Abstract
X-ray crystallography unequivocally shows that protection of the free hydroxyl group of 3,5-O-benzylidene-d-xylono-1,4-lactone with diphenyldiazomethane proceeded smoothly to give the title compound, C25H22O5, with no accompanying epimerization. Unlike the analogously protected lyxono lactone, the isomeric xylono lactone has two molecules present in the asymmetric unit (Z′ = 2). The 5-ring lactones adopt envelope conformations and the 6-ring ketals adopt chair conformations.
Related literature
For related literature, see: Collins & Ferrier (1995 ▶); Draths et al. (1992 ▶); Jackson et al. (1982 ▶); Petursson & Webber (1982 ▶); Petursson et al. (2007 ▶); Petursson (2001 ▶, 2003 ▶); Best et al. (2008 ▶); Jenkinson et al. (2008 ▶); Görbitz (1999 ▶).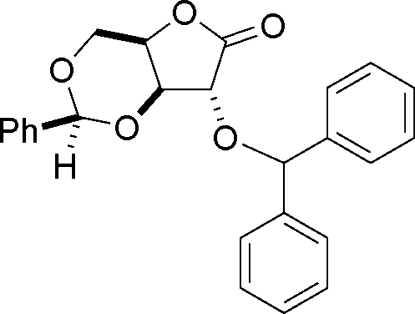
Experimental
Crystal data
C25H22O5
M r = 402.45
Monoclinic,
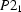
a = 14.8159 (3) Å
b = 9.1959 (2) Å
c = 15.0797 (2) Å
β = 93.7245 (12)°
V = 2050.20 (7) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 150 K
0.80 × 0.30 × 0.10 mm
Data collection
Nonius KappaCCD area-detector diffractometer
Absorption correction: multi-scan (DENZO/SCALEPACK; Otwinowski & Minor, 1997 ▶) T min = 0.55, T max = 0.99
25081 measured reflections
4938 independent reflections
3950 reflections with I > 2σ(I)
R int = 0.047
Refinement
R[F 2 > 2σ(F 2)] = 0.034
wR(F 2) = 0.101
S = 0.89
4938 reflections
542 parameters
1 restraint
H-atom parameters constrained
Δρmax = 0.25 e Å−3
Δρmin = −0.27 e Å−3
Data collection: COLLECT (Nonius, 2001 ▶).; cell refinement: DENZO/SCALEPACK (Otwinowski & Minor, 1997 ▶); data reduction: DENZO/SCALEPACK; program(s) used to solve structure: SIR92 (Altomare et al., 1994 ▶); program(s) used to refine structure: CRYSTALS (Betteridge et al., 2003 ▶); molecular graphics: CAMERON (Watkin et al., 1996 ▶); software used to prepare material for publication: CRYSTALS.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808012567/lh2623sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808012567/lh2623Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Comment
Carbohydrates provide excellent starting materials for the synthesis of small chiral molecules (Collins & Ferrier, 1995). They are relatively inexpensive and provide an almost boundless pool of chiral building blocks (Draths et al., 1992). Much of their synthetic utility is however dependent on developing successful protecting group strategies.
Diazodiphenylmethane has been found to be a useful protecting group in the synthesis of methyl 2,3,6-tri-O-methyl-α-D-glucopyranoside and kojibiose octa-acetate (Jackson et al., 1982); monoalkylations of vicinal diols have been achieved with this reagent and other diaryldiazoalkanes with high regioselectivities (Petursson & Webber, 1982; Petursson et al., 2007; Petursson, 2003; Petursson, 2001). This is of particular interest for the protection of base sensitive sugar lactones as the reaction is carried out under neutral conditions (Best et al. 2008; Jenkinson et al. 2008).
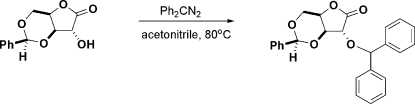
The utility of the benzhydryl group as a protecting group in carbohydrate chemistry has here been demonstrated with the reaction of 3,5-O-benzylidene-D-xylono-1,4-lactone 1 with diphenyldiazomethane (Fig. 1). No epimerization at C2 was observed (Fig. 2).
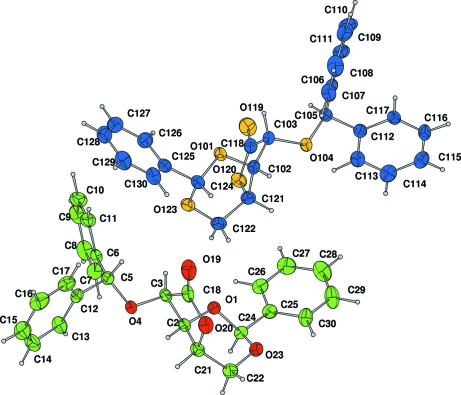
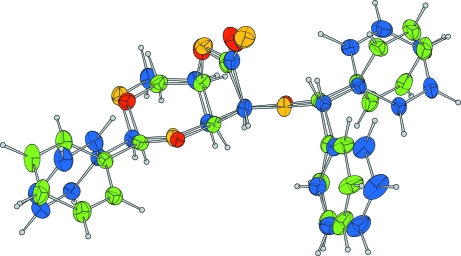
Unlike the analogously protected lyxono lactone (Jenkinson et al., 2008), the asymmetric unit of the isomeric xylono lactone contains two crystallographically distinct molecules which are related by a pseudo 2-fold axis of symmetry. These are similar in geometry with the exception of two of the phenyl rings which sit at approximately 90° to each other (Fig. 3). When the core 20 atoms, of the carbohydrate backbone and 1 phenyl group, are mapped there is good overlap- r.m.s. deviations: posn 0.1139 Å, bond 0.0104 Å, torsion 2.5205°.
