Abstract
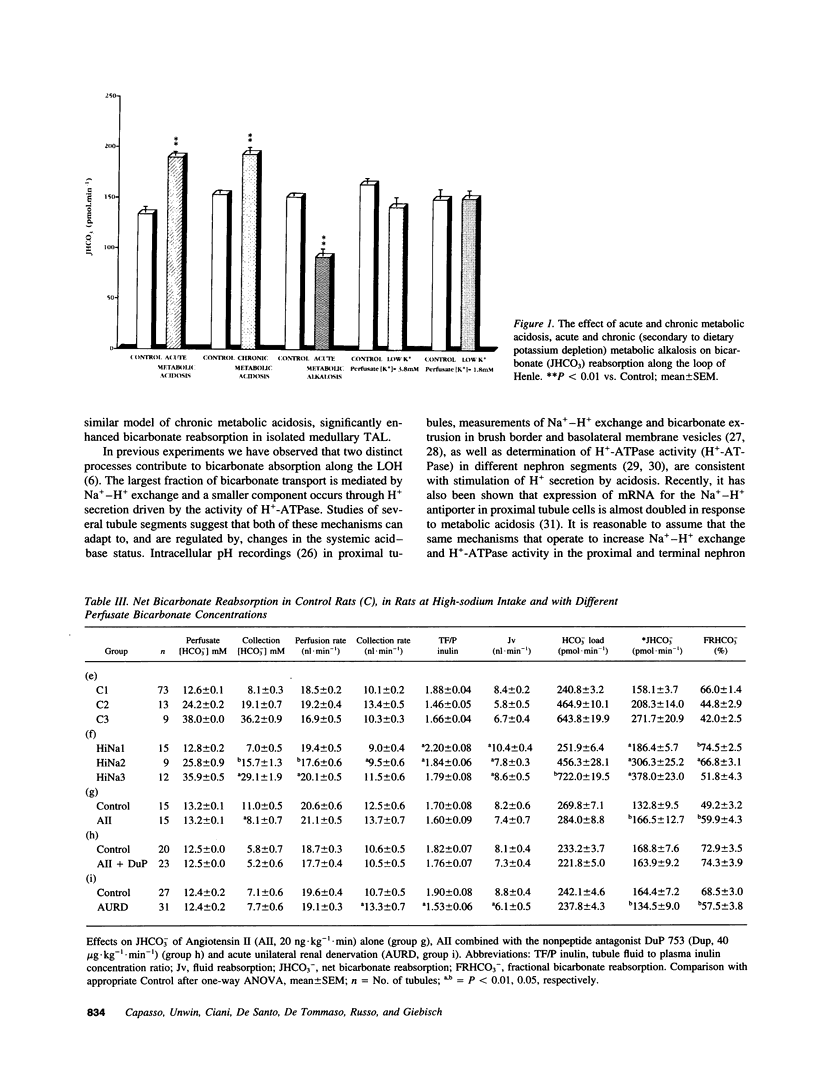
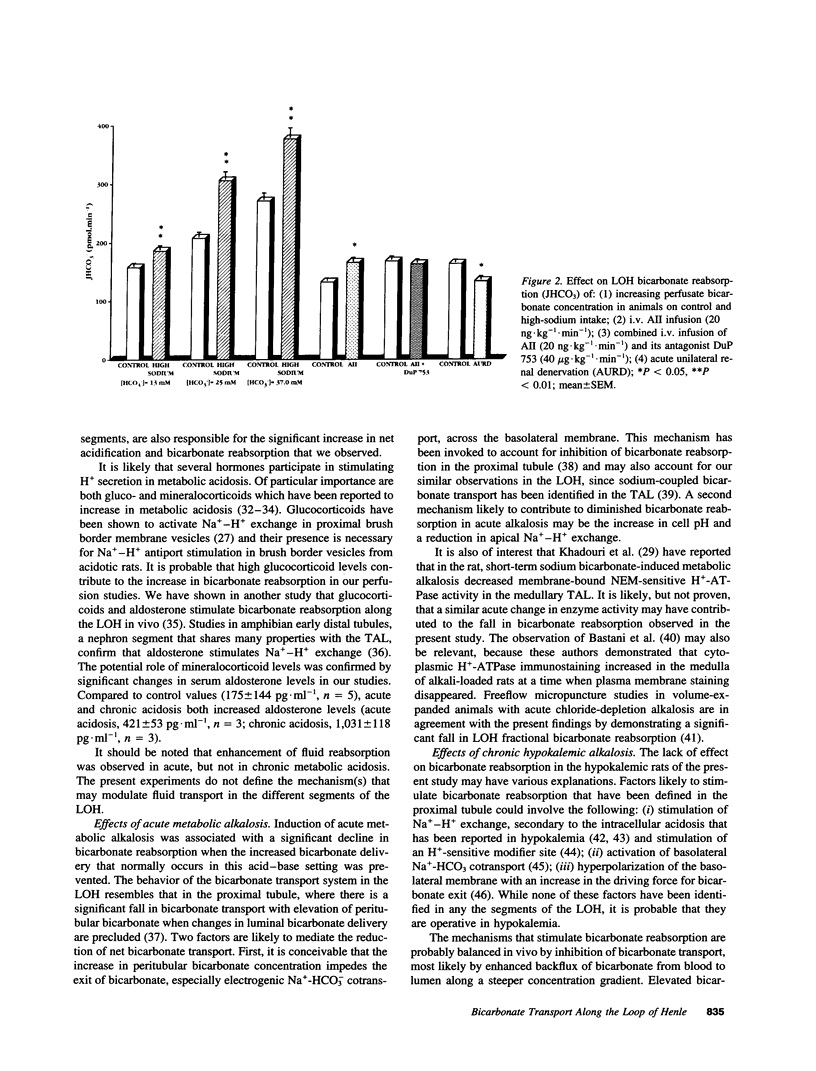
The loop of Henle contributes to renal acidification by reabsorbing about 15% of filtered bicarbonate. To study the effects on loop of Henle bicarbonate transport (JHCO3) of acid-base disturbances and of several factors known to modulate sodium transport, these in vivo microperfusion studies were carried out in rats during: (a) acute and chronic metabolic acidosis, (b) acute and chronic (hypokalemic) metabolic alkalosis, (c) a control sodium diet, (d) a high-sodium diet, (e) angiotensin II (AII) intravenous infusion, (f) simultaneously intravenous infusion of both AII and the AT1 receptor antagonist DuP 753, (g) acute ipsilateral mechanicochemical renal denervation. Acute and chronic metabolic acidosis increased JHCO3; acute metabolic alkalosis significantly reduced JHCO3, whereas chronic hypokalemic alkalosis did not alter JHCO3. Bicarbonate transport increased in animals on a high-sodium intake and following AII administration, and the latter was inhibited by the AII (AT1) receptor antagonist DuP 753; acute renal denervation lowered bicarbonate transport. These data indicate that bicarbonate reabsorption along the loop of Henle in vivo is closely linked to systemic acid-base status and to several factors known to modulate sodium transport.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam W. R., Koretsky A. P., Weiner M. W. 31P-NMR in vivo measurement of renal intracellular pH: effects of acidosis and K+ depletion in rats. Am J Physiol. 1986 Nov;251(5 Pt 2):F904–F910. doi: 10.1152/ajprenal.1986.251.5.F904. [DOI] [PubMed] [Google Scholar]
- Alpern R. J. Mechanism of basolateral membrane H+/OH-/HCO-3 transport in the rat proximal convoluted tubule. A sodium-coupled electrogenic process. J Gen Physiol. 1985 Nov;86(5):613–636. doi: 10.1085/jgp.86.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson P. S., Nee J., Suhm M. A. Modifier role of internal H+ in activating the Na+-H+ exchanger in renal microvillus membrane vesicles. Nature. 1982 Sep 9;299(5879):161–163. doi: 10.1038/299161a0. [DOI] [PubMed] [Google Scholar]
- BROWN J. J., DAVIES D. L., LEVER A. F., ROBERTSON J. I. INFLUENCE OF SODIUM DEPRIVATION AND LOADING ON THE PLASMA-RENIN IN MAN. J Physiol. 1964 Oct;173:408–419. doi: 10.1113/jphysiol.1964.sp007464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barajas L., Liu L., Powers K. Anatomy of the renal innervation: intrarenal aspects and ganglia of origin. Can J Physiol Pharmacol. 1992 May;70(5):735–749. doi: 10.1139/y92-098. [DOI] [PubMed] [Google Scholar]
