobronchial and upper airway blood flow in dogs during thermally induced panting. J Appl Physiol (1985) 1987 Dec;63(6):2240–2246. doi: 10.1152/jappl.1987.63.6.2240. [DOI] [PubMed] [Google Scholar]
- Boucher R. C. Chemical modulation of airway epithelial permeability. Environ Health Perspect. 1980 Apr;35:3–11. doi: 10.1289/ehp.80353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher R. C., Larsen E. H. Comparison of ion transport by cultured secretory and absorptive canine airway epithelia. Am J Physiol. 1988 Apr;254(4 Pt 1):C535–C547. doi: 10.1152/ajpcell.1988.254.4.C535. [DOI] [PubMed] [Google Scholar]
- Boucher R. C., Stutts M. J., Bromberg P. A., Gatzy J. T. Regional differences in airway surface liquid composition. J Appl Physiol Respir Environ Exerc Physiol. 1981 Mar;50(3):613–620. doi: 10.1152/jappl.1981.50.3.613. [DOI] [PubMed] [Google Scholar]
- Chan H. C., Nelson D. J. Chloride-dependent cation conductance activated during cellular shrinkage. Science. 1992 Jul 31;257(5070):669–671. doi: 10.1126/science.1379742. [DOI] [PubMed] [Google Scholar]
- Davis C. W., Dowell M. L., Lethem M., Van Scott M. Goblet cell degranulation in isolated canine tracheal epithelium: response to exogenous ATP, ADP, and adenosine. Am J Physiol. 1992 May;262(5 Pt 1):C1313–C1323. doi: 10.1152/ajpcell.1992.262.5.C1313. [DOI] [PubMed] [Google Scholar]
- Davis C. W., Finn A. L. Cell volume regulation in frog urinary bladder. Fed Proc. 1985 Jun;44(9):2520–2525. [PubMed] [Google Scholar]
- DiBona D. R., Civan M. M. Pathways for movement of ions and water across toad urinary bladder. I. Anatomic site of transepithelial shunt pathways. J Membr Biol. 1973;12(2):101–128. doi: 10.1007/BF01869994. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Clarke C. A., Rothstein A. Activation of Na+/H+ exchange in lymphocytes by osmotically induced volume changes and by cytoplasmic acidification. J Gen Physiol. 1983 Nov;82(5):619–638. doi: 10.1085/jgp.82.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert W. C., Forster B. B., Laird W., Pihl C. E., Walker D. C. An improved method for fixation of the respiratory epithelial surface with the mucous and surfactant layers. Lab Invest. 1982 Oct;47(4):354–363. [PubMed] [Google Scholar]
- MACROBBIE E. A., USSING H. H. Osmotic behaviour of the epithelial cells of frog skin. Acta Physiol Scand. 1961 Nov-Dec;53:348–365. doi: 10.1111/j.1748-1716.1961.tb02293.x. [DOI] [PubMed] [Google Scholar]
- Man S. F., Adams G. K., 3rd, Proctor D. F. Effects of temperature, relative humidity, and mode of breathing on canine airway secretions. J Appl Physiol Respir Environ Exerc Physiol. 1979 Feb;46(2):205–210. doi: 10.1152/jappl.1979.46.2.205. [DOI] [PubMed] [Google Scholar]
- Man S. F., Hulbert W., Park D. S., Thomson A. B., Hogg J. C. Asymmetry of canine tracheal epithelium: osmotically induced changes. J Appl Physiol Respir Environ Exerc Physiol. 1984 Nov;57(5):1338–1346. doi: 10.1152/jappl.1984.57.5.1338. [DOI] [PubMed] [Google Scholar]
- McFadden E. R., Jr, Lenner K. A., Strohl K. P. Postexertional airway rewarming and thermally induced asthma. New insights into pathophysiology and possible pathogenesis. J Clin Invest. 1986 Jul;78(1):18–25. doi: 10.1172/JCI112549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden E. R., Jr, Pichurko B. M. Intraairway thermal profiles during exercise and hyperventilation in normal man. J Clin Invest. 1985 Sep;76(3):1007–1010. doi: 10.1172/JCI112052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel J. A., Davis B., Phipps R. J. Control of mucus secretion and ion transport in airways. Annu Rev Physiol. 1979;41:369–381. doi: 10.1146/annurev.ph.41.030179.002101. [DOI] [PubMed] [Google Scholar]
- Persson B. E., Spring K. R. Gallbladder epithelial cell hydraulic water permeability and volume regulation. J Gen Physiol. 1982 Mar;79(3):481–505. doi: 10.1085/jgp.79.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss L., Finn A. L. Effects of changes in the composition of the mucosal solution on the electrical properties of the toad urinary bladder epithelium. J Membr Biol. 1975;20(1-2):191–204. doi: 10.1007/BF01870636. [DOI] [PubMed] [Google Scholar]
- Spring K. R., Hope A. Fluid transport and the dimensions of cells and interspaces of living Necturus gallbladder. J Gen Physiol. 1979 Mar;73(3):287–305. doi: 10.1085/jgp.73.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange K., Spring K. R. Cell membrane water permeability of rabbit cortical collecting duct. J Membr Biol. 1987;96(1):27–43. doi: 10.1007/BF01869332. [DOI] [PubMed] [Google Scholar]
- Van As A. Fixation of the respiratory epithelial surface. Lab Invest. 1983 May;48(5):650–652. [PubMed] [Google Scholar]
- Welsh M. J. Electrolyte transport by airway epithelia. Physiol Rev. 1987 Oct;67(4):1143–1184. doi: 10.1152/physrev.1987.67.4.1143. [DOI] [PubMed] [Google Scholar]
- Willumsen N. J., Boucher R. C. Shunt resistance and ion permeabilities in normal and cystic fibrosis airway epithelia. Am J Physiol. 1989 May;256(5 Pt 1):C1054–C1063. doi: 10.1152/ajpcell.1989.256.5.C1054. [DOI] [PubMed] [Google Scholar]
- Willumsen N. J., Boucher R. C. Sodium transport and intracellular sodium activity in cultured human nasal epithelium. Am J Physiol. 1991 Aug;261(2 Pt 1):C319–C331. doi: 10.1152/ajpcell.1991.261.2.C319. [DOI] [PubMed] [Google Scholar]
- Willumsen N. J., Davis C. W., Boucher R. C. Intracellular Cl- activity and cellular Cl- pathways in cultured human airway epithelium. Am J Physiol. 1989 May;256(5 Pt 1):C1033–C1044. doi: 10.1152/ajpcell.1989.256.5.C1033. [DOI] [PubMed] [Google Scholar]
- Yankaskas J. R., Cotton C. U., Knowles M. R., Gatzy J. T., Boucher R. C. Culture of human nasal epithelial cells on collagen matrix supports. A comparison of bioelectric properties of normal and cystic fibrosis epithelia. Am Rev Respir Dis. 1985 Dec;132(6):1281–1287. doi: 10.1164/arrd.1985.132.6.1281. [DOI] [PubMed] [Google Scholar]