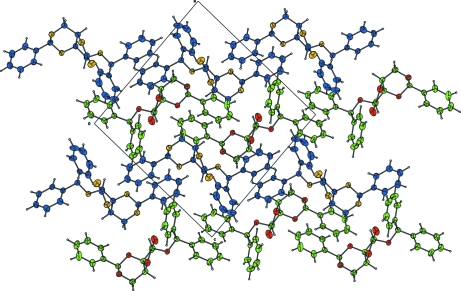
The crystal packing shows alternating layers of molecules in the ac plane (Fig. 4). The 5-ring lactones adopt envelope conformations with C2 or C102 out of the plane. The 6-ring ketals adopt chair conformations. There is no classic hydrogen-bonding.
Experimental
The title lactone was recrystallized from a 1:1 mixture of ethyl acetate and cyclohexane: m.p. 395–397 K; [α]D23 +129.1 (c, 1.02 in CHCl3).
Refinement
In the absence of significant anomalous scattering, Friedel pairs were merged and the absolute configuration was assigned from the starting material.
The relatively large ratio of minimum to maximum corrections applied in the multiscan process (1:1.80) reflect changes in the illuminated volume of the crystal. Changes in illuminated volume were kept to a minimum, and were taken into account (Görbitz, 1999) by the multi-scan inter-frame scaling (DENZO/SCALEPACK, Otwinowski & Minor, 1997).
The H atoms were all located in a difference map, but those attached to carbon atoms were repositioned geometrically. The H atoms were initially refined with soft restraints on the bond lengths and angles to regularize their geometry (C—H in the range 0.93–0.98, O—H = 0.82 Å) and Uiso(H) (in the range 1.2–1.5 times Ueq of the parent atom), after which the positions were refined with riding constraints.
Figures
Fig. 1.
Synthetic Scheme.
Fig. 2.
The title compound with displacement ellipsoids drawn at the 50% probability level. H atoms are shown as spheres of arbitary radius. There are two molecules in the asymmetric repeating unit.
Fig. 3.
Overlay of the two molecules in the asymmetric unit.
Fig. 4.
Packing diagram for the molecule projected along the b-axis.
Crystal data
| C25H22O5 | F000 = 848 |
| Mr = 402.45 | Dx = 1.304 Mg m−3 |
| Monoclinic, P21 | Mo Kα radiation λ = 0.71073 Å |
| Hall symbol: P 2yb | Cell parameters from 4834 reflections |
| a = 14.8159 (3) Å | θ = 5–27º |
| b = 9.1959 (2) Å | µ = 0.09 mm−1 |
| c = 15.0797 (2) Å | T = 150 K |
| β = 93.7245 (12)º | Plate, colourless |
| V = 2050.20 (7) Å3 | 0.80 × 0.30 × 0.10 mm |
| Z = 4 |
Data collection
| Nonius KappaCCD area-detector diffractometer | 3950 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.047 |
| T = 150 K | θmax = 27.5º |
| ω scans | θmin = 5.2º |
| Absorption correction: multi-scan(DENZO/SCALEPACK; Otwinowski & Minor, 1997) | h = −19→19 |
| Tmin = 0.55, Tmax = 0.99 | k = −11→11 |
| 25081 measured reflections | l = −19→19 |
| 4938 independent reflections |
Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.034 | Method, part 1, Chebychev polynomial, (Watkin, 1994, Prince, 1982) [weight] = 1.0/[A0*T0(x) + A1*T1(x) ··· + An-1]*Tn-1(x)] where Ai are the Chebychev coefficients listed below and x = F /Fmax Method = Robust Weighting (Prince, 1982) W = [weight] * [1-(deltaF/6*sigmaF)2]2 Ai are: 16.9 24.9 12.6 3.46 |
| wR(F2) = 0.101 | (Δ/σ)max = 0.0003 |
| S = 0.89 | Δρmax = 0.25 e Å−3 |
| 4938 reflections | Δρmin = −0.27 e Å−3 |
| 542 parameters | Extinction correction: Larson (1970), Equation 22 |
| 1 restraint | Extinction coefficient: 600 (50) |
| Primary atom site location: structure-invariant direct methods |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.44166 (10) | 0.5327 (2) | 0.31715 (10) | 0.0244 | |
| C2 | 0.40499 (15) | 0.5336 (3) | 0.22694 (15) | 0.0242 | |
| C3 | 0.30725 (16) | 0.4866 (3) | 0.23203 (15) | 0.0254 | |
| O4 | 0.25857 (10) | 0.5219 (2) | 0.15093 (10) | 0.0265 | |
| C5 | 0.16129 (15) | 0.5192 (3) | 0.15751 (16) | 0.0257 | |