- Bastani B., Purcell H., Hemken P., Trigg D., Gluck S. Expression and distribution of renal vacuolar proton-translocating adenosine triphosphatase in response to chronic acid and alkali loads in the rat. J Clin Invest. 1991 Jul;88(1):126–136. doi: 10.1172/JCI115268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencsáth P., Szénási G., Takács L. Water and electrolyte transport in Henle's loop and distal tubule after renal sympathectomy in the rat. Am J Physiol. 1985 Aug;249(2 Pt 2):F308–F314. doi: 10.1152/ajprenal.1985.249.2.F308. [DOI] [PubMed] [Google Scholar]
- Buerkert J., Martin D., Trigg D. Segmental analysis of the renal tubule in buffer production and net acid formation. Am J Physiol. 1983 Apr;244(4):F442–F454. doi: 10.1152/ajprenal.1983.244.4.F442. [DOI] [PubMed] [Google Scholar]
- Capasso G., Jaeger P., Giebisch G., Guckian V., Malnic G. Renal bicarbonate reabsorption in the rat. II. Distal tubule load dependence and effect of hypokalemia. J Clin Invest. 1987 Aug;80(2):409–414. doi: 10.1172/JCI113087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso G., Kinne R., Malnic G., Giebisch G. Renal bicarbonate reabsorption in the rat. I. Effects of hypokalemia and carbonic anhydrase. J Clin Invest. 1986 Dec;78(6):1558–1567. doi: 10.1172/JCI112748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso G., Unwin R., Agulian S., Giebisch G. Bicarbonate transport along the loop of Henle. I. Microperfusion studies of load and inhibitor sensitivity. J Clin Invest. 1991 Aug;88(2):430–437. doi: 10.1172/JCI115322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cemerikić D., Wilcox C. S., Giebisch G. Intracellular potential and K+ activity in rat kidney proximal tubular cells in acidosis and K+ depletion. J Membr Biol. 1982;69(2):159–165. doi: 10.1007/BF01872275. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Biagi B., Giebisch G. Control mechanisms of bicarbonate transport across the rat proximal convoluted tubule. Am J Physiol. 1982 May;242(5):F532–F543. doi: 10.1152/ajprenal.1982.242.5.F532. [DOI] [PubMed] [Google Scholar]
- Cogan M. G. Neurogenic regulation of proximal bicarbonate and chloride reabsorption. Am J Physiol. 1986 Jan;250(1 Pt 2):F22–F26. doi: 10.1152/ajprenal.1986.250.1.F22. [DOI] [PubMed] [Google Scholar]
- Cogan M. G., Rector F. C., Jr Proximal reabsorption during metabolic acidosis in the rat. Am J Physiol. 1982 May;242(5):F499–F507. doi: 10.1152/ajprenal.1982.242.5.F499. [DOI] [PubMed] [Google Scholar]