| C6 | 0.13142 (16) | 0.6490 (3) | 0.21052 (15) | 0.0252 | |
| C7 | 0.17991 (18) | 0.7781 (3) | 0.21395 (18) | 0.0330 | |
| C8 | 0.1517 (2) | 0.8947 (3) | 0.26476 (19) | 0.0403 | |
| C9 | 0.0760 (2) | 0.8806 (3) | 0.31222 (18) | 0.0432 | |
| C10 | 0.0264 (2) | 0.7539 (4) | 0.30826 (19) | 0.0437 | |
| C11 | 0.05367 (19) | 0.6375 (3) | 0.25762 (17) | 0.0335 | |
| C12 | 0.12135 (15) | 0.5140 (3) | 0.06274 (15) | 0.0255 | |
| C13 | 0.15139 (19) | 0.6107 (3) | 0.00038 (17) | 0.0339 | |
| C14 | 0.1185 (2) | 0.6028 (4) | −0.08784 (18) | 0.0429 | |
| C15 | 0.0551 (2) | 0.4987 (4) | −0.11333 (19) | 0.0486 | |
| C16 | 0.02472 (19) | 0.4030 (4) | −0.0519 (2) | 0.0479 | |
| C17 | 0.05739 (17) | 0.4106 (4) | 0.03651 (19) | 0.0368 | |
| C18 | 0.31556 (18) | 0.3212 (3) | 0.23435 (18) | 0.0323 | |
| O19 | 0.26098 (14) | 0.2338 (2) | 0.25627 (16) | 0.0478 | |
| O20 | 0.39494 (13) | 0.2813 (2) | 0.20131 (13) | 0.0344 | |
| C21 | 0.44429 (17) | 0.4094 (3) | 0.17435 (16) | 0.0296 | |
| C22 | 0.54422 (17) | 0.3886 (3) | 0.19447 (16) | 0.0335 | |
| O23 | 0.56825 (11) | 0.3965 (2) | 0.28758 (11) | 0.0301 | |
| C24 | 0.53776 (15) | 0.5268 (3) | 0.32352 (15) | 0.0255 | |
| C25 | 0.56837 (16) | 0.5320 (3) | 0.42104 (16) | 0.0277 | |
| C26 | 0.53029 (17) | 0.6335 (3) | 0.47592 (17) | 0.0318 | |
| C27 | 0.5559 (2) | 0.6362 (4) | 0.56635 (19) | 0.0417 | |
| C28 | 0.6204 (2) | 0.5396 (4) | 0.6014 (2) | 0.0479 | |
| C29 | 0.6607 (2) | 0.4425 (4) | 0.5464 (2) | 0.0479 | |
| C30 | 0.63432 (19) | 0.4375 (3) | 0.45597 (19) | 0.0359 | |
| O101 | 0.17514 (11) | 0.3628 (2) | 0.56763 (10) | 0.0280 | |
| C102 | 0.26545 (16) | 0.3497 (3) | 0.60615 (15) | 0.0265 | |
| C103 | 0.26225 (16) | 0.3956 (3) | 0.70212 (16) | 0.0266 | |
| O104 | 0.34196 (11) | 0.3412 (2) | 0.74916 (10) | 0.0274 | |
| C105 | 0.33862 (16) | 0.3463 (3) | 0.84483 (15) | 0.0254 | |
| C106 | 0.27885 (16) | 0.2264 (3) | 0.87746 (17) | 0.0275 | |
| C107 | 0.25628 (19) | 0.1047 (3) | 0.82618 (19) | 0.0368 | |
| C108 | 0.2043 (2) | −0.0056 (4) | 0.8592 (2) | 0.0473 | |
| C109 | 0.17436 (19) | 0.0054 (4) | 0.9435 (3) | 0.0506 | |
| C110 | 0.1966 (2) | 0.1261 (4) | 0.9956 (2) | 0.0512 | |
| C111 | 0.2483 (2) | 0.2372 (4) | 0.9627 (2) | 0.0394 | |
| C112 | 0.43660 (16) | 0.3392 (3) | 0.88150 (15) | 0.0254 | |
| C113 | 0.49799 (18) | 0.4396 (3) | 0.85136 (18) | 0.0320 | |
| C114 | 0.58750 (19) | 0.4422 (3) | 0.8863 (2) | 0.0383 | |
| C115 | 0.61626 (18) | 0.3427 (3) | 0.95059 (18) | 0.0348 | |
| C116 | 0.55682 (18) | 0.2406 (3) | 0.97998 (16) | 0.0318 | |
| C117 | 0.46694 (17) | 0.2387 (3) | 0.94582 (15) | 0.0276 | |
| C118 | 0.27224 (17) | 0.5609 (3) | 0.69587 (17) | 0.0307 | |
| O119 | 0.25584 (16) | 0.6510 (3) | 0.75019 (14) | 0.0446 | |
| O120 | 0.30885 (13) | 0.5960 (2) | 0.61933 (12) | 0.0333 | |
| C121 | 0.32735 (16) | 0.4648 (3) | 0.56966 (16) | 0.0299 | |
| C122 | 0.30971 (17) | 0.4926 (4) | 0.47132 (17) | 0.0357 | |
| O123 | 0.21497 (11) | 0.4979 (2) | 0.44632 (11) | 0.0315 | |
| C124 | 0.17243 (16) | 0.3695 (3) | 0.47308 (15) | 0.0280 | |
| C125 | 0.07503 (16) | 0.3717 (3) | 0.43830 (16) | 0.0286 | |
| C126 | 0.01418 (18) | 0.4674 (3) | 0.47230 (17) | 0.0354 | |
| C127 | −0.07582 (19) | 0.4661 (4) | 0.44074 (19) | 0.0426 | |
| C128 | −0.10512 (19) | 0.3684 (4) | 0.3750 (2) | 0.0417 | |
| C129 | −0.0444 (2) | 0.2749 (4) | 0.3393 (2) | 0.0412 | |
| C130 | 0.0462 (2) | 0.2761 (3) | 0.37099 (18) | 0.0344 | |
| H21 | 0.4102 | 0.6308 | 0.1980 | 0.0310* | |
| H31 | 0.2815 | 0.5292 | 0.2838 | 0.0300* | |
| H51 | 0.1461 | 0.4285 | 0.1912 | 0.0293* | |
| H71 | 0.2332 | 0.7897 | 0.1793 | 0.0378* | |
| H81 | 0.1854 | 0.9828 | 0.2657 | 0.0498* | |
| H91 | 0.0574 | 0.9586 | 0.3462 | 0.0522* | |
| H101 | −0.0270 | 0.7456 | 0.3417 | 0.0499* | |
| H111 | 0.0193 | 0.5507 | 0.2559 | 0.0398* | |
| H131 | 0.1944 | 0.6831 | 0.0155 | 0.0428* | |
| H141 | 0.1417 | 0.6660 | −0.1305 | 0.0511* | |
| H151 | 0.0293 | 0.4918 | −0.1722 | 0.0598* | |
| H161 | −0.0195 | 0.3290 | −0.0668 | 0.0570* | |
| H171 | 0.0384 | 0.3446 | 0.0768 | 0.0465* | |
| H211 | 0.4312 | 0.4257 | 0.1074 | 0.0355* | |
| H221 | 0.5780 | 0.4629 | 0.1587 | 0.0427* | |
| H222 | 0.5617 | 0.2896 | 0.1766 | 0.0439* | |
| H241 | 0.5627 | 0.6105 | 0.2909 | 0.0328* | |
| H261 | 0.4846 | 0.7013 | 0.4543 | 0.0393* | |
| H271 | 0.5298 | 0.7065 | 0.6043 | 0.0510* | |
| H281 | 0.6372 | 0.5419 | 0.6640 | 0.0566* | |
| H291 | 0.7087 | 0.3765 | 0.5704 | 0.0512* | |
| H301 | 0.6622 | 0.3686 | 0.4199 | 0.0430* | |
| H1021 | 0.2909 | 0.2513 | 0.5939 | 0.0322* | |
| H1031 | 0.2066 | 0.3671 | 0.7308 | 0.0308* | |
| H1051 | 0.3131 | 0.4449 | 0.8589 | 0.0297* | |
| H1071 | 0.2755 | 0.0977 | 0.7683 | 0.0447* | |
| H1081 | 0.1886 | −0.0904 | 0.8241 | 0.0582* | |
| H1091 | 0.1414 | −0.0692 | 0.9676 | 0.0609* | |
| H1101 | 0.1777 | 0.1334 | 1.0531 | 0.0637* | |
| H1111 | 0.2619 | 0.3211 | 0.9967 | 0.0452* | |
| H1131 | 0.4790 | 0.5064 | 0.8020 | 0.0373* | |
| H1141 | 0.6288 | 0.5114 | 0.8631 | 0.0459* | |
| H1151 | 0.6769 | 0.3437 | 0.9745 | 0.0399* | |
| H1161 | 0.5788 | 0.1694 | 1.0226 | 0.0391* | |
| H1171 | 0.4223 | 0.1678 | 0.9652 | 0.0343* | |
| H1211 | 0.3914 | 0.4381 | 0.5851 | 0.0389* | |
| H1221 | 0.3378 | 0.5873 | 0.4563 | 0.0426* | |
| H1222 | 0.3394 | 0.4138 | 0.4383 | 0.0433* | |
| H1241 | 0.2031 | 0.2834 | 0.4506 | 0.0363* | |
| H1261 | 0.0340 | 0.5326 | 0.5187 | 0.0406* | |
| H1271 | −0.1184 | 0.5305 | 0.4660 | 0.0513* | |
| H1281 | −0.1677 | 0.3670 | 0.3552 | 0.0470* | |
| H1291 | −0.0649 | 0.2101 | 0.2929 | 0.0471* | |
| H1301 | 0.0895 | 0.2105 | 0.3457 | 0.0399* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0232 (7) | 0.0288 (9) | 0.0210 (7) | −0.0002 (7) | 0.0005 (6) | −0.0019 (7) |
| C2 | 0.0265 (11) | 0.0255 (12) | 0.0202 (10) | 0.0009 (9) | −0.0007 (8) | 0.0009 (9) |
| C3 | 0.0275 (11) | 0.0277 (13) | 0.0209 (10) | −0.0006 (10) | −0.0001 (8) | 0.0048 (10) |
| O4 | 0.0243 (8) | 0.0340 (10) | 0.0209 (7) | 0.0030 (7) | −0.0011 (6) | 0.0044 (7) |
| C5 | 0.0245 (11) | 0.0251 (12) | 0.0276 (11) | −0.0001 (10) | 0.0017 (8) | 0.0027 (10) |
| C6 | 0.0300 (11) | 0.0240 (11) | 0.0211 (10) | 0.0031 (10) | −0.0022 (8) | 0.0024 (9) |
| C7 | 0.0352 (13) | 0.0288 (13) | 0.0346 (13) | −0.0016 (11) | −0.0004 (10) | −0.0027 (11) |
| C8 | 0.0518 (16) | 0.0289 (14) | 0.0387 (14) | −0.0017 (13) | −0.0081 (12) | −0.0037 (12) |
| C9 | 0.0659 (19) | 0.0334 (16) | 0.0298 (13) | 0.0140 (15) | 0.0001 (12) | −0.0040 (12) |
| C10 | 0.0570 (18) | 0.0398 (17) | 0.0361 (15) | 0.0146 (14) | 0.0169 (13) | 0.0055 (13) |
| C11 | 0.0390 (13) | 0.0283 (13) | 0.0341 (13) | 0.0044 (11) | 0.0094 (10) | 0.0046 (11) |
| C12 | 0.0239 (10) | 0.0255 (12) | 0.0269 (11) | 0.0055 (9) | −0.0005 (8) | −0.0051 (10) |
| C13 | 0.0413 (14) | 0.0298 (14) | 0.0296 (12) | −0.0048 (11) | −0.0048 (10) | −0.0012 (11) |
| C14 | 0.0528 (17) | 0.0505 (18) | 0.0244 (13) | 0.0027 (15) | −0.0041 (11) | 0.0001 (13) |
| C15 | 0.0420 (15) | 0.072 (2) | 0.0303 (13) | 0.0034 (16) | −0.0085 (11) | −0.0144 (16) |
| C16 | 0.0305 (13) | 0.066 (2) | 0.0468 (16) | −0.0103 (15) | −0.0029 (11) | −0.0195 (17) |