- DiBona G. F., Sawin L. L. Effect of renal nerve stimulation on NaCl and H2O transport in Henle's loop of the rat. Am J Physiol. 1982 Dec;243(6):F576–F580. doi: 10.1152/ajprenal.1982.243.6.F576. [DOI] [PubMed] [Google Scholar]
- DiBona G. F., Sawin L. L. Renal nerve activity in conscious rats during volume expansion and depletion. Am J Physiol. 1985 Jan;248(1 Pt 2):F15–F23. doi: 10.1152/ajprenal.1985.248.1.F15. [DOI] [PubMed] [Google Scholar]
- DiBona G. F., Sawin L. L. Renal nerves in renal adaptation to dietary sodium restriction. Am J Physiol. 1983 Sep;245(3):F322–F328. doi: 10.1152/ajprenal.1983.245.3.F322. [DOI] [PubMed] [Google Scholar]
- DuBose T. D., Jr, Lucci M. S., Hogg R. J., Pucacco L. R., Kokko J. P., Carter N. W. Comparison of acidification parameters in superficial and deep nephrons of the rat. Am J Physiol. 1983 May;244(5):F497–F503. doi: 10.1152/ajprenal.1983.244.5.F497. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK C. W., LASSITER W. E., MYLLE M. Localization of urine acidification in the mammalian kidney. Am J Physiol. 1960 Mar;198:581–585. doi: 10.1152/ajplegacy.1960.198.3.581. [DOI] [PubMed] [Google Scholar]
- Galla J. H., Bonduris D. N., Luke R. G. Effects of chloride and extracellular fluid volume on bicarbonate reabsorption along the nephron in metabolic alkalosis in the rat. Reassessment of the classical hypothesis of the pathogenesis of metabolic alkalosis. J Clin Invest. 1987 Jul;80(1):41–50. doi: 10.1172/JCI113061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg L. C., Narang N. Stimulation of an N-ethylmaleimide-sensitive ATPase in the collecting duct segments of the rat nephron by metabolic acidosis. Can J Physiol Pharmacol. 1985 Oct;63(10):1291–1296. doi: 10.1139/y85-213. [DOI] [PubMed] [Google Scholar]
- Garvin J. L. Angiotensin stimulates bicarbonate transport and Na+/K+ ATPase in rat proximal straight tubules. J Am Soc Nephrol. 1991 Apr;1(10):1146–1152. doi: 10.1681/ASN.V1101146. [DOI] [PubMed] [Google Scholar]
- Garvin J. L., Knepper M. A. Bicarbonate and ammonia transport in isolated perfused rat proximal straight tubules. Am J Physiol. 1987 Aug;253(2 Pt 2):F277–F281. doi: 10.1152/ajprenal.1987.253.2.F277. [DOI] [PubMed] [Google Scholar]