| C17 | 0.0269 (12) | 0.0416 (16) | 0.0422 (14) | −0.0072 (12) | 0.0034 (10) | −0.0072 (13) |
| C18 | 0.0321 (13) | 0.0315 (14) | 0.0318 (13) | −0.0024 (11) | −0.0098 (10) | 0.0067 (11) |
| O19 | 0.0394 (11) | 0.0384 (12) | 0.0638 (14) | −0.0101 (10) | −0.0106 (10) | 0.0201 (11) |
| O20 | 0.0399 (10) | 0.0248 (9) | 0.0376 (10) | 0.0003 (8) | −0.0035 (8) | −0.0012 (8) |
| C21 | 0.0357 (12) | 0.0308 (13) | 0.0223 (10) | 0.0026 (11) | 0.0027 (9) | −0.0005 (10) |
| C22 | 0.0373 (13) | 0.0370 (14) | 0.0267 (12) | 0.0071 (12) | 0.0052 (10) | −0.0038 (11) |
| O23 | 0.0300 (8) | 0.0340 (10) | 0.0264 (8) | 0.0074 (8) | 0.0021 (6) | −0.0025 (8) |
| C24 | 0.0224 (10) | 0.0262 (12) | 0.0282 (11) | 0.0001 (10) | 0.0032 (8) | −0.0014 (10) |
| C25 | 0.0245 (11) | 0.0280 (12) | 0.0303 (12) | −0.0073 (10) | −0.0004 (9) | −0.0015 (10) |
| C26 | 0.0289 (12) | 0.0327 (14) | 0.0336 (13) | −0.0030 (11) | 0.0009 (9) | −0.0074 (11) |
| C27 | 0.0428 (15) | 0.0469 (18) | 0.0350 (14) | −0.0127 (14) | −0.0017 (11) | −0.0100 (14) |
| C28 | 0.0585 (19) | 0.0486 (18) | 0.0344 (14) | −0.0137 (16) | −0.0135 (13) | −0.0068 (14) |
| C29 | 0.0542 (18) | 0.0407 (18) | 0.0456 (17) | 0.0004 (15) | −0.0219 (14) | −0.0023 (14) |
| C30 | 0.0372 (13) | 0.0318 (14) | 0.0373 (14) | 0.0023 (11) | −0.0080 (11) | −0.0043 (12) |
| O101 | 0.0258 (8) | 0.0354 (10) | 0.0223 (8) | −0.0028 (7) | −0.0020 (6) | 0.0045 (7) |
| C102 | 0.0243 (11) | 0.0282 (12) | 0.0268 (11) | 0.0022 (10) | −0.0011 (8) | 0.0006 (10) |
| C103 | 0.0230 (10) | 0.0315 (13) | 0.0249 (11) | 0.0045 (10) | 0.0002 (8) | 0.0039 (10) |
| O104 | 0.0251 (8) | 0.0349 (10) | 0.0219 (8) | 0.0045 (7) | −0.0009 (6) | 0.0021 (7) |
| C105 | 0.0276 (11) | 0.0253 (12) | 0.0234 (10) | 0.0012 (10) | 0.0017 (8) | 0.0004 (10) |
| C106 | 0.0224 (11) | 0.0293 (13) | 0.0309 (12) | 0.0040 (10) | 0.0017 (9) | 0.0040 (10) |
| C107 | 0.0379 (14) | 0.0348 (15) | 0.0367 (14) | −0.0038 (12) | −0.0050 (11) | 0.0054 (12) |
| C108 | 0.0436 (16) | 0.0373 (17) | 0.0591 (19) | −0.0105 (14) | −0.0108 (13) | 0.0091 (15) |
| C109 | 0.0315 (14) | 0.0453 (19) | 0.075 (2) | 0.0004 (13) | 0.0064 (14) | 0.0245 (18) |
| C110 | 0.0442 (17) | 0.052 (2) | 0.060 (2) | 0.0125 (16) | 0.0272 (15) | 0.0164 (17) |
| C111 | 0.0377 (14) | 0.0385 (16) | 0.0435 (15) | 0.0096 (13) | 0.0152 (12) | 0.0036 (13) |
| C112 | 0.0268 (11) | 0.0262 (12) | 0.0230 (10) | 0.0003 (10) | 0.0003 (8) | −0.0033 (9) |
| C113 | 0.0333 (12) | 0.0267 (13) | 0.0356 (13) | −0.0018 (10) | −0.0012 (10) | 0.0016 (11) |
| C114 | 0.0342 (14) | 0.0329 (15) | 0.0473 (15) | −0.0060 (12) | −0.0002 (11) | −0.0029 (13) |
| C115 | 0.0284 (12) | 0.0387 (15) | 0.0363 (13) | 0.0009 (11) | −0.0051 (10) | −0.0111 (12) |
| C116 | 0.0329 (13) | 0.0377 (15) | 0.0239 (11) | 0.0081 (11) | −0.0051 (9) | −0.0038 (11) |
| C117 | 0.0307 (12) | 0.0303 (13) | 0.0215 (10) | 0.0026 (10) | 0.0008 (9) | −0.0010 (10) |
| C118 | 0.0294 (12) | 0.0332 (14) | 0.0291 (12) | 0.0039 (10) | −0.0005 (9) | 0.0015 (11) |
| O119 | 0.0597 (13) | 0.0362 (11) | 0.0382 (10) | 0.0110 (10) | 0.0052 (9) | −0.0041 (10) |
| O120 | 0.0381 (10) | 0.0317 (10) | 0.0304 (9) | −0.0055 (8) | 0.0030 (7) | 0.0035 (8) |
| C121 | 0.0255 (11) | 0.0375 (14) | 0.0268 (11) | −0.0026 (11) | 0.0028 (9) | −0.0006 (11) |
| C122 | 0.0268 (12) | 0.0527 (18) | 0.0278 (12) | −0.0056 (12) | 0.0041 (9) | 0.0032 (13) |
| O123 | 0.0274 (8) | 0.0407 (11) | 0.0264 (8) | −0.0048 (8) | 0.0019 (6) | 0.0068 (8) |
| C124 | 0.0308 (11) | 0.0300 (13) | 0.0231 (11) | −0.0002 (10) | 0.0019 (9) | 0.0024 (10) |
| C125 | 0.0297 (12) | 0.0313 (13) | 0.0247 (11) | −0.0025 (10) | 0.0010 (9) | 0.0040 (10) |
| C126 | 0.0334 (13) | 0.0430 (16) | 0.0294 (12) | 0.0014 (12) | −0.0004 (10) | −0.0060 (12) |
| C127 | 0.0342 (13) | 0.0577 (19) | 0.0359 (14) | 0.0035 (14) | 0.0024 (11) | 0.0010 (14) |
| C128 | 0.0325 (13) | 0.0510 (19) | 0.0407 (14) | −0.0064 (13) | −0.0058 (11) | 0.0092 (14) |
| C129 | 0.0456 (16) | 0.0385 (16) | 0.0380 (14) | −0.0092 (13) | −0.0091 (12) | −0.0012 (13) |
| C130 | 0.0420 (14) | 0.0284 (13) | 0.0319 (13) | −0.0018 (12) | −0.0050 (11) | −0.0005 (11) |
Geometric parameters (Å, °)
| O1—C2 | 1.432 (3) | O101—C102 | 1.429 (3) |
| O1—C24 | 1.422 (3) | O101—C124 | 1.425 (3) |
| C2—C3 | 1.518 (3) | C102—C103 | 1.511 (3) |
| C2—C21 | 1.527 (4) | C102—C121 | 1.526 (4) |
| C2—H21 | 1.000 | C102—H1021 | 1.002 |
| C3—O4 | 1.416 (3) | C103—O104 | 1.428 (3) |
| C3—C18 | 1.526 (4) | C103—C118 | 1.531 (4) |
| C3—H31 | 0.973 | C103—H1031 | 0.991 |
| O4—C5 | 1.451 (3) | O104—C105 | 1.448 (3) |
| C5—C6 | 1.518 (3) | C105—C106 | 1.516 (3) |
| C5—C12 | 1.512 (3) | C105—C112 | 1.521 (3) |
| C5—H51 | 1.009 | C105—H1051 | 1.011 |
| C6—C7 | 1.387 (4) | C106—C107 | 1.388 (4) |
| C6—C11 | 1.396 (3) | C106—C111 | 1.393 (4) |
| C7—C8 | 1.398 (4) | C107—C108 | 1.385 (4) |
| C7—H71 | 0.980 | C107—H1071 | 0.938 |
| C8—C9 | 1.374 (4) | C108—C109 | 1.377 (5) |
| C8—H81 | 0.952 | C108—H1081 | 0.963 |
| C9—C10 | 1.377 (5) | C109—C110 | 1.387 (5) |
| C9—H91 | 0.933 | C109—H1091 | 0.930 |
| C10—C11 | 1.390 (4) | C110—C111 | 1.388 (5) |
| C10—H101 | 0.969 | C110—H1101 | 0.932 |
| C11—H111 | 0.947 | C111—H1111 | 0.941 |
| C12—C13 | 1.388 (4) | C112—C113 | 1.393 (4) |
| C12—C17 | 1.382 (4) | C112—C117 | 1.393 (3) |
| C13—C14 | 1.389 (4) | C113—C114 | 1.395 (4) |
| C13—H131 | 0.939 | C113—H1131 | 0.991 |
| C14—C15 | 1.378 (5) | C114—C115 | 1.381 (4) |
| C14—H141 | 0.948 | C114—H1141 | 0.964 |
| C15—C16 | 1.374 (5) | C115—C116 | 1.380 (4) |
| C15—H151 | 0.946 | C115—H1151 | 0.946 |
| C16—C17 | 1.390 (4) | C116—C117 | 1.396 (3) |
| C16—H161 | 0.961 | C116—H1161 | 0.960 |
| C17—H171 | 0.916 | C117—H1171 | 0.986 |
| C18—O19 | 1.202 (3) | C118—O119 | 1.201 (3) |
| C18—O20 | 1.357 (3) | C118—O120 | 1.346 (3) |
| O20—C21 | 1.458 (3) | O120—C121 | 1.456 (3) |
| C21—C22 | 1.504 (4) | C121—C122 | 1.511 (3) |
| C21—H211 | 1.026 | C121—H1211 | 0.994 |
| C22—O23 | 1.428 (3) | C122—O123 | 1.430 (3) |
| C22—H221 | 1.021 | C122—H1221 | 0.998 |
| C22—H222 | 0.989 | C122—H1222 | 0.998 |
| O23—C24 | 1.402 (3) | O123—C124 | 1.409 (3) |
| C24—C25 | 1.511 (3) | C124—C125 | 1.503 (3) |
| C24—H241 | 0.997 | C124—H1241 | 0.984 |
| C25—C26 | 1.392 (4) | C125—C126 | 1.382 (4) |
| C25—C30 | 1.385 (4) | C125—C130 | 1.389 (4) |
| C26—C27 | 1.392 (4) | C126—C127 | 1.387 (4) |
| C26—H261 | 0.961 | C126—H1261 | 0.953 |
| C27—C28 | 1.385 (5) | C127—C128 | 1.386 (5) |
| C27—H271 | 0.961 | C127—H1271 | 0.962 |
| C28—C29 | 1.380 (5) | C128—C129 | 1.379 (5) |
| C28—H281 | 0.962 | C128—H1281 | 0.955 |
| C29—C30 | 1.394 (4) | C129—C130 | 1.394 (4) |
| C29—H291 | 0.986 | C129—H1291 | 0.954 |
| C30—H301 | 0.948 | C130—H1301 | 0.977 |
| C2—O1—C24 | 112.39 (17) | C102—O101—C124 | 112.03 (17) |
| O1—C2—C3 | 104.75 (18) | O101—C102—C103 | 106.20 (18) |
| O1—C2—C21 | 110.80 (19) | O101—C102—C121 | 111.3 (2) |
| C3—C2—C21 | 102.2 (2) | C103—C102—C121 | 102.0 (2) |
| O1—C2—H21 | 112.6 | O101—C102—H1021 | 110.8 |
| C3—C2—H21 | 112.2 | C103—C102—H1021 | 117.7 |