- Geibel J., Giebisch G., Boron W. F. Angiotensin II stimulates both Na(+)-H+ exchange and Na+/HCO3- cotransport in the rabbit proximal tubule. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7917–7920. doi: 10.1073/pnas.87.20.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good D. W. Adaptation of HCO-3 and NH+4 transport in rat MTAL: effects of chronic metabolic acidosis and Na+ intake. Am J Physiol. 1990 May;258(5 Pt 2):F1345–F1353. doi: 10.1152/ajprenal.1990.258.5.F1345. [DOI] [PubMed] [Google Scholar]
- Good D. W. Bicarbonate absorption by the thick ascending limb of Henle's loop. Semin Nephrol. 1990 Mar;10(2):132–138. [PubMed] [Google Scholar]
- Good D. W. Sodium-dependent bicarbonate absorption by cortical thick ascending limb of rat kidney. Am J Physiol. 1985 Jun;248(6 Pt 2):F821–F829. doi: 10.1152/ajprenal.1985.248.6.F821. [DOI] [PubMed] [Google Scholar]
- Good D. W. The thick ascending limb as a site of renal bicarbonate reabsorption. Semin Nephrol. 1993 Mar;13(2):225–235. [PubMed] [Google Scholar]
- Kakinuma Y., Fogo A., Inagami T., Ichikawa I. Intrarenal localization of angiotensin II type 1 receptor mRNA in the rat. Kidney Int. 1993 Jun;43(6):1229–1235. doi: 10.1038/ki.1993.174. [DOI] [PubMed] [Google Scholar]
- Khadouri C., Marsy S., Barlet-Bas C., Cheval L., Doucet A. Effect of metabolic acidosis and alkalosis on NEM-sensitive ATPase in rat nephron segments. Am J Physiol. 1992 Apr;262(4 Pt 2):F583–F590. doi: 10.1152/ajprenal.1992.262.4.F583. [DOI] [PubMed] [Google Scholar]
- Kinsella J., Cujdik T., Sacktor B. Na+-H+ exchange activity in renal brush border membrane vesicles in response to metabolic acidosis: The role of glucocorticoids. Proc Natl Acad Sci U S A. 1984 Jan;81(2):630–634. doi: 10.1073/pnas.81.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapf R. Basolateral membrane H/OH/HCO3 transport in the rat cortical thick ascending limb. Evidence for an electrogenic Na/HCO3 cotransporter in parallel with a Na/H antiporter. J Clin Invest. 1988 Jul;82(1):234–241. doi: 10.1172/JCI113576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapf R., Pearce D., Lynch C., Xi X. P., Reudelhuber T. L., Pouysségur J., Rector F. C., Jr Expression of rat renal Na/H antiporter mRNA levels in response to respiratory and metabolic acidosis. J Clin Invest. 1991 Feb;87(2):747–751. doi: 10.1172/JCI115057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunau R. T., Jr, Hart J. I., Walker K. A. Effect of metabolic acidosis on proximal tubular total CO2 absorption. Am J Physiol. 1985 Jul;249(1 Pt 2):F62–F68. doi: 10.1152/ajprenal.1985.249.1.F62. [DOI] [PubMed] [Google Scholar]