| C21—C2—H21 | 113.5 | C121—C102—H1021 | 108.5 |
| C2—C3—O4 | 108.98 (18) | C102—C103—O104 | 107.53 (19) |
| C2—C3—C18 | 102.1 (2) | C102—C103—C118 | 102.1 (2) |
| O4—C3—C18 | 106.5 (2) | O104—C103—C118 | 107.4 (2) |
| C2—C3—H31 | 110.6 | C102—C103—H1031 | 115.0 |
| O4—C3—H31 | 113.3 | O104—C103—H1031 | 112.1 |
| C18—C3—H31 | 114.7 | C118—C103—H1031 | 112.1 |
| C3—O4—C5 | 112.91 (17) | C103—O104—C105 | 113.76 (17) |
| O4—C5—C6 | 110.32 (19) | O104—C105—C106 | 111.0 (2) |
| O4—C5—C12 | 105.39 (18) | O104—C105—C112 | 105.51 (17) |
| C6—C5—C12 | 114.4 (2) | C106—C105—C112 | 114.5 (2) |
| O4—C5—H51 | 107.6 | O104—C105—H1051 | 106.0 |
| C6—C5—H51 | 107.6 | C106—C105—H1051 | 110.5 |
| C12—C5—H51 | 111.3 | C112—C105—H1051 | 108.9 |
| C5—C6—C7 | 121.7 (2) | C105—C106—C107 | 122.0 (2) |
| C5—C6—C11 | 119.1 (2) | C105—C106—C111 | 118.6 (2) |
| C7—C6—C11 | 119.2 (2) | C107—C106—C111 | 119.4 (3) |
| C6—C7—C8 | 120.3 (3) | C106—C107—C108 | 120.6 (3) |
| C6—C7—H71 | 120.5 | C106—C107—H1071 | 119.8 |
| C8—C7—H71 | 119.2 | C108—C107—H1071 | 119.6 |
| C7—C8—C9 | 119.7 (3) | C107—C108—C109 | 119.9 (3) |
| C7—C8—H81 | 119.1 | C107—C108—H1081 | 121.1 |
| C9—C8—H81 | 121.2 | C109—C108—H1081 | 119.0 |
| C8—C9—C10 | 120.6 (3) | C108—C109—C110 | 120.1 (3) |
| C8—C9—H91 | 119.5 | C108—C109—H1091 | 121.1 |
| C10—C9—H91 | 119.9 | C110—C109—H1091 | 118.7 |
| C9—C10—C11 | 120.1 (3) | C109—C110—C111 | 120.2 (3) |
| C9—C10—H101 | 119.7 | C109—C110—H1101 | 120.7 |
| C11—C10—H101 | 120.1 | C111—C110—H1101 | 119.1 |
| C6—C11—C10 | 120.0 (3) | C106—C111—C110 | 119.8 (3) |
| C6—C11—H111 | 120.7 | C106—C111—H1111 | 119.4 |
| C10—C11—H111 | 119.3 | C110—C111—H1111 | 120.8 |
| C5—C12—C13 | 119.8 (2) | C105—C112—C113 | 118.8 (2) |
| C5—C12—C17 | 120.8 (2) | C105—C112—C117 | 122.6 (2) |
| C13—C12—C17 | 119.4 (2) | C113—C112—C117 | 118.6 (2) |
| C12—C13—C14 | 120.5 (3) | C112—C113—C114 | 120.9 (3) |
| C12—C13—H131 | 122.2 | C112—C113—H1131 | 119.8 |
| C14—C13—H131 | 117.3 | C114—C113—H1131 | 119.2 |
| C13—C14—C15 | 119.6 (3) | C113—C114—C115 | 119.7 (3) |
| C13—C14—H141 | 119.9 | C113—C114—H1141 | 119.0 |
| C15—C14—H141 | 120.5 | C115—C114—H1141 | 121.2 |
| C14—C15—C16 | 120.3 (3) | C114—C115—C116 | 120.1 (2) |
| C14—C15—H151 | 122.4 | C114—C115—H1151 | 120.3 |
| C16—C15—H151 | 117.4 | C116—C115—H1151 | 119.6 |
| C15—C16—C17 | 120.4 (3) | C115—C116—C117 | 120.2 (2) |
| C15—C16—H161 | 122.9 | C115—C116—H1161 | 118.9 |
| C17—C16—H161 | 116.7 | C117—C116—H1161 | 120.8 |
| C16—C17—C12 | 119.9 (3) | C116—C117—C112 | 120.3 (2) |
| C16—C17—H171 | 120.0 | C116—C117—H1171 | 122.7 |
| C12—C17—H171 | 120.0 | C112—C117—H1171 | 116.9 |
| C3—C18—O19 | 128.2 (3) | C103—C118—O119 | 128.2 (3) |
| C3—C18—O20 | 109.4 (2) | C103—C118—O120 | 109.7 (2) |
| O19—C18—O20 | 122.3 (3) | O119—C118—O120 | 122.0 (3) |
| C18—O20—C21 | 110.2 (2) | C118—O120—C121 | 110.0 (2) |
| C2—C21—O20 | 104.14 (18) | C102—C121—O120 | 104.57 (19) |
| C2—C21—C22 | 113.2 (2) | C102—C121—C122 | 113.7 (2) |
| O20—C21—C22 | 110.3 (2) | O120—C121—C122 | 109.8 (2) |
| C2—C21—H211 | 110.3 | C102—C121—H1211 | 109.4 |
| O20—C21—H211 | 109.0 | O120—C121—H1211 | 107.1 |
| C22—C21—H211 | 109.7 | C122—C121—H1211 | 111.9 |
| C21—C22—O23 | 111.80 (19) | C121—C122—O123 | 111.63 (19) |
| C21—C22—H221 | 108.5 | C121—C122—H1221 | 108.8 |
| O23—C22—H221 | 112.7 | O123—C122—H1221 | 109.1 |
| C21—C22—H222 | 109.5 | C121—C122—H1222 | 108.2 |
| O23—C22—H222 | 105.2 | O123—C122—H1222 | 110.5 |
| H221—C22—H222 | 109.0 | H1221—C122—H1222 | 108.5 |
| C22—O23—C24 | 110.9 (2) | C122—O123—C124 | 110.2 (2) |
| O1—C24—O23 | 110.67 (19) | O101—C124—O123 | 109.8 (2) |
| O1—C24—C25 | 107.49 (18) | O101—C124—C125 | 108.28 (18) |
| O23—C24—C25 | 108.6 (2) | O123—C124—C125 | 109.0 (2) |
| O1—C24—H241 | 109.8 | O101—C124—H1241 | 109.1 |
| O23—C24—H241 | 109.3 | O123—C124—H1241 | 110.5 |
| C25—C24—H241 | 111.0 | C125—C124—H1241 | 110.2 |
| C24—C25—C26 | 119.5 (2) | C124—C125—C126 | 120.9 (2) |
| C24—C25—C30 | 120.7 (2) | C124—C125—C130 | 119.3 (2) |
| C26—C25—C30 | 119.9 (2) | C126—C125—C130 | 119.8 (2) |
| C25—C26—C27 | 120.0 (3) | C125—C126—C127 | 120.2 (3) |
| C25—C26—H261 | 122.5 | C125—C126—H1261 | 119.7 |
| C27—C26—H261 | 117.5 | C127—C126—H1261 | 120.0 |
| C26—C27—C28 | 120.0 (3) | C126—C127—C128 | 120.0 (3) |
| C26—C27—H271 | 120.1 | C126—C127—H1271 | 119.9 |
| C28—C27—H271 | 119.9 | C128—C127—H1271 | 120.0 |
| C27—C28—C29 | 120.0 (3) | C127—C128—C129 | 120.1 (3) |
| C27—C28—H281 | 119.3 | C127—C128—H1281 | 119.0 |
| C29—C28—H281 | 120.7 | C129—C128—H1281 | 121.0 |
| C28—C29—C30 | 120.3 (3) | C128—C129—C130 | 120.0 (3) |
| C28—C29—H291 | 120.5 | C128—C129—H1291 | 119.4 |
| C30—C29—H291 | 119.2 | C130—C129—H1291 | 120.7 |
| C29—C30—C25 | 119.8 (3) | C129—C130—C125 | 119.9 (3) |
| C29—C30—H301 | 118.6 | C129—C130—H1301 | 120.3 |
| C25—C30—H301 | 121.6 | C125—C130—H1301 | 119.8 |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C8—H81···O19i | 0.95 | 2.57 | 3.520 (4) | 173 |
| C14—H141···O119ii | 0.95 | 2.55 | 3.309 (4) | 137 |
| C28—H281···O19iii | 0.96 | 2.57 | 3.225 (4) | 126 |
Symmetry codes: (i) x, y+1, z; (ii) x, y, z−1; (iii) −x+1, y+1/2, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LH2623).
References
- Altomare, A., Cascarano, G., Giacovazzo, C., Guagliardi, A., Burla, M. C., Polidori, G. & Camalli, M. (1994). J. Appl. Cryst.27, 435.
- Best, D., Jenkinson, S. F., Rule, S. D., Higham, R., Mercer, T. B., Newell, R. J., Weymouth-Wilson, A. C., Fleet, G. W. J. & Petursson, S. (2008). Tetrahedron Lett.49, 2196–2199.
- Betteridge, P. W., Carruthers, J. R., Cooper, R. I., Prout, K. & Watkin, D. J. (2003). J. Appl. Cryst.36, 1487.
- Collins, P. M. & Ferrier, R. J. (1995). In Monosaccharides: Their Chemistry and Their Roles in Natural Products. New York: John Wiley & Sons.
- Draths, K. M., Pompliano, D. L., Conley, D. L., Frost, J. W., Berry, A., Disbrow, G. L., Staversky, R. J. & Lievenset, J. C. (1992). J. Am. Chem. Soc.114, 3956–3962.
- Görbitz, C. H. (1999). Acta Cryst. B55, 1090–1098. [DOI] [PubMed]
- Jackson, G., Jones, H. F., Petursson, S. & Webber, J. M. (1982). Carbohydr. Res.102, 147–157.
- Jenkinson, S. F., Rule, S. D., Booth, K. V., Fleet, G. W. J., Watkin, D. J. & Petursson, S. (2008). Acta Cryst. E64, o26. [DOI] [PMC free article] [PubMed]
- Nonius (2001). COLLECT Nonius BV, Delft, The Netherlands.
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
- Petursson, S. (2001). Carbohydr. Res.331, 239–245. [DOI] [PubMed]
- Petursson, S. (2003). Carbohydr. Res.338, 963–968. [DOI] [PubMed]
- Petursson, S., Jenkinson, S. F., Booth, K. V., Weymouth-Wilson, A. C., Watkin, D. J., Fleet, G. W. J. & Best, D. (2007). Acta Cryst. E63, o4121.
- Petursson, S. & Webber, J. M. (1982). Carbohydr. Res.103, 41–52.
- Watkin, D. J., Prout, C. K. & Pearce, L. J. (1996). CAMERON Chemical Crystallography Laboratory, Oxford, UK.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808012567/lh2623sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808012567/lh2623Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report