- Levine D. Z., Byers M. K., McLeod R. A., Luisello J. A., Raman S. Loop of Henle bicarbonate accumulation in vivo in the rat. J Clin Invest. 1979 Jan;63(1):59–66. doi: 10.1172/JCI109278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F. Y., Cogan M. G. Acidification is inhibited in late proximal convoluted tubule during chronic metabolic alkalosis. Am J Physiol. 1987 Jul;253(1 Pt 2):F89–F94. doi: 10.1152/ajprenal.1987.253.1.F89. [DOI] [PubMed] [Google Scholar]
- Liu F. Y., Cogan M. G. Angiotensin II stimulation of hydrogen ion secretion in the rat early proximal tubule. Modes of action, mechanism, and kinetics. J Clin Invest. 1988 Aug;82(2):601–607. doi: 10.1172/JCI113638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnic G., De Mello Aires M., Giebisch G. Micropuncture study of renal tubular hydrogen ion transport in the rat. Am J Physiol. 1972 Jan;222(1):147–158. doi: 10.1152/ajplegacy.1972.222.1.147. [DOI] [PubMed] [Google Scholar]
- Moe O. W., Tejedor A., Levi M., Seldin D. W., Preisig P. A., Alpern R. J. Dietary NaCl modulates Na(+)-H+ antiporter activity in renal cortical apical membrane vesicles. Am J Physiol. 1991 Jan;260(1 Pt 2):F130–F137. doi: 10.1152/ajprenal.1991.260.1.F130. [DOI] [PubMed] [Google Scholar]
- Mujais S. K., Kauffman S., Katz A. I. Angiotensin II binding sites in individual segments of the rat nephron. J Clin Invest. 1986 Jan;77(1):315–318. doi: 10.1172/JCI112293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord E. P., Howard M. J., Hafezi A., Moradeshagi P., Vaystub S., Insel P. A. Alpha 2 adrenergic agonists stimulate Na+-H+ antiport activity in the rabbit renal proximal tubule. J Clin Invest. 1987 Dec;80(6):1755–1762. doi: 10.1172/JCI113268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberleithner H., Weigt M., Westphale H. J., Wang W. Aldosterone activates Na+/H+ exchange and raises cytoplasmic pH in target cells of the amphibian kidney. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1464–1468. doi: 10.1073/pnas.84.5.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez G. O., Oster J. R., Katz F. H., Vaamonde C. A. The effect of acute metabolic acidosis on plasma cortisol, renin activity and aldosterone. Horm Res. 1979;11(1):12–21. doi: 10.1159/000179033. [DOI] [PubMed] [Google Scholar]
- Perez G. O., Oster J. R., Vaamonde C. A., Katz F. H. Effect of NH4Cl on plasma aldosterone, cortisol and renin activity in supine man. J Clin Endocrinol Metab. 1977 Oct;45(4):762–767. doi: 10.1210/jcem-45-4-762. [DOI] [PubMed] [Google Scholar]
- Preisig P. A., Alpern R. J. Chronic metabolic acidosis causes an adaptation in the apical membrane Na/H antiporter and basolateral membrane Na(HCO3)3 symporter in the rat proximal convoluted tubule. J Clin Invest. 1988 Oct;82(4):1445–1453. doi: 10.1172/JCI113750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzer P., Wald H., Czaczkes J. W. Na-K-ATPase in isolated rabbit tubules after unilateral nephrectomy and Na+ loading. Am J Physiol. 1985 Apr;248(4 Pt 2):F565–F573. doi: 10.1152/ajprenal.1985.248.4.F565. [DOI] [PubMed] [Google Scholar]
- Sealey J. E., Bühler F. R., Laragh J. H., Manning E. L., Brunner H. R. Aldosterone excretion. Physiological variations in man measured by radioimmunoassay or double-isotope dilution. Circ Res. 1972 Sep;31(3):367–378. doi: 10.1161/01.res.31.3.367. [DOI] [PubMed] [Google Scholar]
- Soleimani M., Bizal G. L., McKinney T. D., Hattabaugh Y. J. Effect of in vitro metabolic acidosis on luminal Na+/H+ exchange and basolateral Na+:HCO3- cotransport in rabbit kidney proximal tubules. J Clin Invest. 1992 Jul;90(1):211–218. doi: 10.1172/JCI115838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton B. A., Kaissling B. Regulation of renal ion transport and cell growth by sodium. Am J Physiol. 1989 Jul;257(1 Pt 2):F1–10. doi: 10.1152/ajprenal.1989.257.1.F1. [DOI] [PubMed] [Google Scholar]
- Terada Y., Tomita K., Nonoguchi H., Marumo F. PCR localization of angiotensin II receptor and angiotensinogen mRNAs in rat kidney. Kidney Int. 1993 Jun;43(6):1251–1259. doi: 10.1038/ki.1993.177. [DOI] [PubMed] [Google Scholar]
- Uhlich E., Baldamus C. A., Ullrich K. J. Verhalten von CO2-Druck und Bicarbonat im Gegenstromysystem des Nierenmarks. Pflugers Arch. 1968;303(1):31–48. doi: 10.1007/BF00586825. [DOI] [PubMed] [Google Scholar]
- Wahl M., Schnermann J. Microdissection study of the length of different tubular segments of rat superficial nephrons. Z Anat Entwicklungsgesch. 1969;129(2):128–134. doi: 10.1007/BF00522242. [DOI] [PubMed] [Google Scholar]
- Wang T., Chan Y. L. Neural control of distal tubular bicarbonate and fluid transport. Am J Physiol. 1989 Jul;257(1 Pt 2):F72–F76. doi: 10.1152/ajprenal.1989.257.1.F72. [DOI] [PubMed] [Google Scholar]
- Weigt M., Dietl P., Silbernagl S., Oberleithner H. Activation of luminal Na+/H+ exchange in distal nephron of frog kidney. An early response to aldosterone. Pflugers Arch. 1987 May;408(6):609–614. doi: 10.1007/BF00581163. [DOI] [PubMed] [Google Scholar]
- Welbourne T. C. Acidosis activation of the pituitary-adrenal-renal glutaminase I axis. Endocrinology. 1976 Oct;99(4):1071–1079. doi: 10.1210/endo-99-4-1071. [DOI] [PubMed] [Google Scholar]